ABSTRACT
Normal passerines (n=216) were evaluated for oocysts of Isospora in feces at the Triage Center for Wild Animals (CETAS, IBAMA, Belo Horizonte; August 21 to September 21, 2012). The positive samples with oocysts represented 13.0% of Cardinalidae (n=23), 11.2% of Emberizidae (n=107), 50% of Icteridae (n=10) and 60.3% of Thraupidae (n=68). The probability of fecal oocysts attributable to the host in Thraupidae is higher than in Cardinalidae, Emberizidae, Fringillidae and Turdidae, but similar to Icteridae. No oocysts were found in Fringillidae and Turdidae. Within Thraupidae, Isospora was for the first time described in Paroaria dominicana and Schistochlamys ruficapillus and within Icteridae, in Gnorimopsar chopi. Saltator similis presented a higher risk, 66.9% greater than Lanio pileatus and Sporophila caerulescens and with a 27.9% greater probability than Sporophila nigricolis. The new coccidian species described were Isospora dominicana [ellipsoid oocysts, 25 (30-20) x 25 (28-20) µm] in Paroaria dominicana; Isospora beagai [ovoid oocysts, 28 (32-17) x 25 (29-16) µm] and Isospora ferri [ellipsoid oocysts, 20 (22-16) x 18 (22-15) µm] in Saltator similis; Isospora ruficapillus [spheric to subspherical oocysts, 25 (26-23) x 24 (25-21) µm] in Schistochlamys ruficapillus; and Isospora chopi [spherical to sub-spherical oocysts, 24.5 x 22 (30-20 x 25-20) µm] and Isospora gnorimopsar [sub-spherical to ovoid oocysts, 27 x 23 (32-22 x 28-20) µm] in Gnorimopsar chopi. The morphometry and features were compared with previously described Isospora in passerines. New coccidian species and new passerine hosts are described for Isospora and recommends for constant monitoring during rehabilitation, especially for the hosts of Thraupidae and Icteridae.
Keywords:
Apicomplexa; Gnorimopsar chopi; Isospora chopi; Isospora gnorimopsar; Paroaria dominicana; Isospora dominicana; Saltator similis; Isospora beagai; Isospora ferri; Schistochlamys ruficapillus; Isospora ruficapillus
Coccidiosis in the order Passeriformes is caused mostly by the genus Isospora (Page & Haddad, 1995Page CD, Haddad K. Coccidial infections in birds. Seminars in Avian and Exotic Pet Medicine 1995;4:138-144.; Duszynski et al., 2018; Freitas et al., 2002Freitas MFL, Oliveira JB, Cavalcanti MDB, Leite AS, Magalhães VS, Oliveira RA, et al. Parasitos gastrointestinales de aves silvestres en cautiverio en el estado de Pernambuco, Brasil. Parasitologia Latinoamericana 2002;57:50-54.; Marietto-Gonçalves et al., 2009Marietto-Gonçalves GA, Martins TF, Lima ET, Lopes RS, Filho RLA. Prevalência de endoparasitas em amostras fecais de aves silvestres e exóticas examinadas no laboratório de ornitopatologia e no laboratório de enfermidades parasitárias da FMVZ-UNESP/BOTUCATU, SP. Ciência Animal Brasileira 2009;10:349-354.; Brown et al., 2010Brown MA, Ball SL, Snow KR. Coccidian parasites of British wild birds. Journal of Natural History 2010;44:2669-2691.; Costa et al., 2010Costa IA, Coelho CD, Bueno C, Ferreira I, Freire RB. Ocorrência de parasitos gastrointestinais em aves silvestres no município de Seropédica, Rio de Janeiro, Brasil. Ciência Animal Brasileira 2010;11:914-922.; Berto et al., 2011aBerto BP, Luz HR, Flausino W, Teixeira-Filho WL, Ferreira I, Lopes CWG. Isosporoid Coccidia (Apicomplexa: Eimeriidae) parasites of tanagers (Passeriformes: Thraupidae) from the Marambaia Island, Brazil. Pesquisa Veterinária Brasileira 2011;31:798-805.) and may result in important clinical impacts for passerines during captivity (Page and Haddad, 1995; Friend and Frason, 1999Friend M, Franson JC, editors. Intestinal coccidiosis. In: Friend M, Franson JC. Field manual of wildlife diseases: general field and procedures and diseases of birds. Washington: United States Geological Survey, Biological Resources Division; 1999. p.207-213.; Brown et al., 2010; Berto et al., 2011a). In fact, infection by Isospora represents the most relevant parasitic disease for captive birds (Vilela et al., 2009Vilela DAR, Savernini THOPM, Mendes EJ, Campos SM, Andrade RA, Guimarães RC, et al. Ocorrência de coccídeos intestinais em passeriformes silvestres provenientes do tráfico e encaminhados para o Cetas do IBAMA em Belo Horizonte. Anais do 12º Congresso ABRAVAS; 2009; Águas de Lindóia, São Paulo. Brasil. p.56-57.; Costa et al., 2010; Keeler et al., 2011Keeler SP, Yabsley MG, Fox JM, McGraw SN, Hernandez SM. Isospora troglodytes n. sp. (Apicomplexa:Eimeriidae), a new coccidian species from wrens of Costa Rica. Parasitology Research 2011;110:1723-1725.; Pereira et al., 2011Pereira LQ, Berto BP, Flausino W, Lovato M, Lopes CWG. Isospora bocamontensis n. sp. (Apicomplexa:Eimeriidae) from the yellow cardinal Gubernatrix cristata (Vieillot) (Passeriformes:Emberizidae) in South America. Systematic Parasitology 2011;78:73-80.) and demands diagnostic surveillance and strategic or curative medication to reduce losses (Freitas et al., 2002; Yabsley, 2008Yabsley MJ. Eimeria. In: Atkinson, CT, Thomas NJ, Hunter DB, editors. Parasitic diseases of wild birds. Oxford: Wiley-Blackwell, 2008. p.162-180.; Marietto-Gonçalves et al., 2009). In contrast, subclinical infection (coccidiosis) is the most common form of infection in the wild, representing a minor health impact in free-living birds (Upton et al., 1995Upton SJ, Stamper MA, Whitaker BR. Isospora bellicosa sp. n. (Apicomplexa) from a Peruvian red-breasted meadowlark, Sturnella bellicose (Passeriformes:Icteridae). Archiv für Protistenkunde 1995;145:132-134.). However, most species of coccidians, especially of wild birds, are yet to be described (Moore & Clayton, 1997Moore J, Clayton DH. Conclusion: Evolution of host-parasite interactions. In: Clayton DH, More J, editors. Host-parasite evolution: general principles and avian models. New York: Oxford University Press; 1997. p.370-376.; Lederberg, 1998Lederberg J. Emerging infections:an evolutionary perspective. Emerging Infection Diseases 1998;4:366-371.; Freitas et al., 2002; Wobeser, 2008Wobeser GA. Parasitism: costs and effects. In: Atkinson, CT, Thomas NJ, Hunter DB, editors. Parasitic diseases of wild birds. Oxford: Wiley-Blackwell, 2008. p.3-12.). In captive birds, a fatal disease outbreak by Isospora icterus was reported in Brazil in a group of a native icterid passerine Icterus jamacaii (Campo oriole or Troupial) (Marques et al., 2011Marques MVR, Vilela DAR, Andrade EAG, Galvão GZ, Resende CZ, et al. Fatal coccidiosis by Isospora icterus (Upton & Whitaker 2000) in captive campo troupial (Icterus jamacaii) (Aves, Passeriformes, Icteridae) in Brazil. Journal of Zoo and Wildlife Medicine 2011;42:735-737.).
Twenty-one South-American passerine species are described as hosts for the genus Isospora, however, only 19 out of the 1073 species of Brazilian passerines have been studied for the occurrence of Isospora or other coccidians (Sick, 1997Sick H. Ornitologia Brasileira. Rio de Janeiro: Nova Fronteira; 1997. 862 p.; Berto et al., 2011aBerto BP, Luz HR, Flausino W, Teixeira-Filho WL, Ferreira I, Lopes CWG. Isosporoid Coccidia (Apicomplexa: Eimeriidae) parasites of tanagers (Passeriformes: Thraupidae) from the Marambaia Island, Brazil. Pesquisa Veterinária Brasileira 2011;31:798-805.; CBRO, 2014).
The Thraupidae and Icteridae member species are the most frequently sampled among those admitted at the wild animal triage centers in Brazil (Centros de Triagem de Animais Silvestres, CETAS) due to illegal trade (Ferreira & Glock, 2004Ferreira CM, Glock L. Diagnóstico preliminar sobra a avifauna traficada no Rio Grande do Sul, Brasil. Biociências 2004;12:21-30.; Borges et al., 2006Borges RC, Oliveira A, Bernardo N, Costa RMMC. Diagnóstico da fauna silvestre apreendida e recolhida pela Polícia Militar de Meio Ambiente de Juiz de Fora, MG (1998 e 1999). Revista Brasileira de Zoologia 2006;8:23-33.; Pagano et al., 2009Pagano ISA, Sousa AEB, Wagner PGC, Ramos RTC. Aves depositadas no Centro de Triagem de Animais Silvestres do IBAMA na Paraíba:uma amostra do tráfico de aves silvestres no estado. Ornithologia 2009;3:132-144.; Santos et al., 2011; Vilela, 2012Vilela DAR. Diagnóstico da Avifauna Encaminhada para os Centros de Triagem de Animais Silvestres (CETAS) do Brasil e Ocorrência de Clamidiose Aviária no CETAS de Belo Horizonte, MG [thesis]. Belo Horizonte (MG): Universidade Federal de Minas Gerais; 2012.; CBRO, 2014) and also because most species are declining in population (IUCN, 2018). According to Vilela (2012), and considering the new classification of species (CBRO, 2014), 61% of all Brazilian passerines at the triage centers belong to Thraupidae. This study describes 5 new host species of Isospora, from Thraupidae: (Paroaria dominicana, Schistoclamys ruficapillus and Sporophila nigricolis), of Cardinalidae (Cyanoloxia brisonii) and of Icteridae (Gnorimopsar chopi), and 2 new species of Isospora in the previously known host Saltator similis.
We describe the occurrence of oocystis of Isospora in passerines of families Thraupidae, Cardinalidae, Emberizidae, Fringillidae and Turdidae, and evaluate new and previously described host species, with the characterization of oocysts by morphometry and structural features.
MATERIALS AND METHODS
Birds and sampling
Fecal samples (n = 216) of clinically healthy passerines of six taxonomic families were evaluated: Cardinalidae, Emberizidae, Fringillidae, Icteridae, Thraupidae, and Turdidae. The occurrence of coccidia was evaluated for all passerines at rehabilitation in the Wild Animal Triage Center of Belo Horizonte (CETAS/BH), Brazil, from August 21st to September 21st, 2012 (Table I).
The study was registered at the SISBIO (Sistema de Autorização e Informação em Biodiversidade (SISBIO) do Instituto Chico Mendes de Conservação da Biodiversidade) at under number 35825-1 and at the ethics committee on animal research (CETEA-UFMG) under number 126/2013.
All fecal samples were collected non-invasively from birds that were kept in individual cages throughout the study. Upon arrival, white paper sheets were left overnight on the bottom of the cages to collect feces the following morning. Samples were immediately examined at microscopy and subjected to flotation in saturated NaCl for microscopy on a slide with coverslip (100, 400 and 1,000x). Oocyst positive samples were partially transferred to an aerated flask with 2.5% potassium dichromate solution (22 C) at 1:5 for sporulation (sporogony) for 1 week. Sporulated oocysts of each bird were subjected to morphologic and morphometric evaluation in a Zeiss Axioscop 40 microscope (Carl Zeiss Microscopy GmbH, Göttingen, Germany), equipped with digital imaging, according to the Duszynski & Wilber (1997Duszynski DW, Wilber PG. A guideline for the preparation of species descriptions in the Eimeriidae. Journal of Parasitology 1997;83:333-336.) protocol. Images were captured using a Canon G10 digital camera (Canon S Tower 2-16-6, Konan MINATO-KU, Tokyo, Japan), a 52mm adaptor tube and Axiovision 4.8 software with a Sony Vaio VPCSB35FB (Sony Brasil, São Paulo, SP, Brazil). Only viable oocysts, showing the complete development of sporocysts and sporozoites were evaluated. The numerical data were evaluated for sampling normality and homogeneity (ANOVA). The morphological features and morphometrical values were evaluated in an entirely random design (ERD) (Mann-Whitney, ANOVA) and the specific correlations were verified for the data of each individual. For Saltator similis, data were further evaluated for distribution normality and homogeneity of sampling error (Mann-Whitney, ANOVA) and specific correlation to determine the 2 coccidian species (Sampaio, 2007Sampaio IBM. Estatística aplicada à experimentação animal. Belo Horizonte: Fundação de Ensino E Pesquisa em Medicina Veterinária e Zootecnia; 2007. 265p.).
RESULTS
Fecal samples detected positive for oocysts were from Cyanoloxia brisonii (Cardinalidae), Sicalis flaveola, Sporophila caerulescens and Sporophila nigricolis (Emberizidae), Gnorimopsar chopi (Icteridae), and Schistoclamys ruficapillus, Paroaria dominicana, Saltator similis, and Sporophila nigricolis (Thraupidae). Samples from Fringillidae and Turdidae were negative (Table I). All sporulated oocysts obtained were typical of the Isospora and were further characterized.
DESCRIPTIONS
Isospora dominicana n. sp.
Description of the sporulated oocyst: spherical to subspherical oocysts, 25 x 25 µm (20-28 x 20-30 µm) (n=50); morphometric index (MI, length /width) 1.0 (1.0 - 1.2); smooth bilayered wall (1 µm in thickness); absences of micropyle, polar granule and sporocyst residuum (Table II).
Comparative morphometry (µm) of oocysts and sporocysts, and morphological features of species of genus Isospora described in family Thraupidae (Aves: Passeriformes: Thraupidae).
Description of the sporocyst: Ellipsoidal sporocysts, 11 x 17 µm (9-13 x 15-18 µm), SI 1.5 (1.3-1.8), flattened Stieda body (1 µm in height by 2 µm in width) and prominent substieda body (2 µm in height by 3 µm in width) (Table II).
Oocysts of Isospora dominicana recovered from Paroaria dominicana. Note the spheric to subspheric oocyst, absence of polar granule and oocyst residuum, flat Stieda body (arrow) and prominent substieda (arrow head).
Taxonomy summary
Host: Paroaria dominicana (Aves: Passeriformes: Thraupidae).
Geographical location and date: Samples obtained at the Wild Animal Triage Center of Belo Horizonte (CETAS/BH), Brazil, from August 21st to September 21st, 2012.
Site of infection: Undetermined.
Sporulation: Exogenous.
Frequency of detection: In 4/7 (57%).
Collection material Id. Dominicana: Refrigerated (4 - 8 ºC) for analysis and subsequently frozen feces; oocysts photomicrographs deposited at the Avian Diseases Laboratory, Veterinary College, UFMG.
Etymology: The name given to this species was based on its host name: Paroaria dominicana.
Remarks
Considering the absence of a polar granule found in the coccidian species, this species was similar to 12 others previously described in publications on Thraupidae Isospora marambaiensis, Isospora sepetibensis and Isospora tiesangui,Berto et al. (2008aBerto BP, Flausino W, Luz HR, Ferreira I, Lopes CWG. Three New Coccidian Parasites of Brazilian Tanager (Ramphocelus bresilius dorsalis) from South America. Acta Protozoologica 2008a;47:77-81.) and Berto (2010), Isospora saltatori Berto et al. (2008b), Isospora cadimi and Isospora navarroi Berto et al. (2009a, 2009b), Isospora ramphoceli, Isospora sanhaci and Isospora sayacae Berto et al. (2009c, 2010), Isospora thraupisLainson (1994Lainson R. Observations on some avian coccidia (Apicomplexa:Eimeriidae) in amazonian Brazil. Memórias do Instituto Oswaldo Cruz 1994;89:303-311.), Isospora formarum and Isospora pityliMcQuistion and Capparella (1992McQuistion, TE, Capparella A. Isospora sagittulae, a new coccidian parasite (Apicomplexa:Eimeriidae) from the spotted antbird (Hylophylax naevioides). Transactions of the American Microscopical Society 1992;111:365-368.), and Isospora paroariaeUpton et al. (1985Upton SJ, Current W, Clubb S. Two new species of Isospora (Apicomplexa:Eimeriidae) from passeriform birds of South America. Systematic Parasitology 1985;7:227-229.). Regarding the sporocyst residuum, it was absent for only the described species, in contrast to those compared with Isospora of Thraupidae (Table II). Despite such detail being considered sufficient for describing a new species (Duszynski & Wilber, 1997Duszynski DW, Wilber PG. A guideline for the preparation of species descriptions in the Eimeriidae. Journal of Parasitology 1997;83:333-336.), further data were analyzed using a dichotomic key (Berto et al., 2010). Results indicated a new species characterized by the absence of polar granule, larger than 25µm in length, flat Stieda body, large and easily visible substieda body and no sporocyst residuum (Table II). The absence of sporocyst residuum was not previously described for Isospora of Passeriformes. Considering these exclusive characteristics, the new species was thereby named Isospora dominicana.
Morphometric comparisons of Isospora species described in passerines of family Icteridae (Aves: Passeriformes, Icteridae).
Isospora beagai n. sp.
Description of the sporulated oocyst: Ovoidal oocysts, 25 x 28µm (16 -29 x 17-32µm) (n = 53), with morphometric index (MI, length/width) 1.1 (1..0 - 1.5), smooth bilayer wall approximately 1 µm in thickness. Absence of micropyle and the presence of polar granule (Table II).
Description of the sporocyst: The sporocysts are ovoidal of 11 x 18 µm (9-15 x 12 - 23 µm), SI 1.5 (1.2 - 2.2), prominent club-shaped Stieda body, of 2 µm in height by 3 µm in width and compact sporocysts residuum (Table II).
Oocysts of Isospora beagai recovered from Saltator similis. (3) Note the ovoidal oocyst, the presence of polar granule (arrowhead) and prominent Stieda body (arrow). (4) Note the ovoidal oocyst, the presence of prominent Stieda body (arrowhead) and diffused sporocyst residuum (arrow).
Taxonomy summary
Host: Saltator similis (Aves: Passeriformes: Thraupidae).
Geographical location and date: Samples obtained at the Wild Animal Triage Center of Belo Horizonte (CETAS/BH), Brazil, from August 21st to September 21st, 2012.
Site of infection: Undetermined. Samples were taken from feces.
Sporulation: Exogenous.
Frequency of detection: In 36/54 (68%).
Collection material Id. Beagai: Refrigerated (4-8 ºC) for analysis and subsequently frozen feces; oocycts photomicrographs; stored at the Avian Diseases Laboratory, Veterinary College, UFMG.
Etymology: The name given to this species was based on the acronym of the geographical location in which it was found (BH, Belo Horizonte) (Brazil).
Remarks
Comparing the oocysts possessing a polar granule with previously described species (Table II), it was shown that, among the 23 known species, including the 4 described in this manuscript, only 8 Isospora species have been characterized by the presence of a polar granule. The species described here (Table III) (Isospora beagai) had a bubble-shaped polar granule similar to Isospora vanriperorum, Isospora trincaferrri, Isospora iridornisi, Isospora sepetibensis and Isospora similis. Considering the sporocyst residuum, a compact residuum similar to I. vanriperorum and I. sepetibensis was observed. In order to compare the species described here to the previously described I. vanriperorum, data in Table II along with descriptive images previously published, were employed (Lopes et al., 2007Lopes BB, Berto BP, Massad FV, Lopes CWG. Isospora vanriperorum Levine 1982 (Apicomplexa:Eimeriidae) in the green-winger Saltator, Saltator similis (Passeriformes:Cardinalidae) in the southeastern Brasil. Revista Brasileira de Parasitololgia Veterinária 2007;16:211-214.; Berto et al., 2011aBerto BP, Luz HR, Flausino W, Teixeira-Filho WL, Ferreira I, Lopes CWG. Isosporoid Coccidia (Apicomplexa: Eimeriidae) parasites of tanagers (Passeriformes: Thraupidae) from the Marambaia Island, Brazil. Pesquisa Veterinária Brasileira 2011;31:798-805.; 2011b). Dissimilarities were found when compared to I. vanriperorum, such as a bilayered wall and a large and conspicuous substieda body. The sporocyst, although both ovoidal, also showed differences. Such characteristics are considered sufficient in determining this to be a different species (Duszynski & Wilber, 1997Duszynski DW, Wilber PG. A guideline for the preparation of species descriptions in the Eimeriidae. Journal of Parasitology 1997;83:333-336.).
When considering I. sepetibensis, comparisons using dichotomic keys, descriptive drawings (Berto et al., 2010Berto BP. Morfologia e sistemática de coccídios (Apicomplexa: Eimeriidae) parasitas de aves passeriformes da Ilha de Marambaia [thesis]. Rio de Janeiro (RJ): Universidade Federal Rural do Rio de Janeiro; 2010.; 2011a; 2011b), and data in Table II, show that sporocysts were described as ellipsoidal, in contrast to the ovoidal sporocysts found in the new I. beagai species found in this paper. The sporocyst residuum has been reported as compact, in contrast to the slightly larger and diffused sporocyst described here. Comparing the morphometric data of I. beagai with that of I. sepetibensis, a slight difference of oocyst and sporocyst measurements were revealed, being greater for the former.
The dichotomic key (Berto et al., 2010Berto BP. Morfologia e sistemática de coccídios (Apicomplexa: Eimeriidae) parasitas de aves passeriformes da Ilha de Marambaia [thesis]. Rio de Janeiro (RJ): Universidade Federal Rural do Rio de Janeiro; 2010.) suggests this species be compared to I. iridornisi, which presents larger oocysts and sporocysts than Isospora beagai (p = 0.0001), a compact but not diffused sporocyst residuum in I. beagai, a larger substieda body, and not collar-shaped as seen in I. beagai. These individual features were considered sufficient in order to describe a new species (Duszynski & Wilber, 1997Duszynski DW, Wilber PG. A guideline for the preparation of species descriptions in the Eimeriidae. Journal of Parasitology 1997;83:333-336.). In addition, the described host (Irisidornis analis) for Isospora iridornisi occcurs only in Colombia, Ecuador and Peru (northern South America), while Saltator similis occurs in southcentral South America, including Argentina, Bolivia, Brazil, Paraguay and Uruguay.
The morphologic and morphometric data and images were compared with descriptions in literature (Table II). The differences described for I. beagai, in comparison to I. iridornisi, include a diffused and ovoidal sporocyst for I. beagai, in contrast with a compact and ellipsoidal sporocyst for I. iridornisi.
Considering the morphometrical data of oocysts in Saltator similis (Tables II and III), two different set of data were obtained, and oocyst dimensions, the presence of polar granule, and compact sporocyst residuum, were considered sufficient reasons to describe a new species Isospora beagai (Duszynski & Wilber, 1997Duszynski DW, Wilber PG. A guideline for the preparation of species descriptions in the Eimeriidae. Journal of Parasitology 1997;83:333-336.).
Isospora ferri n.sp.
Oocysts of Isospora ferri recovered from Saltator similis. (5) Note the spheric to subspheric oocyst, absence of polar granule, flat delicate Stieda body (arrowhead), large and conspicuous substieda (arrow), and round oocyst. (6) Note the absence of polar granule, flat and delicate Stieda body (arrowhead), and diffused sporocyst residuum (arrows).
Description of the sporulated oocyst: Spherical to subspherical oocysts, 18 x 20 µm (15-22 x 16-22) (n=59), MI 1.0 (1.0 x 1.2), presenting a smooth double layered wall of approximately 1 µm, and the absence of micropyle, polar granule and diffused sporocyst residuum (Table II).
Description of the sporocyst: Sporocysts are ellipsoidal, 8 x 14 µm (7 - 11 x 12 - 20) SI, 1,6 (1.3 - 2.5), with flat Stieda body 1 µm in height and 2 µm in width, prominent substieda body of approximately 2 µm in height and 3 µm in width (Table II).
Taxonomy summary
Host: Saltator similis (Aves: Passeriformes: Thraupi-dae).
Geographical location and date: samples obtained at the Wild Animal Triage Center of Belo Horizonte (CETAS/BH), Brazil, from August 21st to September 21st, 2012.
Site of infection: Undetermined. Samples were taken from feces.
Sporulation: Exogenous.
Frequency of detection: In 36/54 (68%).
Collection material Id. Ferri: Refrigerated (4-8 ºC) for analysis and subsequently frozen feces; oocysts photomicrographs; stored at the Avian Diseases Laboratory, Veterinary College, UFMG.
Etymology: The name I. ferri was given based on the host’s popular Portuguese name: trinca-ferro.
Remarks
In comparison to the coccidia previously found in feces of S. similis (Table II), the oocysts in this study were larger, thus reinforcing the need to introduce a separate species (Duszynski & Wilber, 1997Duszynski DW, Wilber PG. A guideline for the preparation of species descriptions in the Eimeriidae. Journal of Parasitology 1997;83:333-336.).
Oocysts of Isospora, which do not present polar granule of previously described species in S. similis, were compared to those of this new species, I. ferri (Table II). Out of the 20 known species, in addition to 4 described in this family (Thraupidae), 16 do not present a polar granule oocyst. The dichotomic key (Berto et al., 2010Berto BP. Morfologia e sistemática de coccídios (Apicomplexa: Eimeriidae) parasitas de aves passeriformes da Ilha de Marambaia [thesis]. Rio de Janeiro (RJ): Universidade Federal Rural do Rio de Janeiro; 2010.) suggests this species be compared to Isospora tiesangui of the Brazilian tanager Ramphocelus bresilius. However, comparing the oocyst and sporocyst of these coccidians, I. ferri had a smaller dimension (Table II). The oocyst format was ovoidal for I. ferri but ellipsoidal for I. tiesangui; the Stieda body measured 0.5 x 3 µm in I. tiesangui in comparison to 1 x 2 µm in I. ferri; and the substieda body measured 2.5 x 4 µm in I. tiesangui, but 2 x 3 µm for I. ferri. Such differences are considered sufficient in determining this to be a different species of Isospora in Saltator similis (Duszynski & Wilber, 1997Duszynski DW, Wilber PG. A guideline for the preparation of species descriptions in the Eimeriidae. Journal of Parasitology 1997;83:333-336.).
The differences to Isospora navarroi were visible at the Stieda and substieda bodies (Berto et al., 2011aBerto BP, Luz HR, Flausino W, Teixeira-Filho WL, Ferreira I, Lopes CWG. Isosporoid Coccidia (Apicomplexa: Eimeriidae) parasites of tanagers (Passeriformes: Thraupidae) from the Marambaia Island, Brazil. Pesquisa Veterinária Brasileira 2011;31:798-805., 2011b). The new species presented a larger flat Stieda and larger conspicuous substieda body. The sporocyst residuum was diffused and spread within the sporocyst, and not ring-shaped as in I. navarroi.
Considering the morphometrical data of oocysts in Saltator similis (Tables II and III), two different sets of data were obtained, and oocyst dimensions, the absence of polar granule, and diffuse sporocyst residuum, considered sufficient reasons enough to indicate Isospora ferri as a new species.
Isospora ruficapillus
Oocysts of Isospora ruficapillus recovered from Schistochlamys ruficapillus. (7) Note the mammilliform Stieda body (arrowhead) and the sporocyst diffused residuum (arrow). (8) Note the complex formed by the mammilliform Stieda body (arrowhead) and the prominent substieda (arrow).
Description of the sporulated oocyst: Spherical to subspherical oocysts, 24 x 25 µm (21-25 x 23-26) (n = 20), MI, 1.0 (1.0 - 1.1), presenting a double smooth wall, approximately 1 µm in thickness, the absence of micropyle and polar granule (Table II).
Description of the sporocyst: The ellipsoidal sporocysts, 11 x 17 µm (10 -11 x 16 x 18), with mammilliform Stieda body 1 µm in height per 2 µm in width, prominent substieda 2.5 x 3 µm (2.5 µm in height by 3µm in width), and the presence of a diffused sporocyst residuum (Table II).
Taxonomic summary
Host: Schistoclamys ruficapillus (Aves: Passeriformes: Thraupidae).
Geographical location and date: Samples obtained at the Wild Animal Triage Center of Belo Horizonte (CETAS/BH), Brazil, from August 21st to September 21st, 2012.
Site of infection: Undetermined. Samples were taken from feces.
Sporulation: Exogenous.
Frequency of detection: 1/2 (50%).
Collection material Id. Ruficapillus: Refrigerated (4-8 ºC) for analysis and subsequently frozen feces; oocycsts photomicrographs stored at the Avian Diseases Laboratory, Veterinary College, UFMG.
Etymology: The name I. Ruficapillus was given based on the species’ host Schistoclamys ruficapillus.
Remarks
The morphometric and morphologic data obtained after sporulation were compared to data from the literature (Table II) and using a previously described dichotomic key (Berto et al., 2010Berto BP. Morfologia e sistemática de coccídios (Apicomplexa: Eimeriidae) parasitas de aves passeriformes da Ilha de Marambaia [thesis]. Rio de Janeiro (RJ): Universidade Federal Rural do Rio de Janeiro; 2010.) which enabled the description of a new species: Isospora ruficapillus.
The closest species to Isospora ruficapillus was Isospora sanhaci, based on the dichotomic key (Berto et al., 2010Berto BP. Morfologia e sistemática de coccídios (Apicomplexa: Eimeriidae) parasitas de aves passeriformes da Ilha de Marambaia [thesis]. Rio de Janeiro (RJ): Universidade Federal Rural do Rio de Janeiro; 2010.). I. sanhaci was described as having a roundish delicate substieda body, which differed from I. ruficapillus as observed in the present study, even though it presented similarities in other structures. The sporocyst shape of both species differed, being ellipsoidal for I. ruficapillus and ovoid for I. sanhaci. Considering the sporocyst residuum, the new described species presented a diffused structure.
Taking into account the absence of polar granule, the shape of the Stieda body and sporocyst residuum; the species found in Schistochlamys ruficapillus suggests this species be compared to Isospora navarroi and Isospora marambaiensis previously described in Ramphocelus bresilius dorsalis (Berto et al., 2008aBerto BP, Flausino W, Luz HR, Ferreira I, Lopes CWG. Three New Coccidian Parasites of Brazilian Tanager (Ramphocelus bresilius dorsalis) from South America. Acta Protozoologica 2008a;47:77-81.; 2009b; 2010). I. marambaiensis presented larger oocysts and sporocyts, but the Stieda and substieda bodies were smaller and more delicate than in I. ruficapillus (Berto et al. 2011a, 2011b), and such differences were considered sufficient for describing a new coccidian species (Duszynski & Wilber, 1997Duszynski DW, Wilber PG. A guideline for the preparation of species descriptions in the Eimeriidae. Journal of Parasitology 1997;83:333-336.).
Isospora chopi
Oocysts of Isospora chopi recovered from Gnorimopsar chopi. (9) Note the spheric to subspheric oocyst of Isospora chopi without polar granule, with prominent Stieda body (arrow) and diffused sporocyst residuum (arrowhead). (10) Note the spheric to subspheric Isospora chopi oocyst with sporocyst residuum (arrow), prominent Stieda body (arrowhead) and absence of polar granule.
Description of the sporulated oocyst: Spheric to subspheric oocysts (n = 48) of 22 x 24.5µm (20-25 x 20-30µm), MI of 1.2 (1.0 -1.2), with smooth double layered wall of approximately 1 µm in thickness, without micropyle or polar granule (Table IV).
Description of sporocyst: Ellipsoidal sporocysts of 10 x 15 µm (8-10 x 13-16 µm) with 2 µm in height and 1 µm in width prominent Stieda body and diffused sporocyst residuums (Table IV)
Host: Gnorimopsar chopi (Aves: Passeriformes: Icteridae).
Geographical location and date: Samples obtained at the Wild Animal Triage Center of Belo Horizonte (CETAS/BH), Brazil, from August 21st to September 21st, 2012.
Site of infection: Unknown. Samples were taken from feces.
Sporulation: Exogenous. Frequency of detection: In 5/ 9 (55%).
Collection material Id. Chopi: Refrigerated (4-8 ºC) for analysis and subsequently frozen feces; oocysts photomicrographs stored at the Avian Diseases Laboratory, Veterinary College, UFMG.
Etymology: The name given to this species was based on its host name: Gnorimopsar chopi.
Remarks
The morphometric and morphologic data (Table IV) obtained after sporulation were compared to data from the literature (Table V) for Isospora of Icteridae and using a previously described dichotomic key (Berto et al., 2010Berto BP. Morfologia e sistemática de coccídios (Apicomplexa: Eimeriidae) parasitas de aves passeriformes da Ilha de Marambaia [thesis]. Rio de Janeiro (RJ): Universidade Federal Rural do Rio de Janeiro; 2010.) which enabled the description of a new species, Isospora chopi.
Morphometric comparisons of Isospora species described in passerines of family Icteridae (Aves: Passeriformes, Icteridae).
Analyzing the previously known Isospora of Icteridae, only Isospora divitis (Pellérdy, 1967Pellérdy L. Three new coccidia parasitic in Cuban birds (Protozoa:Sporozoa). Acta Zoologica Academiae Scientiarum Hungaricae 1967;13:227-230.) (Table V) shares common features to the new species (I. chopi), including the presence of sporocyst residuum, even though the morphometric measurements of the sporocyst differed. I. divitis was described in Dives atroviolaceus as an endemic icterid in Cuba. D. atroviolaceus and G. chopi never share the same environment; G. chopi occurs up to the southern borders of the Amazon forest in Brazil and D. atroviolaceus occurs only in Cuba.
Isospora gnorimopsar
Description of the sporulated oocyst: Subspheric to ovoid oocysts of 23 - 27 µm (20-28 x 22-32µm) (n = 59), with morphometric index (MI) 1.2 (1.0-1.4), with a smooth double layered wall of approximately 1 µm in thickness, absent micropyle and with 1 or 2 polar granules (Table IV).
Description of the sporocyst: Ellipsoidal sporocysts, 11-16 µm (9-12 x 14-19 µm), with SI 1.5 (1.3 x 1.), flat Stieda body (1 µm in thickness and 2 µm in width) and diffused sporocyst residuum (Table IV).
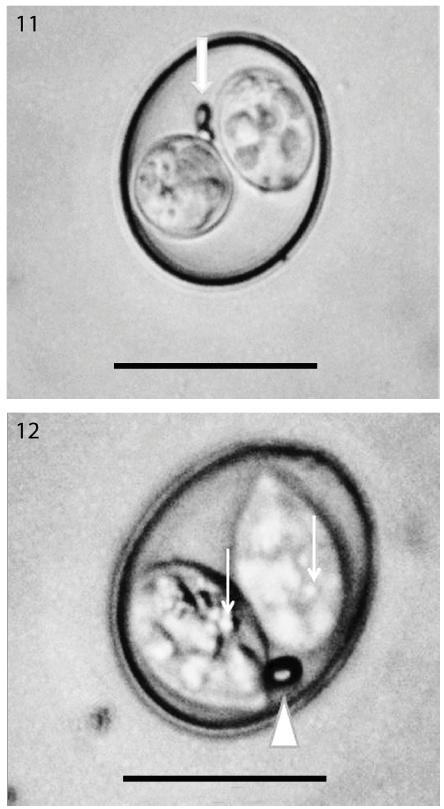
Oocysts of Isospora gnorimopsar recovered from Gnorimopsar chopi. (11) Note the ovoid to subspheric oocyst of Isospora gnorimopsar with two polar granules (arrow). (12) Note the ovoidal to subspheric oocyst of Isospora gnorimopsar with one polar granule (arrowhead) and sporocyst residuum (arrows).
Taxonomic summary
Host: Gnorimopsar chopi (Aves: Passeriformes: Icteridae).
Geographical location and date: Samples obtained at the Wild Animal Triage Center of Belo Horizonte (CETAS/BH), Brazil, from August 21st to September 21st, 2012.
Site of infection: Unknown. Samples were taken from feces.
Sporulation: Exogenous.
Frequency of detection: In 5/9 (55%).
Collection material Id. Gnorimopsar: Refrigerated (4-8 ºC) for analysis and subsequently frozen feces; oocysts photomicrographs stored at the Avian Diseases Laboratory, Veterinary College, UFMG.
Etymology: The name given to this species was based on the genus of the host, Gnorimopsar chopi.
Remarks
The morphometric and morphologic data obtained after sporulation were compared to data from literature (Table II) and using a previously described dichotomic key (Berto et al., 2010Berto BP. Morfologia e sistemática de coccídios (Apicomplexa: Eimeriidae) parasitas de aves passeriformes da Ilha de Marambaia [thesis]. Rio de Janeiro (RJ): Universidade Federal Rural do Rio de Janeiro; 2010.) which enabled the description of a new species, Isospora gnorimopsar.
The presence of the polar granule in I. gnorimopsar, were compared to those of I. cacici (Lainson, 1994Lainson R. Observations on some avian coccidia (Apicomplexa:Eimeriidae) in amazonian Brazil. Memórias do Instituto Oswaldo Cruz 1994;89:303-311.), I. belicosa (Upton et al., 1995Upton SJ, Stamper MA, Whitaker BR. Isospora bellicosa sp. n. (Apicomplexa) from a Peruvian red-breasted meadowlark, Sturnella bellicose (Passeriformes:Icteridae). Archiv für Protistenkunde 1995;145:132-134.), I. icterus (Upton and Whitaker, 2000) and I. graceannae (Upton and Whitaker, 2000). However, the flattened shape of the Stieda body was distinct in I. gnorimopsar. Although I. icterus was described with a small Stieda body, the oocyst and sporocyst average dimensions were significantly different. In addition, no sporocyst residuum was found in I. gnorimopsar, in contrast to a diffused residuum in I. icterus. The analyses were performed based on descriptions by Berto et al. (2011aBerto BP, Luz HR, Flausino W, Teixeira-Filho WL, Ferreira I, Lopes CWG. Isosporoid Coccidia (Apicomplexa: Eimeriidae) parasites of tanagers (Passeriformes: Thraupidae) from the Marambaia Island, Brazil. Pesquisa Veterinária Brasileira 2011;31:798-805.). Such differences are considered sufficient in determining this to be a new coccidian species (Duszynski & Wilber, 1997Duszynski DW, Wilber PG. A guideline for the preparation of species descriptions in the Eimeriidae. Journal of Parasitology 1997;83:333-336.).
DISCUSSION
Out of the 216 fecal samples evaluated, 61 were positive for oocysts, which corresponded to 28% of this total, all being of the genus Isospora. Considering this occurrence, similar results had been previously found in birds by McQuistion (2000), Marietto-Gonçalves et al. (2009Marietto-Gonçalves GA, Martins TF, Lima ET, Lopes RS, Filho RLA. Prevalência de endoparasitas em amostras fecais de aves silvestres e exóticas examinadas no laboratório de ornitopatologia e no laboratório de enfermidades parasitárias da FMVZ-UNESP/BOTUCATU, SP. Ciência Animal Brasileira 2009;10:349-354.), Vilela et al. (2009Vilela DAR, Savernini THOPM, Mendes EJ, Campos SM, Andrade RA, Guimarães RC, et al. Ocorrência de coccídeos intestinais em passeriformes silvestres provenientes do tráfico e encaminhados para o Cetas do IBAMA em Belo Horizonte. Anais do 12º Congresso ABRAVAS; 2009; Águas de Lindóia, São Paulo. Brasil. p.56-57.) and Schoener et al. (2013Schoener ER, Alley MR, Castro I. Coccidia species in endemic and native New Zealand passerines. Parasitology Research 2013;112:2027-2036.). Considering passerines (Passeriformes), results are in agreement with previous reports by Pereira et al., (2011Pereira LQ, Berto BP, Flausino W, Lovato M, Lopes CWG. Isospora bocamontensis n. sp. (Apicomplexa:Eimeriidae) from the yellow cardinal Gubernatrix cristata (Vieillot) (Passeriformes:Emberizidae) in South America. Systematic Parasitology 2011;78:73-80.) and Coelho et al. (2013Coelho CD, Berto BP, Neves DM, Oliveira VM, Flausino W, Lopes CWG. Oocyst shedding by green-winged-saltator (Saltator similis) in the diagnosis of coccidiosis and Isospora similis n. sp. (Apicomplexa: Eimeriidae). Revista Brasileira de Parasitolologia Veterinária 2013;22:64-70.), studying Gubernatrix cristata and Saltator similis, respectively, and found only Isospora.
Although the impact of coccidiosis in free-living passerines is unknown, the disease is highly significant in captive birds (Swayne et al., 1991Swayne DE; Getzy D, Slemons RD, Bocetti C, Kramer L. Coccidiosis as a cause of transmural lymphocytic enterits and mortality in captive Nashville warblers (Vermivora ruficapilla). Journal of Wildlife Diseases 1991;27:615-620.; Cork and Alley, 1999Cork SC, Alley MR. Aspergillosis and other causes of mortality in the stitchbird in New Zealand. Journal of Wildlife Diseases 1999;35:481-48.; Twentyman, 2001Twentyman CM. A study of coccidial parasitis in the hihi (Notionystis cincta) [thesis]. Palmerston North (NZ): Massey University; 2001.; Gill and Paperna, 2008Gill H, Paperna I. Proliferative visceral Isospora (atoxoplasmose) with morbid impacto on the Israel sparrow Passer domesticus biblicus Hartert, 1904. Parasitology Research 2008;103:493-499.; Marques et al., 2011Marques MVR, Vilela DAR, Andrade EAG, Galvão GZ, Resende CZ, et al. Fatal coccidiosis by Isospora icterus (Upton & Whitaker 2000) in captive campo troupial (Icterus jamacaii) (Aves, Passeriformes, Icteridae) in Brazil. Journal of Zoo and Wildlife Medicine 2011;42:735-737.; Vasconcelos et al., 2012Vasconcelos TCB, Longa CS, Campos SDE, Costa CHC, Bruno SF. Coccidiose em Sporophila maximilliani (Passeriformes:Emberizidae):relato de dois casos. Revista Brasileira de Medicina Veterinária 2012;34:261-264). Results indicate that the triage birds evaluated, despite being temporarily in captivity, have undergone challenge, which could eventually have become clinically significant for susceptible birds, with the increasing concentration of oocysts in the housing environment. The adequate cleaning and disinfection of the premises should be intensified using more efficient principles such as ammonium hydroxide (Kahrs, 1995Kahrs RF. General disinfection guidelines. Scientific and Technical Review of the Office International des Epizooties 1995;14:105-122.). Considering the risk of coccidiosis for birds in triage, in agreement with monitoring recommendations, the authors suggest routine evaluations of feces to be performed weekly (Friend and Franson, 1999Friend M, Franson JC, editors. Intestinal coccidiosis. In: Friend M, Franson JC. Field manual of wildlife diseases: general field and procedures and diseases of birds. Washington: United States Geological Survey, Biological Resources Division; 1999. p.207-213.).
In this study, not all oocysts found were characterized. A few samples, although adequate for determining the genus, were not adequate for the description of species due to the lack of development, presentation or preservation of discernible features. New host species presenting Isospora in this study include Cyanoloxia brisonii, Gnorimopsar chopi, Paroaria dominicana, Schistochlamys ruficapillus, Sicalis flaveola, Sporophila caerulescens and Sporophila nigricolis. The coccidians found in the host species Cyanoloxia brisonii, Sicalis flaveola, Sporophila caerulescens and Sporophila nigricolis belong to the genus Isospora, and a detailed morphology of the oocysts was not evaluated.
The morphological comparisons were performed for characterizing Isospora species in the host family taxon. The careful analysis of oocysts included the determination of oocyst and sporocyst metrics (length and width), oocyst wall, including layers, projections, texture and thickness, and the presence and characteristics of structures (micropyle, polar granule, oocyst residuum, Stieda and substieda bodies, refractile bodies, nucleus, residuum, adherent membranes, sporodium and sutures) (Duszynski & Wilber, 1997Duszynski DW, Wilber PG. A guideline for the preparation of species descriptions in the Eimeriidae. Journal of Parasitology 1997;83:333-336.). When comparing features, Isospora gnorimopsar differed from Isospora cacici (Lainson 1994Lainson R. Observations on some avian coccidia (Apicomplexa:Eimeriidae) in amazonian Brazil. Memórias do Instituto Oswaldo Cruz 1994;89:303-311.), Isospora belicosa (Upton et al., 1995Upton SJ, Stamper MA, Whitaker BR. Isospora bellicosa sp. n. (Apicomplexa) from a Peruvian red-breasted meadowlark, Sturnella bellicose (Passeriformes:Icteridae). Archiv für Protistenkunde 1995;145:132-134.), Isospora icterus and Isospora graceannae (Upton and Whitaker 2000). Isospora chopi was compared to Isospora divitis (Pellérdy 1967Pellérdy L. Three new coccidia parasitic in Cuban birds (Protozoa:Sporozoa). Acta Zoologica Academiae Scientiarum Hungaricae 1967;13:227-230.) and differed from this species.
Isospora dominicana, I. beagai, I. ferri and I. ruficapillus, which were found in the Thraupidae feces, were compared to and differed from I. vanriperorum (Levine et al., 1980Levine ND, Van-Riper S, Van-Riper C. Five new species of Isospora from Hawaiian birds. Journal of Protozoology 1980;27:258-259.; Lopes et al., 2007Lopes BB, Berto BP, Massad FV, Lopes CWG. Isospora vanriperorum Levine 1982 (Apicomplexa:Eimeriidae) in the green-winger Saltator, Saltator similis (Passeriformes:Cardinalidae) in the southeastern Brasil. Revista Brasileira de Parasitololgia Veterinária 2007;16:211-214.), I. paroariae (Upton et al., 1985Upton SJ, Current W, Clubb S. Two new species of Isospora (Apicomplexa:Eimeriidae) from passeriform birds of South America. Systematic Parasitology 1985;7:227-229.), I. pityli and I. formarum (McQuistion and Capparella, 1992McQuistion, TE, Capparella A. Isospora sagittulae, a new coccidian parasite (Apicomplexa:Eimeriidae) from the spotted antbird (Hylophylax naevioides). Transactions of the American Microscopical Society 1992;111:365-368.), I. saltatori (Berto et al. 2008bBerto BP, Balthazar LMC, Flausino W, Lopes CWG. Two new coccidian parasites of green-winged Saltator (Saltator similis) from South America. Acta Protozoologica 2008b;47:263-267.), I. trincaferri, (Berto et al., 2008b), I. thraupis (Lainson, 1994Lainson R. Observations on some avian coccidia (Apicomplexa:Eimeriidae) in amazonian Brazil. Memórias do Instituto Oswaldo Cruz 1994;89:303-311.; Berto, 2010), I. andesensis (Templar et al., 2004Templar AC, McQuistion TE, Capparella AP. A new coccidian parasite, Isospora andesensis, from the common bush tanager Chlorospingus ophthalmicus of South America. Acta Protozoologica 2004;43:369-371.; Berto, 2010), I. irisidornisi (Metzelaars et al., 2005Metzelaars HT, Spaargaren T, McQuistion TE, Capparella AP. Isospora iridosornisi, a new coccidian parasite (Apicomplexa, Eimeriidae) from the yellow-throated tanager, Iridosornis analis of South America. Acta Parasitologica 2005;50:191-193.; Berto, 2010), I. tiesangui (Berto et al., 2008a; Berto, 2010), I. marambaiensis (Berto et al. 2008a; 2010), I. sepetibensis (Berto et al., 2008a; 2010), I. cadimi (Berto et al., 2009a; 2010), I. navarroi (Berto et al., 2009b; 2010), I. ramphoceli (Berto et al, 2010), I. sanhaci (Berto et al., 2009c; 2010), I. silvasouzai (Berto et al., 2009c; 2010), I. sayacae (Berto et al., 2009c; 2010) and I. similisi (Coelho et al., 2013Coelho CD, Berto BP, Neves DM, Oliveira VM, Flausino W, Lopes CWG. Oocyst shedding by green-winged-saltator (Saltator similis) in the diagnosis of coccidiosis and Isospora similis n. sp. (Apicomplexa: Eimeriidae). Revista Brasileira de Parasitolologia Veterinária 2013;22:64-70.).
The morphometric and morphologic comparisons enabled the identification of new Isospora species in previously known and unknown passerine host species. However, more reliable and conclusive studies should be developed, including phylogenetic studies, in order to evaluate, compare and group genotypes, using molecular techniques.
In addition to previous studies, we have demonstrated the occurrence of new host species and new coccicidian species in native avian host passerine species. The higher risk for the host species of Thraupidae became clear, as compared to Cardinalidae, Emberizidae, Fringillidae and Turdidae. Within Thraupidae, Saltator similis is the most frequently described host species. Isospora was unpublished in Paroaria dominicana, Schistochlamys ruficapillus and Gnorimopsar chopi. The new species of Isospora described were Isospora dominicana, Isospora ferri, Isospora ruficapillus, Isospora chopi and Isospora gnorimopsar. The new coccidian species and new passerine hosts add to the complexity of Isospora in passerines and indicates for frequent monitoring during rehabilitation.
ACKNOWLEDGMENTS
The authors are indebted to IBAMA (Instituto Brasileiro de Meio Ambiente e Recursos Naturais Renováveis), for the sampled birds. This project is part of the National Institute for Science and Technology (INCT) - Brazilian Livestock Genetic and Health Information (IGSPB) and was partially supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG).
LITERATURE CITED
- Berto BP, Balthazar LMC, Flausino W, Lopes CWG. New isosporoid coccidian parasites of sayaca tanager, Thraupis sayaca, from South America. Acta Parasitologica 2009b;54:90-94.
- Berto BP, Balthazar LMC, Flausino W, Lopes CWG. Three new species of Isospora Schneider, 1881 (Apicomplexa: Eimeriidae) from the buffy-fronted seedeater Sporophila frontalis Verreaux, 1869 (Passeriformes: Emberizidae) from South America. Systematic Parasitology 2009a;73:65-69.
- Berto BP, Balthazar LMC, Flausino W, Lopes CWG. Two new coccidian parasites of green-winged Saltator (Saltator similis) from South America. Acta Protozoologica 2008b;47:263-267.
- Berto BP, Flausino W, Luz HR, Ferreira I, Lopes CWG. Three New Coccidian Parasites of Brazilian Tanager (Ramphocelus bresilius dorsalis) from South America. Acta Protozoologica 2008a;47:77-81.
- Berto BP, Flausino W, Luz HR, Ferreira I, Lopes CWG.. Two new Isospora species from Brazilian tanager (Ramphocelus bresilius dorsalis) of South America. Parasitology Research 2009b;105:635-639.
- Berto BP, Flausino W, McIntosh D, Teixeira-Filho WL, Lopes CWG. Coccidia of new world passerine birds (Aves: Passeriformes): a review of Eimeria Schneider, 1875 and Isospora Schneider, 1881 (Apicomplexa: Eimeriidae). Systematic Parasitology 2011a;80:159-204.
- Berto BP, Luz HR, Ferreira I, Flausino W, Lopes CWG. A diagnostic tool to identify species of the genus Isospora Schneider, 1881(Apicomplexa: Eimeriidae) based on sporulated oocysts from Thaupidae family (Aves: Passeriformes): a dichotomous key. Revista Brasileira de Medicina Veterinária 2010;32:182-186.
- Berto BP, Luz HR, Flausino W, Teixeira-Filho WL, Ferreira I, Lopes CWG. Isosporoid Coccidia (Apicomplexa: Eimeriidae) parasites of tanagers (Passeriformes: Thraupidae) from the Marambaia Island, Brazil. Pesquisa Veterinária Brasileira 2011;31:798-805.
- Berto BP. Morfologia e sistemática de coccídios (Apicomplexa: Eimeriidae) parasitas de aves passeriformes da Ilha de Marambaia [thesis]. Rio de Janeiro (RJ): Universidade Federal Rural do Rio de Janeiro; 2010.
- Borges RC, Oliveira A, Bernardo N, Costa RMMC. Diagnóstico da fauna silvestre apreendida e recolhida pela Polícia Militar de Meio Ambiente de Juiz de Fora, MG (1998 e 1999). Revista Brasileira de Zoologia 2006;8:23-33.
- Brown MA, Ball SL, Snow KR. Coccidian parasites of British wild birds. Journal of Natural History 2010;44:2669-2691.
- CBRO- Comitê Brasileiro de Registros ornitológicos. Listas das aves do Brasil [cited 2014 April 1]. Available from: http://www.cbro.org.br
» http://www.cbro.org.br - Coelho CD, Berto BP, Neves DM, Oliveira VM, Flausino W, Lopes CWG. Oocyst shedding by green-winged-saltator (Saltator similis) in the diagnosis of coccidiosis and Isospora similis n. sp. (Apicomplexa: Eimeriidae). Revista Brasileira de Parasitolologia Veterinária 2013;22:64-70.
- Cork SC, Alley MR. Aspergillosis and other causes of mortality in the stitchbird in New Zealand. Journal of Wildlife Diseases 1999;35:481-48.
- Costa IA, Coelho CD, Bueno C, Ferreira I, Freire RB. Ocorrência de parasitos gastrointestinais em aves silvestres no município de Seropédica, Rio de Janeiro, Brasil. Ciência Animal Brasileira 2010;11:914-922.
- Duszynski DW, Upton SJ, Couch L. The Coccidia of the world [cited 2018 Apr 09]. Available from: http://biology.unm. edu/coccidia/home.html
» http://biology.unm. edu/coccidia/home.html - Duszynski DW, Wilber PG. A guideline for the preparation of species descriptions in the Eimeriidae. Journal of Parasitology 1997;83:333-336.
- Ferreira CM, Glock L. Diagnóstico preliminar sobra a avifauna traficada no Rio Grande do Sul, Brasil. Biociências 2004;12:21-30.
- Freitas MFL, Oliveira JB, Cavalcanti MDB, Leite AS, Magalhães VS, Oliveira RA, et al. Parasitos gastrointestinales de aves silvestres en cautiverio en el estado de Pernambuco, Brasil. Parasitologia Latinoamericana 2002;57:50-54.
- Friend M, Franson JC, editors. Intestinal coccidiosis. In: Friend M, Franson JC. Field manual of wildlife diseases: general field and procedures and diseases of birds. Washington: United States Geological Survey, Biological Resources Division; 1999. p.207-213.
- Gill H, Paperna I. Proliferative visceral Isospora (atoxoplasmose) with morbid impacto on the Israel sparrow Passer domesticus biblicus Hartert, 1904. Parasitology Research 2008;103:493-499.
- IUCN. Red list of threatened species. Version 2013.2 [cited 2018 May 12]. Available from: www.iucnredlist.org.
» www.iucnredlist.org - Kahrs RF. General disinfection guidelines. Scientific and Technical Review of the Office International des Epizooties 1995;14:105-122.
- Keeler SP, Yabsley MG, Fox JM, McGraw SN, Hernandez SM. Isospora troglodytes n. sp. (Apicomplexa:Eimeriidae), a new coccidian species from wrens of Costa Rica. Parasitology Research 2011;110:1723-1725.
- Lainson R. Observations on some avian coccidia (Apicomplexa:Eimeriidae) in amazonian Brazil. Memórias do Instituto Oswaldo Cruz 1994;89:303-311.
- Lederberg J. Emerging infections:an evolutionary perspective. Emerging Infection Diseases 1998;4:366-371.
- Levine ND, Van-Riper S, Van-Riper C. Five new species of Isospora from Hawaiian birds. Journal of Protozoology 1980;27:258-259.
- Lopes BB, Berto BP, Massad FV, Lopes CWG. Isospora vanriperorum Levine 1982 (Apicomplexa:Eimeriidae) in the green-winger Saltator, Saltator similis (Passeriformes:Cardinalidae) in the southeastern Brasil. Revista Brasileira de Parasitololgia Veterinária 2007;16:211-214.
- Marietto-Gonçalves GA, Martins TF, Lima ET, Lopes RS, Filho RLA. Prevalência de endoparasitas em amostras fecais de aves silvestres e exóticas examinadas no laboratório de ornitopatologia e no laboratório de enfermidades parasitárias da FMVZ-UNESP/BOTUCATU, SP. Ciência Animal Brasileira 2009;10:349-354.
- Marques MVR, Vilela DAR, Andrade EAG, Galvão GZ, Resende CZ, et al. Fatal coccidiosis by Isospora icterus (Upton & Whitaker 2000) in captive campo troupial (Icterus jamacaii) (Aves, Passeriformes, Icteridae) in Brazil. Journal of Zoo and Wildlife Medicine 2011;42:735-737.
- McQuistion TE. The frequency of detection of coccidian parasites in passerine birds from South America. Transactions of the Illinois State Academy of Science 93:221-227.
- McQuistion, TE, Capparella A. Isospora sagittulae, a new coccidian parasite (Apicomplexa:Eimeriidae) from the spotted antbird (Hylophylax naevioides). Transactions of the American Microscopical Society 1992;111:365-368.
- Metzelaars HT, Spaargaren T, McQuistion TE, Capparella AP. Isospora iridosornisi, a new coccidian parasite (Apicomplexa, Eimeriidae) from the yellow-throated tanager, Iridosornis analis of South America. Acta Parasitologica 2005;50:191-193.
- Moore J, Clayton DH. Conclusion: Evolution of host-parasite interactions. In: Clayton DH, More J, editors. Host-parasite evolution: general principles and avian models. New York: Oxford University Press; 1997. p.370-376.
- Pagano ISA, Sousa AEB, Wagner PGC, Ramos RTC. Aves depositadas no Centro de Triagem de Animais Silvestres do IBAMA na Paraíba:uma amostra do tráfico de aves silvestres no estado. Ornithologia 2009;3:132-144.
- Page CD, Haddad K. Coccidial infections in birds. Seminars in Avian and Exotic Pet Medicine 1995;4:138-144.
- Pellérdy L. Three new coccidia parasitic in Cuban birds (Protozoa:Sporozoa). Acta Zoologica Academiae Scientiarum Hungaricae 1967;13:227-230.
- Pereira LQ, Berto BP, Flausino W, Lovato M, Lopes CWG. Isospora bocamontensis n. sp. (Apicomplexa:Eimeriidae) from the yellow cardinal Gubernatrix cristata (Vieillot) (Passeriformes:Emberizidae) in South America. Systematic Parasitology 2011;78:73-80.
- Sampaio IBM. Estatística aplicada à experimentação animal. Belo Horizonte: Fundação de Ensino E Pesquisa em Medicina Veterinária e Zootecnia; 2007. 265p.
- Santos EAM, Bueno M, Araújo AS, Barros IFA, Paes NNG, Rodrigues SRW, et al. Aves do centro de triagem de animais silvestres do Estado do Amapá. Ornithologia 2001;4:86-90.
- Schoener ER, Alley MR, Castro I. Coccidia species in endemic and native New Zealand passerines. Parasitology Research 2013;112:2027-2036.
- Sick H. Ornitologia Brasileira. Rio de Janeiro: Nova Fronteira; 1997. 862 p.
- Swayne DE; Getzy D, Slemons RD, Bocetti C, Kramer L. Coccidiosis as a cause of transmural lymphocytic enterits and mortality in captive Nashville warblers (Vermivora ruficapilla). Journal of Wildlife Diseases 1991;27:615-620.
- Templar AC, McQuistion TE, Capparella AP. A new coccidian parasite, Isospora andesensis, from the common bush tanager Chlorospingus ophthalmicus of South America. Acta Protozoologica 2004;43:369-371.
- Twentyman CM. A study of coccidial parasitis in the hihi (Notionystis cincta) [thesis]. Palmerston North (NZ): Massey University; 2001.
- Upton SJ, Current W, Clubb S. Two new species of Isospora (Apicomplexa:Eimeriidae) from passeriform birds of South America. Systematic Parasitology 1985;7:227-229.
- Upton SJ, Stamper MA, Whitaker BR. Isospora bellicosa sp. n. (Apicomplexa) from a Peruvian red-breasted meadowlark, Sturnella bellicose (Passeriformes:Icteridae). Archiv für Protistenkunde 1995;145:132-134.
- Upton SJ, Whitaker B. New species of Isospora (Apicomplexa, Eimeriidae) from the troupial and white-edged oriole (Icterus spp.) (Aves, Passeriformes, Icteridae). Acta Parasitologica 2000;45:67-70.
- Vasconcelos TCB, Longa CS, Campos SDE, Costa CHC, Bruno SF. Coccidiose em Sporophila maximilliani (Passeriformes:Emberizidae):relato de dois casos. Revista Brasileira de Medicina Veterinária 2012;34:261-264
- Vilela DAR, Savernini THOPM, Mendes EJ, Campos SM, Andrade RA, Guimarães RC, et al. Ocorrência de coccídeos intestinais em passeriformes silvestres provenientes do tráfico e encaminhados para o Cetas do IBAMA em Belo Horizonte. Anais do 12º Congresso ABRAVAS; 2009; Águas de Lindóia, São Paulo. Brasil. p.56-57.
- Vilela DAR. Diagnóstico da Avifauna Encaminhada para os Centros de Triagem de Animais Silvestres (CETAS) do Brasil e Ocorrência de Clamidiose Aviária no CETAS de Belo Horizonte, MG [thesis]. Belo Horizonte (MG): Universidade Federal de Minas Gerais; 2012.
- Wobeser GA. Parasitism: costs and effects. In: Atkinson, CT, Thomas NJ, Hunter DB, editors. Parasitic diseases of wild birds. Oxford: Wiley-Blackwell, 2008. p.3-12.
- Yabsley MJ. Eimeria. In: Atkinson, CT, Thomas NJ, Hunter DB, editors. Parasitic diseases of wild birds. Oxford: Wiley-Blackwell, 2008. p.162-180.
Publication Dates
-
Publication in this collection
05 June 2020 -
Date of issue
2020
History
-
Received
11 Apr 2019 -
Accepted
23 Nov 2019