ABSTRACT
The control of Salmonella in the poultry production chain combined with biosecurity measures is an important tool to maintain and guarantee the sanitary status of Brazilian flocks. The aim of this work was to compare official laboratory data on molecular typification of Salmonella isolates from poultry breeding flocks in different Brazilian states between 2016 and 2018 and identify the production category with the most positive flocks, in light of current legislation. Surveillance data of positive samples from the official Brazilian Salmonella Control Programme sent to Federal Agricultural Defence Laboratory of São Paulo (LFDA-SP) after molecular characterization were analysed. These data were subject to an exploratory study, undergoing a descriptive statistical analysis followed by the use of frequency and non-parametric hypothesis tests. Overall, 49 serovars were detected in poultry broiler-breeder and layer-breeder flocks. Salmonella ser. Heidelberg, Salmonella ser. Anatum, Salmonella ser. Newport, Salmonella ser. Schwarzengrund and Salmonella ser. Mbandaka were the five most common isolated serovars. The data shows that there is an opportunity to improve biosecurity measures in parent breeder flocks. A total of 16 serovars were identified in turkey-breeders. Salmonella ser. Anatum, Salmonella ser. Newport, Salmonella ser. Brandenburg, Salmonella ser. Litchfield, and Salmonella ser. Livingstone were the most common ones. The four official controlled serovars represented a small part of the isolated strains. These data demonstrate the importance of an official program in Brazil for Salmonella surveillance in breeder flocks combined with biosecurity measures.
Keywords:
Biosecurity; Salmonella official control; Salmonella serovars; Poultry breeders´ categories
INTRODUCTION
Brazil is one of the world’s largest producers of animal protein, the third largest producer of broiler chicken, and the first global exporter of chicken meat and its products. Its privileged position is the result of the country’s natural capacity for producing food, as well as the sanitary status of its flocks. Such position allows industries to stand out in their competitiveness up to the present days (ABPA, 2021).
The southern region of the country, comprising the states of Paraná, Santa Catarina, and Rio Grande do Sul, accounts for approximately 65% of Brazilian chicken meat production and 80% of its exports. On the other hand, the south-eastern region is responsible for the core of the country’s egg production, which has been developing over the years.
Meanwhile, Brazilian turkey meat production has declined considerably after 2017, along with its exports. The southern states of Santa Catarina and Rio Grande do Sul are responsible for 99% of turkey meat exports (ABPA, 2021).
Due to the importance of poultry production, the Ministry of Agriculture, Livestock, and Food Supply (MAPA) launched the National Poultry Health Program (PNSA) in 1994 (Brasil,1994Brasil. Ministério da Agricultura, Pecuária e do Abastecimento. Portaria ministerial nº193, de 19 de setembro de 1994. Institui o programa nacional de sanidade avícola no âmbito do dsa e cria o comitê consultivo do programa de sanidade avícola. Diário Oficial da União, Seção I; 1994.), which has allowed Brazil’s poultry production to move forward. In order to maintain and guarantee the sanitary status of Brazilian flocks, biosecurity and surveillance public policies have been established (Brasil, 2003; 2007; 2016). Nevertheless, the prevention and control of pathogens in those flocks are a permanent challenge, in which the official veterinary service plays a crucial role.
Among such pathogens, Salmonella is a major concern in the poultry production chain worldwide, considering its importance for public and animal health (WOAH, 2021a; WOAH, 2021b). From farm to fork, there are many possible routes for Salmonella dissemination and contamination in poultry and their products, thus examination of each step of the process is necessary (Rajan et al., 2016Rajan K, Shi Z, Ricke SC. Current aspects of Salmonella contamination in the US poultry production chain and the potential application of risk strategies in understanding emerging hazards. Critical Reviews in Microbiology 2016;43(3):370-92.). Nevertheless, Salmonella is a sanitary barrier to the export of multiplication material (WOAH, 2021b) and poultry products, according to bilateral sanitary agreements in force. Furthermore, the World Organisation for Animal Health (WOAH) suggests that countries should identify the prevalent Salmonella serotypes in humans and poultry in order to establish a control programme (WOAH, 2021a). Therefore, the PNSA implemented the official Salmonella Control Programme (Brasil, 2003Brasil. Ministério da Agricultura, Pecuária e do Abastecimento. Instrução normativa nº 78, de 03 de novembro de 2003. Normas técnicas para controle e certificação de núcleos e estabelecimentos avícolas como livres de Salmonella Gallinarum e de Salmonella Pullorum e Livres ou Controlados para Salmonella Enteritidis e para Salmonella Typhimurium. Diário Oficial da União, Seção I; 2003.).
In this context, the control of this pathogen must start in breeding establishments, considering their potential for dissemination to the rest of the poultry production chain (Sivaramalingam et al., 2013Sivaramalingam T, McEwen SA, Pearl DL, Ojkic D, Guerin MT. A temporal study of Salmonella serovars from environmental samples from poultry breeder flocks in Ontario between 1998 and 2008. Canadian Journal of Veterinary Research 2013;77(1):1-11.; Rajan et al., 2016Rajan K, Shi Z, Ricke SC. Current aspects of Salmonella contamination in the US poultry production chain and the potential application of risk strategies in understanding emerging hazards. Critical Reviews in Microbiology 2016;43(3):370-92.). The Brazilian breeding flocks are constantly monitored to be certified as being free from typhoidal and non-typhoidal Salmonella. To monitor Salmonella in the poultry production, samples are collected on a regular basis from all breeder flocks, which includes broiler-breeders, layer-breeders, turkey-breeders, and other bird breeders such as quail, duck, and geese (Brasil, 2003Brasil. Ministério da Agricultura, Pecuária e do Abastecimento. Instrução normativa nº 78, de 03 de novembro de 2003. Normas técnicas para controle e certificação de núcleos e estabelecimentos avícolas como livres de Salmonella Gallinarum e de Salmonella Pullorum e Livres ou Controlados para Salmonella Enteritidis e para Salmonella Typhimurium. Diário Oficial da União, Seção I; 2003.). Additionally, control and monitoring of Salmonella in commercial broilers and turkey establishments was established, as well as measures to control this pathogen in slaughterhouses (Brasill, 2016).
At present, breeding flocks are certified free from 4 (four) Salmonella serovars (Brasil, 2003Brasil. Ministério da Agricultura, Pecuária e do Abastecimento. Instrução normativa nº 78, de 03 de novembro de 2003. Normas técnicas para controle e certificação de núcleos e estabelecimentos avícolas como livres de Salmonella Gallinarum e de Salmonella Pullorum e Livres ou Controlados para Salmonella Enteritidis e para Salmonella Typhimurium. Diário Oficial da União, Seção I; 2003.): Salmonella ser. Enteritidis and Salmonella ser. Typhimurium, which are non typhoidal serovars and important foodborne pathogens worldwide related to poultry products (WOAH, 2021a), and Salmonella ser. Gallinarum e Salmonella ser. Pullorum, typhoidal serovars which concern poultry health and can cause severe economic losses (Markos & Abdela, 2016Markos T, Abdela N. Epidemiology and economic importance of pullorum disease in poultry: A review. Global Veterinaria 2016;17:228-37.; Celis-Estupiñan et al., 2017Celis-Estupiñan ALDP, Batista DFA, Cardozo MV, De-Souza AIS, Alves LBR, De-Almeida AM, et al. Further investigations on the epidemiology of fowl typhoid in Brazil. Avian Pathology 2017;46(4):416-25.; De Carli et al., 2017De Carli S, Gräf T, Kipper K, Lehmann FKM, Zanetti N, Siqueira FM, et al. Molecular and phylogenetic analyses of SalmonellaGallinarum trace the origin and diversification of recent outbreaks of fowl typhoid in poultry farms. Veterinary Microbiology 2017;212:80-6.; WOAH, 2021b).
The aim of this work was to compare official laboratory data on molecular typification of Salmonella isolates from poultry breeding flocks from different Brazilian states dated between 2016 and 2018, received by the Federal Agricultural Defence Laboratory of São Paulo (LFDA-SP), and to identify the production category with the most positive flocks in light of the current legislation.
MATERIALS AND METHODS
The study analysed the surveillance data of Brazil’s official Salmonella Control Programme, studying positive samples sent to Federal Agricultural Defence Laboratory of São Paulo (LFDA-SP) after molecular characterization. The samples were collected from poultry breeder flocks for certification of epidemiological units, which mostly comprises broiler-breeders, layer-breeders, and a few samples from turkey-breeders, from different states of Brazil (BRASIL, 2003Brasil. Ministério da Agricultura, Pecuária e do Abastecimento. Instrução normativa nº 78, de 03 de novembro de 2003. Normas técnicas para controle e certificação de núcleos e estabelecimentos avícolas como livres de Salmonella Gallinarum e de Salmonella Pullorum e Livres ou Controlados para Salmonella Enteritidis e para Salmonella Typhimurium. Diário Oficial da União, Seção I; 2003.). Other poultry breeder species such as quail-breeders and duck-breeders were excluded from the analyses because of the small number of samples. The data comprises 318 positive Salmonella samples collected in 2016, 2017, and 2018, which are used to describe the major isolated serovars and determine which breeder category had the greatest number of serovars identified.
Types of samples collected in epidemiological units
The surveillance had been performed in broiler-breeders, layer-breeders, and turkey-breeders during rearing and production period in great-grandparent, grandparent, and parent flocks.
Samples were collected between one and five days of life, in the middle of the growing period, at the beginning of production, and then every three months for periodic control of the epidemiological unit.
The type of material used for bacteriological or molecular diagnosis depends on the production stage. In the initial growing phase, dead chicks, drag swabs, and paper from the chick’s transport boxes were collected. Flocks that were vaccinated against paratific Salmonella had their samples collected before vaccination. Cloacal swabs, fresh faeces, and boot swabs were the samples collected during the rearing period from great-grandmothers, grandmothers, and parents. In addition to these, the samples collected in breeders’ flocks vaccinated with inactivated vaccines for paratific Salmonella were pipped embryo, meconium, and organs. After the first certification of the epidemiological unit by the government, the control is conducted on a quarterly basis, collecting samples of cloacal swab, fresh faeces, drag and boot swabs, and pipped embryo (Brasil, 2003Brasil. Ministério da Agricultura, Pecuária e do Abastecimento. Instrução normativa nº 78, de 03 de novembro de 2003. Normas técnicas para controle e certificação de núcleos e estabelecimentos avícolas como livres de Salmonella Gallinarum e de Salmonella Pullorum e Livres ou Controlados para Salmonella Enteritidis e para Salmonella Typhimurium. Diário Oficial da União, Seção I; 2003.).
Salmonella isolation
The samples were sent to accredited laboratories in the National Accredited Laboratories Network. All isolates tested positive for Salmonella in accredited laboratories were sent to the official MAPA laboratory, the Federal Agricultural Defence Laboratory of São Paulo (LFDA-SP), where they are received, identified, purified, and typified by DNA microarray.
Strains purification and biochemical tests
As a confirmation of a diagnosis already made by accredited laboratories with previously isolated strains, the next step performed was purification in agar plate (Rambach). After purification, the colonies were subjected to molecular characterization by DNA microarray test.
Typing
Molecular characterization followed the methodo-logy described in the Routine Salmonella Serotype Identification Check & Trace manual (CHECK-POINTS, 2020). Soon after, three to five colonies isolated on agar plate (Rambach) were collected to be typified when there was suspicion of Salmonella ser. Gallinarum or Salmonella ser. Pullorum, or a single colony for the other Salmonellae. The colony was homogenised in 100 µl of lysis buffer. From the 1.5 mL microtubes, the homogenate was transferred to the thermoblock previously heated to 99°C for 15 min. Afterwards, the homogenate was cooled to room temperature by vortexing. Then, three steps of DNA recognition and detection were performed, as described in the manual. The reading of Salmonella typification results was performed using software available in the analysis system.
Results analysis
Accredited laboratories provided the official laboratory data sheets collection and the Salmonella isolates on a monthly basis. To select the strains considered in this work, the following filters were applied from the database: year, state, region, type of poultry (chicken, turkey, quail and duck); category (e.g. great-grandmother, grandmother); surveillance type (certified as free or controlled epidemiological unit for Salmonella) and type of sample. Exploratory analysis was used on these data, followed by descriptive statistical analysis. Frequency and non-parametric tests (Kruskal-Wallis) were performed using RStudio software.
RESULTS AND DISCUSSION
Surveillance for Salmonella is not only important for public health, but also for the economic losses due to high morbidity and mortality in poultry flocks. Furthermore, Salmonella is responsible for losses and waste of animal protein that reduce global food availability in a world where hunger is a reality. Therefore, Salmonella control in early phases of production could help reducing these losses (FAO, 2011).
Many studies report that Salmonella control and prevention strategies in the poultry chain should cover the whole production chain, from farm to fork (Andino & Hanning, 2015Andino A, Hanning I. Salmonella enterica: survival, colonization, and virulence differences among serovars. The Scientific World Journal 2015;2015.; Rajan et al., 2016Rajan K, Shi Z, Ricke SC. Current aspects of Salmonella contamination in the US poultry production chain and the potential application of risk strategies in understanding emerging hazards. Critical Reviews in Microbiology 2016;43(3):370-92.; Machado et al., 2020Machado SCA, Pereira VLA, Aquino MHC, Giombeli A, Rodrigues DP, Nascimento ER. Qualitative and quantitative analysis of salmonella spp. in broilers technological processing and determination of a performance objective (PO) for frozen chicken breast. Brazilian Journal of Poultry Science 2020;22(1):1-12.;). Sivaramalingam et al (2013Sivaramalingam T, McEwen SA, Pearl DL, Ojkic D, Guerin MT. A temporal study of Salmonella serovars from environmental samples from poultry breeder flocks in Ontario between 1998 and 2008. Canadian Journal of Veterinary Research 2013;77(1):1-11.) suggest that interventions at the breeder flock level would likely reduce transmission from breeder flocks to lower levels of the production chain and possibly also to the retail level. When a Salmonella Control Programme for birds is applied at all production stages, including hatcheries, it is possible to keep Salmonella prevalence at a low level (EFSA, 2019EFSA BP, Koutsoumanis K, Allende A, Alvarez-Ordóñez A, Bolton D, Bover-Cid S, et al. Salmonella control in poultry flocks and its public health impact. EFSA Journal 2019;17(2):e05596.). Likewise, the European Commission and European Food Safety Authority (EFSA) attributed the reduction in reported human salmonellosis cases across the European Union to, at least in part, successful control of Salmonella in breeding hens, broiler, laying flocks, and eggs through the National Control Programmes for Salmonella implemented by the United Kingdom in the poultry sector (O’Brien, 2013O'Brien SJ. The "decline and fall" of nontyphoidal Salmonella in the United Kingdom. Clinical Infectious Diseases 2013;56(5):705-10.). Moreover, the National Poultry Improvement Plan (NPIP) executed by the United States Federal Government eradicated Salmonella ser. Pullorum and Salmonella ser. Gallinarum from poultry flocks (Andino & Hanning, 2015). As other countries, in order to reduce Salmonella in the poultry production chain, the Brazilian government implemented a National Surveillance Program for Salmonella in breeder flocks (Brasil, 2003Brasil. Ministério da Agricultura, Pecuária e do Abastecimento. Instrução normativa nº 78, de 03 de novembro de 2003. Normas técnicas para controle e certificação de núcleos e estabelecimentos avícolas como livres de Salmonella Gallinarum e de Salmonella Pullorum e Livres ou Controlados para Salmonella Enteritidis e para Salmonella Typhimurium. Diário Oficial da União, Seção I; 2003.).
The present study analysed data from the National Surveillance Program for Salmonella and the result (Table 1) has shown a great variety of serovars isolated from poultry broiler-breeder and layer-breeder flocks in the main producing states. Some isolates could not be characterised and were described as Salmonella spp., while others were characterised as two possible serovars due to limitations of the microarray assay (CHECK-POINTS, 2020).
Overall, 49 different serovars were detected in poultry broiler-breeder and layer-breeder flocks throughout the three analysed years. Salmonella ser. Heidelberg (10,7%), Salmonella ser. Anatum (6,3%), Salmonella ser. Newport (6,3%), Salmonella ser. Schwarzengrund (5,9%) and Salmonella ser. Mbandaka (5,1%) were the five most common isolated in poultry breeder flocks, accounting for 34,4% of all characterised strains (Figure 1). According to our findings, a Canadian study based on their official data on those types of breeder flocks, between 1998 and 2008, identified the majority of the serovars isolated in Brazil (Sivaramalingam et al., 2013Sivaramalingam T, McEwen SA, Pearl DL, Ojkic D, Guerin MT. A temporal study of Salmonella serovars from environmental samples from poultry breeder flocks in Ontario between 1998 and 2008. Canadian Journal of Veterinary Research 2013;77(1):1-11.). Salmonella ser. Heidelberg was the most common serovar in both studies.
Fifthteen most common serovars isolated in poultry broiler-breeder and layer-breeder flocks between 2016 and 2018.
In terms of Brazilian regions, 39 distinct serovars were identified in the Southern region, where most breeders’ establishments are located. In the Midwestern region, 25 distinct serovars were identified, while in the southeast region there were 18 distinct serovars. On the other hand, only four and six distinct serovars were isolated through the analysed years in the Northern and Northeastern regions, respectively (Table 1). The most common isolated serovars in the Southern region were Salmonella ser. Heidelberg, Salmonella ser. Newport, and Salmonella ser. Anatum. Salmonella breeder data in Brazil are scarce and a great number of studies are based on broiler data. Some of those studies have demonstrated the presence of Salmonella ser. Heidelberg in broiler establishments in the Southern region (Pandini et al., 2015Pandini JA, Pinto FGDS, Muller JM, Weber LD, Moura ACD. Ocorrência e perfil de resistência antimicrobiana de sorotipos de Salmonella spp. isolados de aviários do Paraná, Brasil. Arquivos do Instituto Biológico 2015;82:1-6.; Voss-Rech et al., 2015Voss-Rech D, Vaz CS, Alves L, Coldebella A, Leao JA, Rodrigues DP, et al. A temporal study of Salmonella enterica serotypes from broiler farms in Brazil. Poultry Science 2015;94(3):433-41.) which emphasises that this serovar is more resistant than others in the environment (Voss-Rech et al., 2019). Indeed, our study shows that Salmonella ser. Heidelberg has been constantly isolated in this region, especially in the two main producer states, Paraná and Santa Catarina (Figure 2).
Most common serovars isolated in the Southern region of Brazil in poultry broiler-breeder and layer-breeder flocks between 2016 and 2018.
Our results demonstrated that the number of serovars identified in Paraná and São Paulo has increased through the analysed years (Table 1). A review was performed on the number of poultry establishments through those years and the increase in the variety of serovars could not be related to the increase in the number of poultry producing establishments. On the other hand, layer breeder population and egg production have clearly risen in those years, which might have contributed for the increase of Salmonella isolations in breeder flocks; particularly in São Paulo, where the production of laying hens is predominant (ABPA, 2021). Another possibility to explain the increase in the number of serovars detected is a failure in the biosecurity programs, which may facilitate the spread of local Salmonella strains (De Carli et al., 2017De Carli S, Gräf T, Kipper K, Lehmann FKM, Zanetti N, Siqueira FM, et al. Molecular and phylogenetic analyses of SalmonellaGallinarum trace the origin and diversification of recent outbreaks of fowl typhoid in poultry farms. Veterinary Microbiology 2017;212:80-6.).
Some serovars are common in broiler-breeders regardless of the production region. Data analysis has shown that the frequency of appearance of different serovars in poultry production in the regions of Brazil is statistically significant. The Southern and Midwestern regions presented the greatest variety of serovars throughout the analysed period.
Frequency of distinct serovar in Brazil per region in poultry broiler-breeder and layer-breeder flocks between 2016 and 2018.
The aim of Brazilian breeding flock’s Salmonella certification is to eradicate the four official controlled serovars from great-grandparent and grandparent flocks; to eradicate Salmonella ser. Pullorum and Salmonella ser. Gallinarum from parent flocks; and to control Salmonella ser. Enteritidis and Salmonella ser. Typhimurium in parent flocks (Brasil, 2003Brasil. Ministério da Agricultura, Pecuária e do Abastecimento. Instrução normativa nº 78, de 03 de novembro de 2003. Normas técnicas para controle e certificação de núcleos e estabelecimentos avícolas como livres de Salmonella Gallinarum e de Salmonella Pullorum e Livres ou Controlados para Salmonella Enteritidis e para Salmonella Typhimurium. Diário Oficial da União, Seção I; 2003.). In addition to the Salmonella surveillance programme, poultry breeder establishments have also implemented compulsory biosecurity measures in order to prevent and control pathogens (Brasil, 2007).
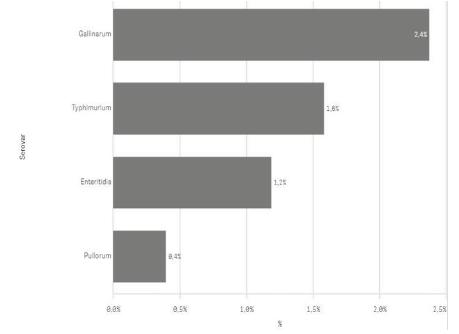
The four serovars controlled by the official surveillance programme, Salmonella ser. Enteritidis, Salmonella ser. Typhimurium, Salmonella ser. Gallinarum and Salmonella ser. Pullorum, represent a small part of the strains isolated (5,5%). Among those serovars, Salmonella ser. Gallinarum was the most common one isolated, followed by Salmonella ser. Typhimurium, Salmonella ser. Enteritidis, and Salmonella ser. Pullorum (Figure 4). The results show that the Salmonella control programme combined with biosecurity measures (Brasil, 2007Brasil. Ministério da Agricultura, Pecuária e do Abastecimento. Instrução normativa nº 56, de 04 de dezembro de 2007. Procedimentos para registro, fiscalização e controle de estabelecimentos avícolas de reprodução, comerciais e de ensino ou pesquisa. Diário Oficial da União, Seção I; 2007.) is fulfilling its purpose, even though the aim of the official control program has not been achieved.
Percentage of controlled serovars isolated in Brazil in poultry broiler-breeder and layer-breeder flocks between 2016 and 2018.
Regarding the four serovars, a Canadian survey showed that less than 1% of Salmonella-positive isolates in breeders between 1998 and 2008 were Salmonella ser. Enteritidis, whereas Salmonella ser. Gallinarum and Salmonella ser. Pullorum were not isolated. Although in the same study Salmonella ser. Typhimurium was the fourth most common serovar, it represented only 2.8% of the isolates (Sivaramalingam et al., 2013Sivaramalingam T, McEwen SA, Pearl DL, Ojkic D, Guerin MT. A temporal study of Salmonella serovars from environmental samples from poultry breeder flocks in Ontario between 1998 and 2008. Canadian Journal of Veterinary Research 2013;77(1):1-11.). Costa et al. (2013Costa RG, Festivo ML, Araujo MS, Reis EM, Lázaro NS, Rodrigues DP. Antimicrobial susceptibility and serovars of Salmonella circulating in commercial poultry carcasses and poultry products in Brazil. Journal of Food Protection 2013;76(12):2011-7.) suggested that the decline in the prevalence of Salmonella ser. Enteritidis and the lack of identification of Salmonella ser. Pullorum and Salmonella ser. Gallinarum in commercial poultry carcass and poultry products in Brazil might reflect the actions of the government programs geared to its control. Nevertheless, those serovars can still be isolated, which may indicate biosecurity gaps.
Salmonella phylogenetic analysis shows common populations and similar strains of Salmonella ser. Enteritidis between humans and chickens, considering that poultry products are the primary vehicle of this serovar transmission to humans, this may be attributable to infected breeding flocks (Li et al., 2021Li S, He Y, Mann DA, Deng X. Global spread of Salmonella Enteritidis via centralized sourcing and international trade of poultry breeding stocks. Nature Communications 2021;12(1):1-12.). Moreover, the control of Salmonella ser. Gallinarum and Salmonella ser. Pullorum can be complicated due to vertical transmission, as hens can become sub clinically infected carriers and pass the infection on to their embryos (Berhanu & Fulasa, 2020Berhanu G, Fulasa A. Pullorum disease and fowl typhoid in poultry: a review. British Journal of Poultry Sciences 2020;9(3):48-56.). Consequently, a lot of effort still needs to be made to eradicate and control those serovars in the poultry chain.
Poultry breeder categories differed in the number of positive epidemiological units for Salmonella. The percentage of positive Salmonella epidemiological units is much higher in parents than in other categories such as great-grandparent and grandparent poultry in all three years. Even in the rearing period, parent-breeder was the category with the most Salmonella positive epidemiological units identified, despite the fact that the same legal biosecurity measures are required for all breeder categories (Table 2).
Percentage of Salmonella positive epidemiological unit per broiler-breeder and layer-breeder category and year in Brazil.
Current knowledge about risk/protective factor categories in relation to the farming system of breeder flocks to improve Salmonellae control highlight outdoor access, group size, stocking density, genetic, farm hatching, existence of enrichment (such as perches, windows, nest boxes), type of litter, factors relating to biosecurity measures (e.g. disinfection, pest control), and occurrence of other diseases. Also, animal welfare indicators listed were stress (other than heat stress), heat stress, activity/behaviour, body status (e.g. foot pad dermatitis - FPD) and other diseases (EFSA et al., 2019EFSA BP, Koutsoumanis K, Allende A, Alvarez-Ordóñez A, Bolton D, Bover-Cid S, et al. Salmonella control in poultry flocks and its public health impact. EFSA Journal 2019;17(2):e05596.).
Although biosecurity legal requirements are the same for these different breeder categories (Brasil, 2007Brasil. Ministério da Agricultura, Pecuária e do Abastecimento. Instrução normativa nº 56, de 04 de dezembro de 2007. Procedimentos para registro, fiscalização e controle de estabelecimentos avícolas de reprodução, comerciais e de ensino ou pesquisa. Diário Oficial da União, Seção I; 2007.), great-grandparents flocks probably have a higher sanitary status, which implies more strict biosecurity measures. Based on these study data, it is possible to assume that the correct application of biosecurity measures may differ between categories. Therefore, data shows that there is an opportunity to improve biosecurity measures in parent breeder flocks.
When the data about turkey breeder flocks was analysed, a total of 16 serovars were identified. The most common isolated serovars were Salmonella ser. Anatum, Salmonella ser. Newport, Salmonella ser. Brandenburg, Salmonella ser. Litchfield, and Salmonella ser. Livingstone, representing 72% of identified strains. It is important to highlight that none of the official controlled serovars were detected (Table 3).
The data presented on Table 3 differs from other studies which isolated Salmonella ser. Typhimurium and Salmonella ser. Enteritidis in turkey facilities, even though with very low prevalence (O´Brian, 2013; Sivaramalingam et al., 2013Sivaramalingam T, McEwen SA, Pearl DL, Ojkic D, Guerin MT. A temporal study of Salmonella serovars from environmental samples from poultry breeder flocks in Ontario between 1998 and 2008. Canadian Journal of Veterinary Research 2013;77(1):1-11.). Another research regarding official data with Salmonella isolates from turkey carcasses was realised in the Southern region of Brazil, between 2004 and 2006, and identified the occurrence of Salmonella ser. Enteritidis and Salmonella ser. Typhimurium in turkey products (Palmeira et al., 2016Palmeira A, Santos LR, Borsoi A, Rodrigues LB, Calasans M, Nascimento VPD. Serovars and antimicrobial resistance of Salmonella spp. isolated from turkey and broiler carcasses in southern Brazil between 2004 and 2006. Revista do Instituto de Medicina Tropical de São Paulo 2016;58.). Brazil’s turkey production is currently represented by a few establishments. The lack of isolation of official serovars in this study may be caused by the ongoing official certification focused on the eradication and control of those serovars and the decrease in Brazilian turkey production (ABPA, 2021).
We analysed different types of samples used to certify the breeder flocks. It was possible to asses that most Salmonella positive isolates were from drag swabs, boot swabs, and pipped embryo samples (Table 4).
Regarding different sample types used to isolate Salmonella in breeders, Berghaus et al. (2012Berghaus RD, Mathis DL, Bramwell RK, Macklin KS, Wilson JL, Wineland MJ, et al. Multilevel analysis of environmental salmonella prevalences and management practices on 49 broiler breeder farms in four South-Eastern States, USA. Zoonoses and Public Health 2012;59(5):365-74.) conducted a serial survey of 49 broiler breeder farms across the United States of America. Different sample types such as drag swabs, boot swabs, and litter samples yielded comparable prevalence of Salmonella positive results, while the prevalence was significantly lower for the slat and egg belt sponge samples. The study by Kahya et al. (2013Kahya S, Eyigör A, Çarli KT. Comparison of different sample types for Salmonella detection from chicken layer breeder flocks. Uludag Üniversitesi Veteriner Fakültesi Dergisi 2013;32(2):19-24.) compared different types of samples for Salmonella detection in chicken layer breeder flock, and positive Salmonella samples were 8% out of pooled cloacal swab, 90,9% out of pooled embryonated chicken egg, and 21,4% of pooled wet faeces. PCR and ISO 6579 culture methods results were 100% in agreement (100% sensitivity and specificity) with culture results for all sample types. These results showed that pipped embryo was the most meaningful sample for Salmonella detection from breeder flocks, followed by wet faeces.
As expected, Salmonella ser. Gallinarum and Salmonella ser Pullorum were isolated from organs. According to the WOAH (2021b), post mortem tissues are preferable to isolate those serovars. It is important to highlight Salmonella ser. Gallinarum isolationfrom dead embryo. This matrix is easy to collect and include in poultry industries’ biosecurity programs. Finally, Salmonella ser. Enteritidis, was detected from boot and drag swabs and Salmonella ser. Typhimurium was isolated from a large number of sample sources, like day old chicks, meconium, boot, and drag swabs. These data reinforce the importance of collecting tissues to detect typhoid Salmonella and suggest that the embryo has a great value in monitoring the presence of this pathogen.
CONCLUSION
Salmonellosis is a disease of economic and public health importance throughout the world, especially for those countries that are major exporters of poultry products, such as Brazil, requiring constant monitoring of the prevalent strains and their characteristics. Especially in the case of Brazil, the adoption of the verticalized poultry production model requires special attention in relation to the control of poultry salmonellosis in breeder flocks.
Our results showed that the number of serovars has increased between 2016 and 2018 in the main broiler and layer poultry producer states; the frequency of distinct serovars in the different regions of Brazil were statistically significant; parent-breeders was the category with the most Salmonella positive epidemiological units identified; and Salmonella ser. Enteritidis, Salmonella ser. Typhimurium, Salmonella ser. Gallinarum, and Salmonella ser. Pullorum represent a small part of the isolated strains.
Therefore, the present study demonstrates the importance of an official program in Brazil for the surveillance for salmonella in breeder flocks, especially when combined with biosecurity measures designed to prevent and control the disease.
ACKNOWLEDGEMENT
To the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), ProcessE-26/211.075/2021- Edital E-29-Apoio aos Programas de Pós Graduação Stricto Senso do Estado Do Rio de Janeiro, for financial support for the publication of this work.
REFERENCES
- ABPA - Associação Brasileira de Proteína Animal. Relatório anual ABPA 2021 [cited 2021 Nov 10). Available from: https://abpa-br.org/relatorios/.
» https://abpa-br.org/relatorios - Andino A, Hanning I. Salmonella enterica: survival, colonization, and virulence differences among serovars. The Scientific World Journal 2015;2015.
- Berghaus RD, Mathis DL, Bramwell RK, Macklin KS, Wilson JL, Wineland MJ, et al. Multilevel analysis of environmental salmonella prevalences and management practices on 49 broiler breeder farms in four South-Eastern States, USA. Zoonoses and Public Health 2012;59(5):365-74.
- Berhanu G, Fulasa A. Pullorum disease and fowl typhoid in poultry: a review. British Journal of Poultry Sciences 2020;9(3):48-56.
- Brasil. Ministério da Agricultura, Pecuária e do Abastecimento. Portaria ministerial nº193, de 19 de setembro de 1994. Institui o programa nacional de sanidade avícola no âmbito do dsa e cria o comitê consultivo do programa de sanidade avícola. Diário Oficial da União, Seção I; 1994.
- Brasil. Ministério da Agricultura, Pecuária e do Abastecimento. Instrução normativa nº 78, de 03 de novembro de 2003. Normas técnicas para controle e certificação de núcleos e estabelecimentos avícolas como livres de Salmonella Gallinarum e de Salmonella Pullorum e Livres ou Controlados para Salmonella Enteritidis e para Salmonella Typhimurium. Diário Oficial da União, Seção I; 2003.
- Brasil. Ministério da Agricultura, Pecuária e do Abastecimento. Instrução normativa nº 56, de 04 de dezembro de 2007. Procedimentos para registro, fiscalização e controle de estabelecimentos avícolas de reprodução, comerciais e de ensino ou pesquisa. Diário Oficial da União, Seção I; 2007.
- Brasil. Ministério da Agricultura, Pecuária e do Abastecimento. Instrução normativa nº 20, de 21 de outubro de 2016. Estabelece o controle e o monitoramento de Salmonella spp. nos estabelecimentos avícolas comerciais de frangos e perus de corte e nos estabelecimentos de abate de frangos, galinhas, perus de corte e reprodução, registrados no Serviço de Inspeção Federal (SIF). Diário Oficial da União, Seção I; 2016.
- Celis-Estupiñan ALDP, Batista DFA, Cardozo MV, De-Souza AIS, Alves LBR, De-Almeida AM, et al. Further investigations on the epidemiology of fowl typhoid in Brazil. Avian Pathology 2017;46(4):416-25.
- Check-Points. Check & trace Salmonella user manual version 2020-03 [cited 2021 Nov 25). Available from: https://www.woah.org/app/uploads/2021/03/official-cts-user-manual-shortened-version-2020-09.pdf
» https://www.woah.org/app/uploads/2021/03/official-cts-user-manual-shortened-version-2020-09.pdf - Costa RG, Festivo ML, Araujo MS, Reis EM, Lázaro NS, Rodrigues DP. Antimicrobial susceptibility and serovars of Salmonella circulating in commercial poultry carcasses and poultry products in Brazil. Journal of Food Protection 2013;76(12):2011-7.
- De Carli S, Gräf T, Kipper K, Lehmann FKM, Zanetti N, Siqueira FM, et al. Molecular and phylogenetic analyses of SalmonellaGallinarum trace the origin and diversification of recent outbreaks of fowl typhoid in poultry farms. Veterinary Microbiology 2017;212:80-6.
- EFSA BP, Koutsoumanis K, Allende A, Alvarez-Ordóñez A, Bolton D, Bover-Cid S, et al. Salmonella control in poultry flocks and its public health impact. EFSA Journal 2019;17(2):e05596.
- FAO - Food and Agriculture Organization of the United Nations. Global food losses and food waste - Extent, causes and prevention; 2011 [cited 2021 Nov 10]. Available from: https://www.fao.org/3/i2697e/i2697e.pdf.
- Kahya S, Eyigör A, Çarli KT. Comparison of different sample types for Salmonella detection from chicken layer breeder flocks. Uludag Üniversitesi Veteriner Fakültesi Dergisi 2013;32(2):19-24.
- Li S, He Y, Mann DA, Deng X. Global spread of Salmonella Enteritidis via centralized sourcing and international trade of poultry breeding stocks. Nature Communications 2021;12(1):1-12.
- Machado SCA, Pereira VLA, Aquino MHC, Giombeli A, Rodrigues DP, Nascimento ER. Qualitative and quantitative analysis of salmonella spp. in broilers technological processing and determination of a performance objective (PO) for frozen chicken breast. Brazilian Journal of Poultry Science 2020;22(1):1-12.
- Markos T, Abdela N. Epidemiology and economic importance of pullorum disease in poultry: A review. Global Veterinaria 2016;17:228-37.
- O'Brien SJ. The "decline and fall" of nontyphoidal Salmonella in the United Kingdom. Clinical Infectious Diseases 2013;56(5):705-10.
- Palmeira A, Santos LR, Borsoi A, Rodrigues LB, Calasans M, Nascimento VPD. Serovars and antimicrobial resistance of Salmonella spp. isolated from turkey and broiler carcasses in southern Brazil between 2004 and 2006. Revista do Instituto de Medicina Tropical de São Paulo 2016;58.
- Pandini JA, Pinto FGDS, Muller JM, Weber LD, Moura ACD. Ocorrência e perfil de resistência antimicrobiana de sorotipos de Salmonella spp. isolados de aviários do Paraná, Brasil. Arquivos do Instituto Biológico 2015;82:1-6.
- Rajan K, Shi Z, Ricke SC. Current aspects of Salmonella contamination in the US poultry production chain and the potential application of risk strategies in understanding emerging hazards. Critical Reviews in Microbiology 2016;43(3):370-92.
- Sivaramalingam T, McEwen SA, Pearl DL, Ojkic D, Guerin MT. A temporal study of Salmonella serovars from environmental samples from poultry breeder flocks in Ontario between 1998 and 2008. Canadian Journal of Veterinary Research 2013;77(1):1-11.
- Voss-Rech D, Vaz CS, Alves L, Coldebella A, Leao JA, Rodrigues DP, et al. A temporal study of Salmonella enterica serotypes from broiler farms in Brazil. Poultry Science 2015;94(3):433-41.
- Voss-Rech D, Kramer B, Silva VS, Rebelatto R, Abreu PG, Coldebella A, et al. Longitudinal study reveals persistent environmental Salmonella Heidelberg in Brazilian broiler farms. Veterinary Microbiology 2019;233:118-23.
- WOAH - World Organisation for Animal Health. Prevention, detection and control of Salmonella in poultry. In: WOAH. Terrestrial animal health code; 2021a [cited 2021 Nov 10]. Available from: https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-code-online-access/?id=169&L=1&htmfile=chapitre_prevent_salmonella.htm
» https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-code-online-access/?id=169&L=1&htmfile=chapitre_prevent_salmonella.htm - WOAH - World Organisation for Animal Health. Fowl typhoid and pullorum disease. In: WOAH. Terrestrial animal health code; 2021b [cited 2021 November 10]. Available from: https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-code-online-access/?id=169&L=1&htmfile=chapitre_fowl_typhoid_pullorum_disease.htm
» https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-code-online-access/?id=169&L=1&htmfile=chapitre_fowl_typhoid_pullorum_disease.htm
Publication Dates
-
Publication in this collection
20 Jan 2023 -
Date of issue
2023
History
-
Received
15 Mar 2022 -
Accepted
25 July 2022








 Kruskal-Wallis chi-squared = 12.049, df = 4, p-value = 0.01699.
Kruskal-Wallis chi-squared = 12.049, df = 4, p-value = 0.01699.