Abstract
Imatinib mesylate was the first BCR-ABL-target agent approved for the treatment of chronic myeloid leukemia. Although most patients respond well to imatinib therapy, the literature shows that one third develops resistance or intolerance. The timing of second-line treatment after failure of initial treatment may have a significant impact on long-term outcome. Thus, appropriate monitoring to identify resistance and/or intolerance is crucial to early intervention with second generation tyrosine kinase inhibitors and attainment of better results
Leukemia, myelogenous, chronic, BCR-ABL positive; Drug resistance; Drug monitoring, neoplasm; Receptor Protein-Tyrosine Kinases; Pyrimidines; Antineoplastic agents; Monitoring; Prognosis
REVIEW ARTICLES
Importance of monitoring and early switch to second generation tyrosine kinase inhibitors for the prognosis of patients with chronic myeloid leukemia with imatinib resistance or intolerance
Belinda Pinto SimõesI; José Wilson Ramos Braga JuniorII; Maria Aparecida do Carmo RegoII; Cármino Antônio de SouzaIII
IFaculdade de Medicina de Ribeirão Preto, Universidade de São Paulo USP, Ribeirão Preto (SP), Brazil
IIDepartamento de Oncologia, Bristol-Myers Squibb, Rio de Janeiro (RJ), Brazil
IIIUniversidade Estadual de Campinas Unicamp, Campinas (SP), Brazil
Correspondence Correspondence: Cármino Antonio de Souza Centro de Hematologia e Hemoterapia de Campinas, Universidade Estadual de Campinas Unicamp Rua Carlos Chagas, 450 Cidade Universitária "Prof. Zeferino Vaz" Distrito de Barão Geraldo 13083- 878 Campinas (SP), Brazil Phone: 55 19-3521 8740 carmino@unicamp.br
ABSTRACT
Imatinib mesylate was the first BCR-ABL-target agent approved for the treatment of chronic myeloid leukemia. Although most patients respond well to imatinib therapy, the literature shows that one third develops resistance or intolerance. The timing of second-line treatment after failure of initial treatment may have a significant impact on long-term outcome. Thus, appropriate monitoring to identify resistance and/or intolerance is crucial to early intervention with second generation tyrosine kinase inhibitors and attainment of better results.
Keywords: Leukemia, myelogenous, chronic, BCR-ABL positive/drug therapy; Drug resistance; Drug monitoring, neoplasm; Receptor Protein-Tyrosine Kinases; Pyrimidines/therapeutic use; Antineoplastic agents/administration & dosage; Monitoring; Prognosis
Introduction
Chronic Myeloid Leukemia (CML) is a clonal hematological disease associated with a reciprocal chromosomal translocation between chromosomes 9 and 22, resulting in the Philadelphia (Ph) chromosome.(1) This gene fusion codes for a chimeric protein, BCR-ABL, which is associated with the uncontrolled activity of ABL tyrosine kinase (TK). (1) The Ph chromosome is detected in 95% of patients with CML.(2) The estimated incidence of CML is 1 to 2 cases per 100,000 people per year.(3)
The International Randomized Study of Interferon versus STI-571 (IRIS) study established the superiority of imatinib, an ABL kinase inhibitor, over the interferon-alpha and cytarabine combination in terms of hematologic response (HR), cytogenetic response (CyR) and molecular response (MolR).(4,5) Since then, imatinib is the first-line treatment of choice for CML. The intent-to-treat analysis of the IRIS study showed an accumulated incidence of complete cytogenetic response (CCyR) of 82.7%, event-free survival (EFS) of 81.3% and overall survival (OS) of 83.2% over 60 months.(6) Patients who reached a CyR and a MolR during treatment showed a longer EFS and protection against progression to the advanced phases of the disease.(7)
After seven years of follow-up of the IRIS study, 40% of patients were found to have discontinued the treatment, and adverse events accounted for 5% of the cases, lack of efficacy for 15%, undergoing bone marrow transplant for 3%, death for 2% and the remaining 15% withdrew for other reasons (protocol violation, consented withdrawal or non-renewal, lost to follow-up).(8) A similar result was observed during follow-up of patients with CML on imatinib outside a clinical trial, by the Hammersmith Hospital (De Lavallade) group. After five years of follow-up, the EFS was 62.7%, imatinib was then suspended for nearly 40% of the patients. The definition of event for this study was death for any cause, loss of hematologic response or major cytogenetic response, white blood cell increase, absence of major cytogenetic response and imatinib intolerance.
Imatinib resistance is generally due to the appearance of clones expressing mutated forms of BCR-ABL, in which the amino acid replacements in the ABL kinase domain prevent imatinib binding but sustain kinase activity.(9-11) In the IRIS study, the accumulated mutation rate over five years was 8.6% (95% CI: 4.5% to 15.8%).(6) Recent studies suggest that these mutations occur in 40% of patients with imatinib failure.(9,10,12) Soverini et al.(13) evaluated the frequency of mutations in 297 patients with hematologic or cytogenetic resistance to imatinib. Mutations were observed in 127 (43%) patients; 27% during the chronic phase, 52% during the accelerated phase, 75% during myeloid blast crisis, and 83% during lymphoid blast crisis/Ph-positive ALL. Jabbour et al. evaluated the frequency of mutations in 171 patients after imatinib treatment failure with 66 mutations being identified in 62 (36%) patients.(14)
Mascarenhas et al. correlated the presence of mutations with OS in 93 imatinib resistant patients. A mutation was detected in 25% of patients. The OS over 30 months was 87% for patients without mutations and 56% for the mutation group (RR = 68; p < 0.0001). Due to the association of some mutations and imatinib resistance, it is clear that approximately 30-40% of patients will eventually need a more effective treatment. Early detection of the presence of mutated clones may help the therapeutic decision and the choice of alternative treatments.(15)
Dasatinib is an ABL kinase activity inhibitor which is different from imatinib as it binds to both to the active and inactive conformation in the ABL domain, in addition to inhibiting other kinases, such as the Src family (Src, Lck, Yes, Fyn), ckit, EphA2 and PDGPR-ß, providing lasting responses in patients with and without BCR-ABL mutations.(16) Dasatinib was evaluated in clinical trials (Phase I, II and III) in adults with Ph-positive leukemias after imatinib failure or intolerance and showed effectiveness in the chronic, accelerated and blast phases of CML.
Dasatinib has been approved by the FDA since 2006 for CML treatment during the three phases and also for Ph-positive ALL.
Nilotinib is a BCR-ABL kinase, c-KIT, platelet-derived growth factor receptor (PDGFR) and ephrin receptor inhibitor.(17) It proved to be 43 to 60 times more potent than imatinib in cell lines. Similar to imatinib and dasatinib, nilotinib showed no activity against T315L mutations.(18)
As imatinib, nilotinib only binds to the BCR-ABL inactive conformation and does not inhibit Src kinases.(18) Nilotinib has been approved by the FDA in the treatment of patients with chronic or accelerated phase CML, who are imatinib intolerant or resistant.(17)
Unlike dasatinib, bosutinib does not inhibit PDGFR and c-kit.(19) It may bind both to inactive and intermediate BCR-ABL conformations, but it does not show activity against T315L mutations.(19) In Phase II studies it has also demonstrated activity in patients with accelerated and blast phase CML, previously treated with imatinib and other TKIs.(20,21)
Monitoring CML treatment
Since imatinib was introduced, it is clear that the appropriate monitoring of the treatment response is an essential and relevant part of the therapeutic strategy.(22) The objectives of monitoring minimal residual disease in CML include: to demonstrate the efficacy of the initial treatment, to detect treatment relapse or resistance and to identify the mechanisms of treatment failure to help in the choice of alternative treatments.(23)
The initial evaluation before the start of the treatment must include the calculation of the prognosis rates using the Sokal(24,25) or Hasford Score(26) methods and demonstrated in Tables 1 and 2. Despite having been described in the pre-imatinib era, they maintain an important prognostic role, even with the use of imatinib.(4,25)
After treatment starts, for the evaluation of imatinib response it is required that complete blood counts with differential, cytogenetic and molecular examinations be performed to evaluate the BCR-ABL transcript level. Monitoring of the percentage of Ph-positive cells is the best validated system to evaluate the response to interferon-alpha and TKIs, as cytogenetic response is the best survival marker.(4) Thus, cytogenetic response remains the gold standard to evaluate CML response.(23) Notwithstanding, most sites perform the evaluation through molecular analysis to monitor treatment response in addition to cytogenetic evaluation.(27-29) Reaching a major molecular response (MMolR) after 12 months of imatinib treatment has been associated with a longer progression-free survival (PFS).(5,7) In general, the use of molecular studies to detect mutations has been recommended only if there is evidence of hematologic, cytogenetic relapse or resistance and a 2- to 5-fold increase in BCR-ABL transcript levels.(23)
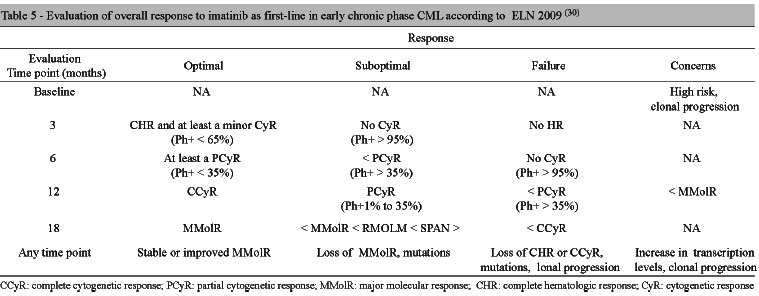
The European LeukemiaNet (ELN) updated the recommendations published in 2006 in order to optimize and standardize CML management.(30) The response definitions according to the ELN are presented in Table 3.(30)
The ELN recommendations for imatinib response monitoring are presented in Table 4.(30) Based on HR, CyR, and MolR and time to response, imatinib overall response can be defined as: optimal, suboptimal and absence of response as shown in Table 5.
The recommendations further include some alert situations. Optimal response means that switching medication will not increase survival, which is anticipated at 100% after six to seven years of treatment.(30) Suboptimal response means the patient will still receive further benefit from the continued treatment, but the chances of optimal response are reduced. Treatment failure suggests switching the approach, as the chances of favorable outcomes are low.
Regarding previous recommendations,(29) a new definition for optimal response has been introduced. In case some type of cytogenetic response does not occur at three months (introducing, therefore, bone marrow puncture at this point, differing from 2006 recommendations), we consider it a suboptimal response. In case there is no complete hematologic response at three months, we refer to it as failure, as well as in case no cytogenetic response is achieved at six months. Clonal progression during treatment was defined as treatment failure. The deletion of the long arm of chromosome 9, considered in previous works as an alert signal, is no longer considered a cause for special concern.
There is evidence in the literature claiming that early introduction of treatment with a second generation TKI, such as dasatinib, improves CML prognosis, making adequate monitoring of patients using first-line medication, such as imatinib, imperative.(31
Importance of early response in prognosis
Imatinib response is the most important prognostic factor to achieve prolonged survival in CML.(7,32) The response grade achieved during treatment is also an important prognostic factor. (32) The 5-year follow-up of the IRIS study showed longer PFS for patients who achieved a CCyR compared to the ones who achieved only a PCyR.(7)
Sixty-nine percent of patients with early chronic phase CML are known to achieve a CCyR after 12 months of imatinib treatment.(4) With continued treatment, only a fraction (13%) may still achieve this response.(7) It is controversial whether achieving an early or delayed complete cytogenetic response is directly related to disease progression. Some recent analyses suggest that the risk of disease progression among patients who achieve a CCyR during the first 12 months of imatinib treatment is similar to that of those who achieve this response later.(7,33)
However, other studies suggest early responses predict a better prognosis.(4,34) In a retrospective analysis, Quintás-Cardama et al.(32) evaluated 258 patients with chronic phase CML regarding the probability of achieving a CCyR, a major molecular response (MMolR) and progression at three, six, and 12 months after imatinib was started. The initial dose of imatinib was 800 mg/day in 208 patients and 400 mg/day in 50 patients. The probability of achieving a CCyR or MMolR decreased significantly over time for patients who did not achieve a CCyR at three months, six months and 12 months, while the progression risk increased at each assessment period. Thus, a patient who did not achieve a CCyR at six months still had a 57% probability of achieving this response and a 34% probability of having an event (defined as evolution to more advanced phases, loss of response and death). For patients who did not achieve a CCyR up to 12 months, the probability of still achieving this response eventually dropped to 42%, and the risk of events increased to 38%. The authors suggest that failure to achieve a CCyR within the first 12 months of imatinib treatment is associated with higher disease progression rates. The same result was seen when the molecular response was used as response parameter. Thus, the authors claim that the evaluation already at three months may guide the rational use of therapeutic strategies able to provide higher rates of CyR and MolR.
Marin et al. evaluated 224 patients with chronic phase CML treated with imatinib regarding response and prognosis in order to validate the 2006 ELN recommendations.(35) For the few patients who did not achieve a CHR after three months of treatment, the probability of achieving a CCyR was zero (p=0.0003); OS over five years was 60% (p=0.003) and PFS was 56% (p=0.002), thus significantly lower than those who had achieved a CCyR.
The introduction of cytogenetic analysis at three months is based on the data that patients who remain without a cytogenetic response at three months have a lower probability of achieving a CCyR with continued treatment.(6,29) Other retrospective analyses have shown that patients who did not achieve any cytogenetic response at six months (Ph+ >95%) have a low probability of achieving a CCyR (25%) or a CMolR, (12%) and are, therefore, considered treatment failure.(6,36) In addition, patients who achieved a CCyR at six months present higher PFS and EFS than those with a delayed response.(37,38) The advantage of achieving a complete cytogenetic remission at 12 months was also evidenced in terms of OS and PFS,(6,7) as well as at 18 months (CCyR versus PCyR, 99% versus 87% and 98% versus 76%, respectively).(6,7)
These results suggest the early identification of high risk patients is fundamental for a better therapeutic planning, such as switching to second generation TKIs. The data are clearer when cytogenetic response is considered, differently from the data where molecular response is considered.
Cortes et al. evaluated 280 patients who achieved a CCyR on imatinib, including 117 after interferon-alpha failure and 163 treatment-naïve patients. Patients who did not achieve a MMolR within 12 months of treatment had a higher chance of losing CyR than those who achieved it (37% versus 5%; p=0.0001). Patients who achieved a decrease of <1-log after three months of treatment had a lower probability of achieving a MMolR at 24 months compared to those who had a decrease higher than 1-log or 2-log (84% and 95%, respectively. P=0.0002).(34)
Iacobucci et al. conducted a retrospective trial of 284 patients with late chronic phase CML treated with imatinib 400 mg daily after interferon-alpha failure. The pattern and time to imatinib response were evaluated, comparing CyR and MolR, PFS and OS in patients who achieved a CCyR within one year of treatment (early responders) and in patients with a CCyR detected after 12 months (late responders). After three to four years of follow-up, patients who had a delayed CyR presented a MolR rate that was similar to that of those who had had an early CyR. No difference was seen in the measure of residual disease through quantitative PCR at 36 months and at 48 months among the groups. Estimated PFS at 4 years was 88% for early responders and 100% for late responders, while the estimated OS was 92% and 100%, respectively. The authors suggest that at least 12 months are required to assess imatinib response.(33)
A study with 120 patients with chronic phase CML after interferon-alpha failure assessed whether early MolR (at one and two months) measured by real time PCR could predict or not the CyR at six months.(39) The authors concluded that a BCR-ABL/ABL ratio < 20% after two months of imatinib treatment was correlated to CyR at 6 months (p=0.0008).
From the studies referenced, we observed that the exact value of MolR is more difficult to evaluate.(30) The first analysis of the IRIS study showed that achieving a CMolR at 12 months of treatment predicted a higher PFS.(5) However, this difference became borderline in the subsequent analysis (100% versus 98%; p=0.11).(7) Patients who did not achieve a MolR during treatment are at a higher risk of losing CyR and developing resistance to imatinib and other TKIs.(40)
Thus, the prognostic value of MMolR is still controversial, although there is a consensus that not achieving a MMolR and the increase in BCR-ABL transcript levels require more careful monitoring.(6,29,37)
When to start a second generation TKI?
Quintás-Cardama et al.(31) evaluated 293 patients with chronic phase CML and imatinib resistance as to whether early intervention (in cytogenetic recurrence) with dasatinib was superior to delayed intervention (in hematologic recurrence). Out of 293 eligible patients, 151 patients had loss of major cytogenetic response (MCyR) (Group 1), 33 patients had loss of MCyR and CHR (Group 2) and 109 patients had loss of CHR without loss of MCyR (Group 3).
The results from dasatinib treatment may be observed on Table 6. Among the patients with loss of MCyR during imatinib treatment (Group 1, early intervention), 72% achieved CCyR with dasatinib, compared to 42% of those who had lost both responses, MCyR and CHR (Group 2, delayed intervention) and 26% of those who lost CHR without loss of previous MCyR (Group 3). The MMolR rate was higher (60%) in Group 1 compared to Groups 2 (29%) and 3 (26%). EFS at 24 months was also higher in the group given early intervention (89% in Group 1 versus 29% in Group 2 versus 64% in Group 3). The transformation-free survival (TFS) for accelerated and blast phases at 24 months was 98% in Group 1 and 93% in Group 2, while the OS was 98% and 93%, respectively. In Group 3, TFS at 24 months was 79% and OS was 86%. The authors suggest regular monitoring of response during imatinib treatment is essential to identify recurrence through loss of MCyR without the occurrence of loss of hematologic response.
Another important factor when deciding whether to start a second generation TKI relates to mutations. The appearance of a mutation with an imatinib resistance profile is considered by the 2009 ELN definitions as failure and requires switching medications. Different mutations may confer distinct levels of resistance, depending on the location and their effect on the kinase.(23)
Some imatinib resistance mechanisms may be overcome by dose escalation.(41,42) A retrospective analysis was carried out for IRIS study patients who underwent imatinib dose escalation from 400 mg to 600 mg or 800 mg in the event of treatment failure or suboptimal response (ELN criteria) or according to the IRIS study criteria.(43) These criteria included: failure to achieve CHR at three months, failure to achieve at least a minor CyR at 12 months and loss of major CyR at any time point. Out of 533 patients initially randomized to receive imatinib in the IRIS study, 106 (19%) patients had their dose escalated to 600 mg or 800 mg daily. In this study, 67% to 86% of patients achieved or recovered a HR, and 38% to 42% a CyR within up to 12 months after dose escalation, depending on the failure or suboptimal response criterion used.
The PFS for the accelerated or blast phase within three years was 89% and the OS was 84%. Other studies, however, suggest the escalation benefit is transient.(44, 45)
Some mutations are known to make the disease completely resistant to imatinib, for example, the T315l mutation. Thus, in case other mutations are acquired, switching to a second generation TKI such as dasatinib or nilotinib may yield superior results.(46-48)
START-R was a Phase II randomized trial comparing dasatinib to high doses of imatinib with imatinib failure at a conventional dose.(48) Imatinib resistant patients with chronic phase CML at daily doses of 400 mg or 600 mg were randomized to receive dasatinib (140 mg daily) or imatinib at a higher dose (800 mg). This trial enrolled 150 patients with a 2:1 randomization (101 received dasatinib and 49 received imatinib). The primary endpoint analyzed was the MCyR rate over 12 months and the secondary endpoints were the MCyR and CHR rates at any period before cross-over, duration of the MCyR and CHR and time to MCyR and CHR before cross-over. The endpoints were also evaluated after cross-over. The response rates are illustrated in Table 3. The mean time to treatment failure was longer for dasatinib, with an 84% decrease in relative risk (HR=0.16; 95% CI=0.1 to 0.26; p<0.001). Progression-free survival was also favorable towards dasatinib, with an 86% decrease in relative risk (HR=0.14; 95% CI=0.05 to 0.4; p<0.001).(49)
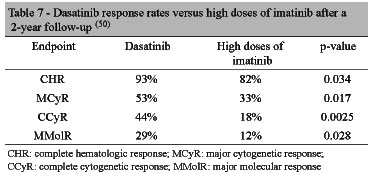
Kantarjian et al. published a phase II study with a 2-year follow-up, demonstrating that dasatinib sustained higher responses (Table 7) compared to high doses of imatinib, in addition to longer progression-free survival (p=0.0012).(50)
Thus, an investigation of mutations in patients during progression is fundamental when planning the best therapeutic strategy. The presence of the T315l mutation confers resistance both to imatinib and to second generation TKIs, such as dasatinib and nilotinib.(51) Although there is no head-to-head comparison of the efficacy of second generation agents, the identification of specific mutations could help towards the choice of best option.(30,51)
Conclusions
The introduction of TKIs had an important impact on the prognosis of patients with CML over the last years. The rising increase of effective therapeutic options justifies the need for adequate monitoring, for the choice of the best second-line treatment option and its introduction at the most adequate time point. In the same manner that CML treatment has been rapidly evolving, the concepts related to monitoring, including the available techniques and the interpretation of their results, in addition to the best use of all this information, also evolve constantly.
Submitted: 4/27/2010
Accepted: 9/24/2010
Conflict-of-interest disclosure: JWRBS and MACR are employees of Bristol-Meyers Squibb, Brazil
This article was written with the support of Bristol-Myers Squibb
- 1. Sawyers CL. Chronic myeloid leukemia. N Engl J Med. 1999; 340(17):1330-40. Comment in: N Engl J Med. 1999;341 (10):765.
- 2. Khoury HJ, Guilhot F, Hughes TP, Kim DW, Cortes JE. Dasatinib treatment for Philadelphia chromosome-positive leukemias: practical considerations. Cancer. 2009;115(7):1381-94.
- 3. Redaelli A, Bell C, Casagrande J, Stephens J, Botteman M, Laskin B, et al. Clinical and epidemiologic burden of chronic myelogenous leukemia. Expert Rev Anticancer Ther. 2004;4(1):85-96.
- 4. O'Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, Cornelissen JJ, Fischer T, Hochhaus A, Hughes T, Lechner K, Nielsen JL, Rousselot P, Reiffers J, Saglio G, Shepherd J, Simonsson B, Gratwohl A, Goldman JM, Kantarjian H, Taylor K, Verhoef G, Bolton AE, Capdeville R, Druker BJ; IRIS Investigators. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348(11):994-1004. Comment in: Curr Hematol Rep. 2004;3(1):37-8. N Engl J Med. 2003; 348(11): 1048-50. Clin Lab Haematol. 2005;27(6):416-7.
- 5. Hughes TP, Kaeda J, Branford S, Rudzki Z, Hochhaus A, Hensley ML, Gathmann I, Bolton AE, van Hoomissen IC, Goldman JM, Radich JP; International Randomised Study of Interferon versus STI571 (IRIS) Study Group. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003; 349(15):1423-32. Comment in: N Engl J Med. 2003;349 (15): 1399-401.
- 6. de Lavallade H, Apperley JF, Khorashad JS, Milojkovic D, Reid AG, Bua M, et al. Imatinib for newly diagnosed patients with chronic myeloid leukemia: incidence of sustained responses in an intention-to-treat analysis. J Clin Oncol. 2008;26(20):3358-63. Comment in: J Clin Oncol. 2008;26(20):3308-9. Comment in: J Clin Oncol. 2008;26(20):3308-9.
- 7. Druker BJ, Guilhot F, O'Brien SG, Gathmann I, Kantarjian H, Gattermann N, Deininger MW, Silver RT, Goldman JM, Stone RM, Cervantes F, Hochhaus A, Powell BL, Gabrilove JL, Rousselot P, Reiffers J, Cornelissen JJ, Hughes T, Agis H, Fischer T, Verhoef G, Shepherd J, Saglio G, Gratwohl A, Nielsen JL, Radich JP, Simonsson B, Taylor K, Baccarani M, So C, Letvak L, Larson RA; IRIS Investigators. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408-17. Comment in: N Engl J Med. 2007;356(17):1780; author reply 1780.
- 8. Deininger M, editor. International Randomized Study of Interferon Vs STI571 (IRIS) 8-year follow up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib. Presented in: 51ş ASH Annual Meeting and Exposition. New Orleans, La; 2009 dec 5-8. [abstract 1126]. Available from: http://ash.confex.com/ash/2009/webprogram/Paper23968.html
- 9. Branford S, Rudzki Z, Walsh S, Parkinson I, Grigg A, Szer J, et al. Detection of BCR-ABL mutations in patients with CML treated with imatinib is virtually always accompanied by clinical resistance, and mutations in the ATP phosphate-binding loop (P-loop) are associated with a poor prognosis. Blood. 2003;102(1):276-83.
- 10. Hochhaus A, La Rosée P. Imatinib therapy in chronic myelogenous leukemia: strategies to avoid and overcome resistance. Leukemia. 2004;18(8):1321-31.
- 11. Branford S, Rudzki Z, Walsh S, Grigg A, Arthur C, Taylor K, et al. High frequency of point mutations clustered within the adenosine triphosphate-binding region of BCR/ABL in patients with chronic myeloid leukemia or Ph-positive acute lymphoblastic leukemia who develop imatinib (STI571) resistance. Blood. 2002;99(9): 3472-5. Comment in: Blood. 2002 ;100(3):1105.
- 12. Kantarjian HM, Giles F, Quintás-Cardama A, Cortes J. Important therapeutic targets in chronic myelogenous leukemia. Clin Cancer Res. 2007;13(4):1089-97.
- 13. Soverini S, Colarossi S, Gnani A, Rosti G, Castagnetti F, Poerio A, Iacobucci I, Amabile M, Abruzzese E, Orlandi E, Radaelli F, Ciccone F, Tiribelli M, di Lorenzo R, Caracciolo C, Izzo B, Pane F, Saglio G, Baccarani M, Martinelli G; GIMEMA Working Party on Chronic Myeloid Leukemia. Contribution of ABL kinase domain mutations to imatinib resistance in different subsets of Philadelphia-positive patients: by the GIMEMA Working Party on Chronic Myeloid Leukemia. Clin Cancer Res. 2006; 12(24):7374-9.
- 14. Jabbour E, Kantarjian H, Jones D, Talpaz M, Bekele N, O'Brien S, et al. Frequency and clinical significance of BCR-ABL mutations in patients with chronic myeloid leukemia treated with imatinib mesylate. Leukemia. 2006;20(10):1767-73.
- 15. Mascarenhas CC, Cunha AF, Miranda EC, Zulli R, Silveira RA, Costa FF, et al. New mutations detected by denaturing high performance liquid chromatography during screening of exon 6 bcr-abl mutations in patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. Leuk Lymphoma. 2009;50 (7):1148-54.
- 16. Müller MC, Cortes JE, Kim DW, Druker BJ, Erben P, Pasquini R, et al. Dasatinib treatment of chronic-phase chronic myeloid leukemia: analysis of responses according to preexisting BCR-ABL mutations. Blood. 2009;114(24):4944-53. Comment in: Blood. 2009; 114(24):4914-5. Comment in: Blood. 2009; 114(24):4914-5.
- 17. McFarland KL, Wetzstein GA. Chronic myeloid leukemia therapy: focus on second-generation tyrosine kinase inhibitors. Cancer Control. 2009;16(2):132-40.
- 18. Martinelli G, Iacobucci I, Soverini S, Palandri F, Castagnetti F, Rosti G, et al. Nilotinib: a novel encouraging therapeutic option for chronic myeloid leukemia patients with imatinib resistance or intolerance. Biologics. 2007;1(2):121-7.
- 19. Puttini M, Coluccia AM, Boschelli F, Cleris L, Marchesi E, Donella-Deana A, et al. In vitro and in vivo activity of SKI-606, a novel Src-Abl inhibitor, against imatinib-resistant Bcr-Abl+ neoplastic cells. Cancer Res. 2006;66(23):11314-22.
- 20. Gambacorti-Passerini C, editor. Activity and tolerance of bosutinib in patients with AP and BPCML and Ph+ ALL [Internet]. Poster presented in: ASCO Annual Meeting 2008. Alexandria: American Society of Clinical Oncology. [cited 2010 Jan 20]. Available from: http://www.asco.org/ascov2/Meetings/Abstracts?&vmview=abst_detail_view&confID=55&abstractID=35805
- 21. Melo JV, Chuah C. Novel agents in CML therapy: tyrosine kinase inhibitors and beyond. Hematology Am Soc Hematol Educ Program. 2008:427-35.
- 22. Baccarani M, Pane F, Saglio G. Monitoring treatment of chronic myeloid leukemia. Haematologica. 2008;93(2):161-9. Comment in: Haematologica. 2008;93(2):186-92.
- 23. Kantarjian H, Schiffer C, Jones D, Cortes J. Monitoring the response and course of chronic myeloid leukemia in the modern era of BCR-ABL tyrosine kinase inhibitors: practical advice on the use and interpretation of monitoring methods. Blood. 2008;111(4): 1774-80.
- 24. Sokal JE, Cox EB, Baccarani M, Tura S, Gomez GA, Robertson JE, et al. Prognostic discrimination in "good-risk" chronic granulocytic leukemia. Blood. 1984;63(4):789-99.
- 25. Baccarani M, Rosti G, Castagnetti F, Palandri F, Gugliotta G, Ferrara F, et al. SOKAL Score and response to imatinib in early chronic phase CML: the GIMEMA CML Working Party Experience on 559 patients. Haematologica [Internet]. 2009 [cited 2010 Jan 20](suppl2):255 abs. Available from: http://online.haematologica.org/EHA14/browserecord.php?-action=browse&-recid=3732
- 26. Thomas MJ, Irving JA, Lennard AL, Proctor SJ, Taylor PR; On behalf of the Northern Region Haematology Group. Validation of the Hasford score in a demographic study in chronic granulocytic leukaemia. J Clin Pathol. 2001;54(6):491-3.
- 27. Branford S, Rudzki Z, Harper A, Grigg A, Taylor K, Durrant S, et al. Imatinib produces significantly superior molecular responses compared to interferon alpha plus cytarabine in patients with newly diagnosed chronic myeloid leukemia in chronic phase. Leukemia. 2003;17(12):2401-9.
- 28. Hughes T, Deininger M, Hochhaus A, Branford S, Radich J, Kaeda J, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108(1):28-37. Comment in: Blood. 2007;109 (5): 2263; author reply 2263-4.
- 29. Baccarani M, Saglio G, Goldman J, Hochhaus A, Simonsson B, Appelbaum F, Apperley J, Cervantes F, Cortes J, Deininger M, Gratwohl A, Guilhot F, Horowitz M, Hughes T, Kantarjian H, Larson R, Niederwieser D, Silver R, Hehlmann R; European LeukemiaNet. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108(6): 1809-20.
- 30. Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, Cervantes F, Deininger M, Gratwohl A, Guilhot F, Hochhaus A, Horowitz M, Hughes T, Kantarjian H, Larson R, Radich J, Simonsson B, Silver RT, Goldman J, Hehlmann R; European LeukemiaNet. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27(35):6041-51. Comment in: J Clin Oncol. 2010;28(18):e310; author reply e311.
- 31. Quintás-Cardama A, Cortes JE, O'Brien S, Ravandi F, Borthakur G, Liu D, et al. Dasatinib early intervention after cytogenetic or hematologic resistance to imatinib in patients with chronic myeloid leukemia. Cancer. 2009;115(13):2912-21.
- 32. Quintás-Cardama A, Kantarjian H, Jones D, Shan J, Borthakur G, Thomas D, et al. Delayed achievement of cytogenetic and molecular response is associated with increased risk of progression among patients with chronic myeloid leukemia in early chronic phase receiving high-dose or standard-dose imatinib therapy. Blood. 2009;113(25):6315-21.
- 33. Iacobucci I, Rosti G, Amabile M, Poerio A, Soverini S, Cilloni D, et al. Comparison between patients with Philadelphia-positive chronic phase chronic myeloid leukemia who obtained a complete cytogenetic response within 1 year of imatinib therapy and those who achieved such a response after 12 months of treatment. J Clin Oncol. 2006;24(3):454-9.
- 34. Cortes J, Talpaz M, O'Brien S, Jones D, Luthra R, Shan J, et al. Molecular responses in patients with chronic myelogenous leukemia in chronic phase treated with imatinib mesylate. Clin Cancer Res. 2005;11(9):3425-32.
- 35. Marin D, Milojkovic D, Olavarria E, Khorashad JS, de Lavallade H, Reid AG, et al. European LeukemiaNet criteria for failure or suboptimal response reliably identify patients with CML in early chronic phase treated with imatinib whose eventual outcome is poor. Blood. 2008;112(12):4437-44.
- 36. Alvarado Y, Kantarjian H, O'Brien S, Faderl S, Borthakur G, Burger J, et al. Significance of suboptimal response to imatinib, as defined by the European LeukemiaNet, in the long-term outcome of patients with early chronic myeloid leukemia in chronic phase. Cancer. 2009;115(16):3709-18.
- 37. Kantarjian H, O'Brien S, Shan J, Huang X, Garcia-Manero G, Faderl S, et al. Cytogenetic and molecular responses and outcome in chronic myelogenous leukemia: need for new response definitions? Cancer. 2008;112(4):837-45.
- 38. Hochhaus A, O'Brien SG, Guilhot F, Druker BJ, Branford S, Foroni L, Goldman JM, Müller MC, Radich JP, Rudoltz M, Mone M, Gathmann I, Hughes TP, Larson RA; IRIS Investigators. Six-year follow-up of patients receiving imatinib for thefirst-line treatment of chronic myeloid leukemia. Leukemia. 2009;23(6):1054-61. Erratum in: Leukemia. 2010;24(5):1102.
- 39. Merx K, Müller MC, Kreil S, Lahaye T, Paschka P, Schoch C, et al. Early reduction of BCR-ABL mRNA transcript levels predicts cytogenetic response in chronic phase CML patients treated with imatinib after failure of interferon alpha. Leukemia. 2002; 16(9):1579-83.
- 40. Branford S, Rudzki Z, Parkinson I, Grigg A, Taylor K, Seymour JF, et al. Real-time quantitative PCR analysis can be used as a primary screen to identify patients with CML treated with imatinib who have BCR-ABL kinase domain mutations. Blood. 2004;104 (9):2926-32.
- 41. Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305(5682):399-401. Comment in: Science. 2004;305 (5692):319-21.
- 42. Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293 (5531):876-80. Comment on: Science. 2001;293(5538):2163.
- 43. Kantarjian HM, Larson RA, Guilhot F, O'Brien SG, Mone M, Rudoltz M, Krahnke T, Cortes J, Druker BJ; International Randomized Study of Interferon and STI571 (IRIS) Investigators. Efficacy of imatinib dose escalation in patients with chronic myeloid leukemia in chronic phase. Cancer. 2009;115(3):551-60. Erratum in: Cancer. 2010;116(15):3750. Santini, Valeria [added]
- 44. Zonder JA, Pemberton P, Brandt H, Mohamed AN, Schiffer CA. The effect of dose increase of imatinib mesylate in patients with chronic or accelerated phase chronic myelogenous leukemia with inadequate hematologic or cytogenetic response to initial treatment. Clin Cancer Res. 2003;9(6):2092-7.
- 45. Marin D, Goldman JM, Olavarria E, Apperley JF. Transient benefit only from increasing the imatinib dose in CML patients who do not achieve complete cytogenetic remissions on conventional doses. Blood. 2003;102(7):2702-3; Comment on: Blood. 2003; 101(2):473-5.
- 46. Hochhaus A, Kantarjian HM, Baccarani M, Lipton JH, Apperley JF, Druker BJ, et al. Dasatinib induces notable hematologic and cytogenetic responses in chronic-phase chronic myeloid leukemia after failure of imatinib therapy. Blood. 2007;109(6):2303-9. Erratum in: Blood. 2007;110(5):1438.
- 47. Hughes T, Saglio G, Branford S, Soverini S, Kim DW, Müller MC, et al. Impact of baseline BCR-ABL mutations on response to nilotinib in patients with chronic myeloid leukemia in chronic phase. J Clin Oncol. 2009;27(25):4204-10.
- 48. Breccia M, Alimena G. Nilotinib: a second-generation tyrosine kinase inhibitor for chronic myeloid leukemia. Leuk Res. 2010; 34(2):129-34.
- 49. Kantarjian H, Pasquini R, Hamerschlak N, Rousselot P, Holowiecki J, Jootar S, et al. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia after failure of first-line imatinib: a randomized phase 2 trial. Blood. 2007;109(12):5143-50.
- 50. Kantarjian H, Pasquini R, Lévy V, Jootar S, Holowiecki J, Hamerschlak N, et al. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia resistant to imatinib at a dose of 400 to 600 milligrams daily: two-year follow-up of a randomized phase 2 study (START-R). Cancer. 2009;115(18): 4136-47.
- 51. Jabbour E, Hochhaus A, Cortes J, La Rosée P, Kantarjian HM. Choosing the best treatment strategy for chronic myeloid leukemia patients resistant to imatinib: weighing the efficacy and safety of individual drugs with BCR-ABL mutations and patient history. Leukemia. 2010;24(1):6-12.
Correspondence:
Publication Dates
-
Publication in this collection
05 May 2011 -
Date of issue
Feb 2011
History
-
Received
27 Apr 2010 -
Accepted
24 Sept 2010