Abstract
BACKGROUND: New criteria for the diagnosis and classification of chronic graft-versus-host disease were developed in 2005 for the purpose of clinical trials with a consensus sponsored by the National Institute of Health. OBJECTIVES: The aim of this study is to present the results of a multicenter pilot study performed by the Brazil-Seattle chronic graft-versus-host disease consortium to determine the feasibility of using these criteria in five Brazilian centers. METHODS: The study was performed after translation of the consensus criteria into Portuguese and training. A total of 34 patients with National Institute of Health chronic graft-versus-host disease were enrolled in the pilot study between June 2006 and May 2009. RESULTS: Of the 34 patients, 26 (76%) met the criteria of overlap syndrome and eight (24%) the classic subcategory. The overall severity of disease was moderate in 21 (62%) and severe in 13 (38%) patients. The median time from transplant to onset of chronic graft-versus-host disease was 5.9 months (Range: 3 - 16 months); the median time for the overlap syndrome subcategory was 5.9 months (Range: 3 - 10 months) and for the classic subcategory, it was 7.3 months (Range: 3 - 16 months). At a median follow up of 16.5 months (Range: 4 - 39 months), overall survival was 75%. CONCLUSIONS: It was feasible to use the National Institute of Health consensus criteria for the diagnosis and scoring of chronic graft-versus-host disease in a Brazilian prospective multicenter study. More importantly, a collaborative hematopoietic cell transplantation network was established in Brazil offering new opportunities for future clinical trials in chronic graft-versus-host disease and in other areas of research involving hematopoietic stem cell transplantation.
Hematopoietic stem cell transplantation; Chronic graft versus host disease; NIH consensus
ORIGINAL ARTICLE
A multicenter feasibility study of chronic graft-versus-host disease according to the National Institute of Health criteria: efforts to establish a Brazil-Seattle consortium as a platform for future collaboration in clinical trials
Afonso Celso VigoritoI; Luis Fernando da Silva BouzasII; Maria Cláudia Rodrigues MoreiraII,III; Vaneuza Araújo Moreira FunkeIV; Virgílio Antonio Rensi ColturatoV; Andréia PedroV; Clarissa Vasconcellos de SouzaI; Elenaide Coutinho NunesIV; Eliana Cristina Martins MirandaI; Kátia CamachoII; Marcos Augusto MauadV; Maria Elvira Pizzigatti CorreaI; Márcia de Matos SilvaII; Mair Pedro de SousaV; Rita de Cássia Barbosa da Silva TavaresII; Stephanie Joi LeeVI; Mary Evelyn Dantas FlowersVI
IUniversidade Estadual de Campinas - UNICAMP, Campinas, SP, Brazil
IIBone Marrow Transplantation Center, Instituto Nacional de Câncer - INCA, Rio de Janeiro, RJ, Brazil
IIIUniversidade Federal do Rio de Janeiro - UFRJ, Rio de Janeiro, RJ, Brazil
IVUniversidade Federal do Paraná - UFPR, Curitiba, PR, Brazil
VHospital Amaral Carvalho - HAC, Jaú, SP, Brazil
VIDivision of Clinical Research, Fred Hutchinson Cancer Research Center and University of Washington School of Medicine, Seattle, WA, USA
Corresponding author Corresponding author: Afonso Celso Vigorito Hemocentro/Universidade Estadual de Campinas Rua Carlos Chagas, 480 - Cx.P. 6198 Cidade Universitária Zeferino Vaz 13083-878 - Campinas, SP, Brazil Phone: 55 19 3521-8740 afonso@unicamp.br
ABSTRACT
BACKGROUND: New criteria for the diagnosis and classification of chronic graft-versus-host disease were developed in 2005 for the purpose of clinical trials with a consensus sponsored by the National Institute of Health.
OBJECTIVES: The aim of this study is to present the results of a multicenter pilot study performed by the Brazil-Seattle chronic graft-versus-host disease consortium to determine the feasibility of using these criteria in five Brazilian centers.
METHODS: The study was performed after translation of the consensus criteria into Portuguese and training. A total of 34 patients with National Institute of Health chronic graft-versus-host disease were enrolled in the pilot study between June 2006 and May 2009.
RESULTS: Of the 34 patients, 26 (76%) met the criteria of overlap syndrome and eight (24%) the classic subcategory. The overall severity of disease was moderate in 21 (62%) and severe in 13 (38%) patients. The median time from transplant to onset of chronic graft-versus-host disease was 5.9 months (Range: 3 - 16 months); the median time for the overlap syndrome subcategory was 5.9 months (Range: 3 - 10 months) and for the classic subcategory, it was 7.3 months (Range: 3 - 16 months). At a median follow up of 16.5 months (Range: 4 - 39 months), overall survival was 75%.
CONCLUSIONS: It was feasible to use the National Institute of Health consensus criteria for the diagnosis and scoring of chronic graft-versus-host disease in a Brazilian prospective multicenter study. More importantly, a collaborative hematopoietic cell transplantation network was established in Brazil offering new opportunities for future clinical trials in chronic graft-versus-host disease and in other areas of research involving hematopoietic stem cell transplantation.
Keywords: Hematopoietic stem cell transplantation; Chronic graft versus host disease; NIH consensus
Introduction
Chronic graft-versus-host disease (GvHD) remains an elusive disorder to characterize and represents the major cause of morbidity and mortality affecting long-term survivors of allogeneic hematopoietic stem cell transplantation (HSCT).(1-4)
Approximately 50% of patients develop chronic GvHD. The rate varies between 6% to 80% depending on several factors including recipient and donor ages, prior acute GvHD, donor type, patient/recipient gender mismatch, stem cell source, graft manipulation, use of post-transplantation donor lymphocyte infusions (DLI) and the clinical diagnosis criteria used.(5-8) This immunologic complication after allogeneic HSCT resembles an autoimmune disease with clinical manifestations of collagen vascular diseases, such as oral ulcerations (lichen planus), keratoconjunctivitis sicca, xerostomia, polyserositis, esophagitis and stricture, vaginal ulceration and stricture, intrahepatic obstructive liver disease, obstructive pulmonary disease, scleroderma, morphea, fasciitis and myositis.(2,4,9) These manifestations are associated with debilitating consequences, such as reduced quality of life, impaired functional status, need for extended immune suppression, profound chronic immune suppression leading to recurrent or life-threatening infections and death.(1,10-17)
The historical classification of chronic GvHD proposed in 1980 included limited disease (i.e., only localized skin and/or liver involvement) and extensive disease (i.e., generalized skin or limited disease plus involvement of other organs).(9) In 2001, the classification was revised to distinguish patients who required systemic immune suppression from those who did not.(3) Although highly reproducible among transplant centers, the Seattle revised chronic GvHD criteria did not stratify patients according to outcomes.(18,19)
Unlike other innovations in HSCT, progress in chronic GvHD has been slow due to the variability of the clinical manifestations of this syndrome, the lack of consistent diagnostic laboratory findings, the limited understanding of its pathogenesis and the slow accrual of patients in clinical trials.(20-22) In the last 6 years however, the growing interest in chronic GvHD research has been spurred by the National Institute of Health (NIH) sponsored consensus project in chronic GvHD that included six working groups of multidisciplinary experts on diagnosis and staging, histopathology, biomarkers, response criteria, supportive care and design of clinical trials. Each of the six working groups presented their reports at the June 2005 NIH Consensus Meeting on critical issues for future research in chronic GvHD.(20) Recommendations for future clinical trials by each of the NIH consensus working groups have been published based on experts' opinion due to insufficient data at the time to provide evidence-based recommendations.(23-28)
In an effort to foster collaboration and expand the network for future clinical trials in chronic GvHD, a Brazil-Seattle consortium was established between HSCT investigators from the Fred Hutchinson Cancer Research Center (FHCRC) and from five Brazilian institutions. The current manuscript describes the establishment of the Seattle-Brazil consortium as a platform to conduct future collaborative studies in chronic GvHD and other HSCT areas and presents the results of the first 34 patients enrolled in a pilot study to determine the feasibility of conducting a multicenter, prospective, observational clinical trial in chronic GvHD using the NIH criteria(23) in five Brazilian institutions between June 2006 and May 2009.
Methods
The consortium
The Brazil-Seattle consortium was established in 2008 by a Brazilian-American expert in chronic GvHD and other late effects of HSCT at the FHCRC in Seattle and by investigators with common research interests from five Brazilian institutions (Universidade Estadual de Campinas, Instituto Nacional de Câncer in Rio de Janeiro, Universidade Federal do Rio de Janeiro, Universidade Federal do Paraná in Curitiba and Hospital Amaral Carvalho in Jaú). The objective of the consortium was to establish a platform to conduct future collaborative studies in chronic GvHD and other research areas in HSCT between investigators in Brazil and Seattle. The consortium consists of a multidisciplinary team of HSCT physicians, one dermatologist, dentists, psychiatrists, psychologists, physiotherapists and two data managers. Medical training was conducted at the FHCRC for most of the physicians and one dentist, and was extended to others members of the teams in Brazil.
The consortium held monthly teleconferences with investigators from Brazil and Seattle and met at least twice yearly during annual meetings of the American and the Brazilian Societies of HSCT and Hematology. After completion of the chronic GvHD training and translation to Portuguese of the NIH consensus criteria, a pilot study to determine the feasibility of conducting a multicenter, prospective, observational clinical trial in chronic GvHD was initiated by the consortium upon approval of the institutional review boards of each of the five participating centers.
Inclusion criteria and definitions
The inclusion criteria were over 2-year-old patients with a diagnosis of chronic GvHD by NIH criteria(23) that required systemic immunosuppression treatment and who were within the first 3 years of their allogeneic HSCT. Only patients with no evidence of primary disease relapse/progression after transplantation were included, although the presence of minimal residual disease by molecular testing was allowed. Patients with an inability to comply with study procedures or anticipated survival of less than 6 months due to co-morbid disease were excluded. The criteria for diagnosis and staging of chronic GvHD were according to the 2005 NIH consensus recommendations for clinical trials.(23)
Patients enrolled in the study within 3 months from the initial diagnosis of chronic GvHD were categorized as 'incidence cases' and those enrolled after 3 months from the initial diagnosis of chronic GvHD were classified as 'prevalent cases'. When patients received only topical therapy at the initial diagnosis of chronic GvHD, but subsequently required systemic treatment and then enrolled in the study, they were classified as incident or prevalent cases according to the date of the initial diagnosis of chronic GvHD and not the date when treatment with systemic immunosuppression was started.
Study procedure
No therapeutic intervention was indicated by this study. Appropriate patients were identified by individual physicians at each center at the time of clinical visits. Potentially eligible patients were approached and the study procedures outlined. Signed informed consent was required for study enrollment. The patients were registered at the local institution. They were assigned a unique sequential study identification number after verification of eligibility and receipt of informed consent. Each institution retained a file of the patients enrolled in the study.
Patients were evaluated at the time of study enrollment and at 6-month intervals. Newly diagnosed patients (incident cases) had an additional assessment 3 months after enrollment. Physicians provided standardized information about organ involvement and symptoms at each evaluation. Only enrollment data were analyzed in the pilot study.
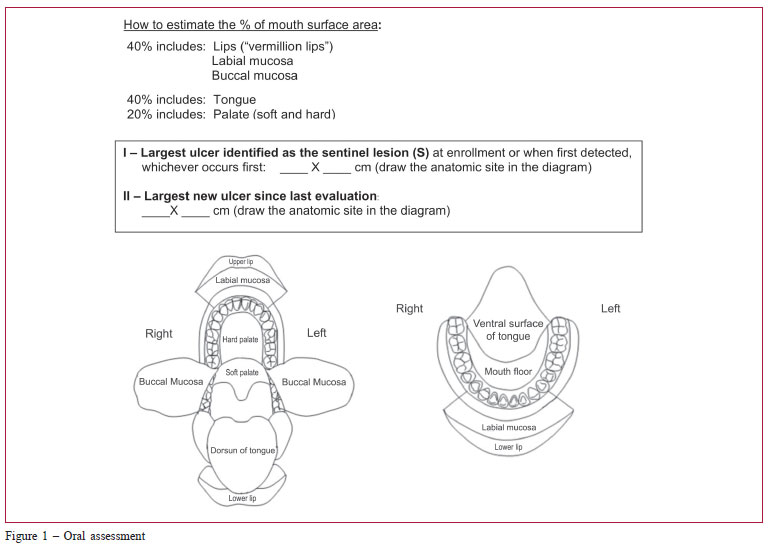
The medical assessment included the NIH chronic GvHD diagnosis and staging consensus,(20,23) as well the Hopkins and Vienna(29) scoring systems. Data were collected on skin, mouth, eye, gastrointestinal, liver and lung involvement, overall severity ratings (mild, moderate and severe), and perceptions of changes by physicians. As the mouth is the most common site of NIH chronic GvHD symptoms,(21) an oral cavity diagram was included in our study (Figure 1) to help to estimate the percentage of oral cavity surface involvement when grading GvHD. The length and width of the largest oral ulcer was also added to monitor response.
Statistical analysis
The objective of this multicenter, prospective, observational pilot study was to determine its feasibility of using the diagnosis and staging of chronic GvHD according to the NIH criteria at five institutions in Brazil. Each participating institution collected data prospectively.
Demographic and transplant related information were calculated as proportions. The comparison between groups was performed using chi-squared tests. Overall survival after onset of chronic GvHD was estimated by the Kaplan-Meier method. Analysis was carried out as of December 2009.
Results
The Brazil-Seattle chronic GvHD multidisciplinary study group (GeDECH-Brasil/Seattle) was conceived by several Brazilian investigators who had been post-doctor fellows or visiting physicians at the FHCRC and by Mary Flowers, a collaborator and mentor in Seattle. The first initiative of the GeDECH-Brasil-Seattle consortium was to conduct a feasibility, multicenter, prospective, observational pilot study in a chronic GvHD Brazilian population applying NIH criteria similar to those being tested in a larger multicenter observational chronic GvHD study in the United States by Stephanie Lee et al. since 2007.
A total of 34 patients were enrolled in this pilot study. At least 5 patients were enrolled from each of the five participating institutions with the first patient enrolled in March 2008 and the last one in September 2009.
Patient and transplant characteristics are shown in Table 1. The median patient age was 36 years old (range: 15 - 65 years) and 67% (n = 23) were male. The majority of patients (94%) had HLA matched related donors (n=32) and most (91%) received a high-dose pre-transplant conditioning regimen with chemotherapy but without total body irradiation (n = 31). The source of stem cells for the HSCT was peripheral blood in 21 patients (64%). Twenty patients (59%) received their first allogeneic HSCT between 2008 and 2009 and 14 patients (41%) between 2006 and 2007.
Chronic GvHD characteristics are shown in Table 2. The median time from HSCT to diagnosis of chronic GvHD was 5.9 months (range: 3 - 16 months) and from initial diagnosis of chronic GvHD to enrollment was 2.2 months (range: 0.4 - 15 months).
Of the 34 patients, 24 (71%) were incident cases and 10 (29%) prevalent cases. Twenty-six (76%) patients met the NIH chronic GvHD subcategory of overlap syndrome and 8 (24%) the classic subcategory. The median time from transplant to chronic GvHD was shorter for overlap syndrome at 5.9 months (range: 3 - 10 months) compared to 7.3 months (range: 3 - 16 months) for the classic subcategory. Among the 34 patients, 4 (12%) had one organ/site involvement, 11 (32%) had two organ/site involvement, 11 (32%) had three organ/site involvement and 8 (24%) had four or more organ/ site involvement at the time of study enrollment. The organs/sites involved by chronic GvHD at initial diagnosis are shown in Figure 2.
Overall, 21/34 (62%) patients had no prior history of grade 2-4 acute GvHD with 5 of 8 (62%) in the classic and 16 of 26 (47%) in overlap chronic GvHD subcategories. Of the 13/34 (38%) patients with prior grade 2-4 acute GvHD, 10/26 (38%) belonged to the overlap chronic GvHD subcategory and 3/8 (38%) to the classic subtype. The distribution of patients with and without prior acute GvHD was not statistically significant between the chronic GvHD subcategories.
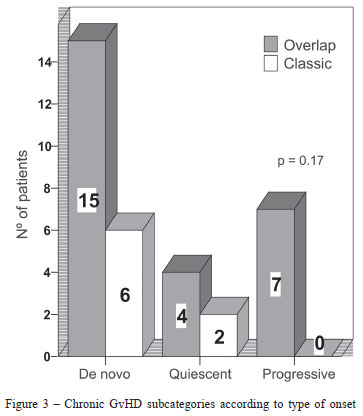
Most patients (21/34; 62%) had de novo onset of chronic GvHD, 15 in the overlap and 6 in the classic subcategories. No statistically significant differences between type of onset and NIH chronic GvHD subcategories were found (p-value = 0.17) (Figure 3).
The frequency of selected high risk features at the time of initial diagnosis of chronic GvHD associated with poor survival or prolonged immunosuppression(1,5,17,28) are shown in Table 2. At the time of initial diagnosis, 7 patients (20%) had progressive onset of chronic GvHD, 5 patients (15%) had platelet counts below 100 x 109/L, 10 patients (29%) had skin involvement of more than 50% of the total body surface area and 17 patients (50%) were receiving corticosteroids at a dose equal to or greater than 0.5 mg/kg/day.
The severity of chronic GvHD in the cohort studied is shown in Figure 4. Overall, the severity of chronic GvHD was graded as moderate in 21/34 (62%) and severe in 13/34 (38%) patients. Among the 21 patients with moderate disease, 16 were in the overlap and 5 in the classic chronic GvHD subcategories. Of the 13 patients with severe disease, 10 were in the overlap and 3 in the classic chronic GvHD groups. There was no significant statistical difference between moderate and severe disease between the subcategories (p-value = 0.64).
After a median follow-up of 16.5 months, the overall survival from the initial chronic GvHD diagnosis in our patient population was 75% (Figure 5).
Discussion
Two points should be highlighted in this paper. The first relates to describing the establishment of a consortium between investigators at the FHCRC and at Brazilian institutions to promote collaboration in research areas of common interest in HSCT, such as the Brazil-Seattle chronic GvHD multidisciplinary study group. The second relates to reporting the feasibility of conducting a multicenter pilot observational study in chronic GvHD applying the diagnostic, staging and response criteria as recommended by the 2005 NIH consensus conference for clinical trials.
In this pilot study we describe the characteristics of patients with NIH defined chronic GvHD who met the overlap syndrome and classic subcategories. The most prevalent subcategory of chronic GvHD observed was the overlap syndrome (76%), a result similar to that reported in a large North American retrospective study(30) but different to others reports.(31-33) Most patients in our study had de novo onset of chronic GvHD (i.e., no prior acute GvHD) equally distributed among patients in the overlap and classic chronic GvHD subcategories. The majority of patients had 2 or 3 organs/sites involved with the mouth being the most prevalent site (91%), thus corroborating with previous findings.(21) Similar to other studies,(31,32) the overall severity of chronic GvHD of most patients in our study cohort was moderate in both overlap syndrome and classic chronic GvHD subcategories.
Presence of high risks features associated with poor survival at the initial diagnosis of chronic GvHD (i.e., thrombocytopenia, progressive onset of chronic GvHD and skin involvement of more than 50% of the body surface area) was observed in approximately one third of patients in our study. While the follow-up is short, the 75% overall survival is an encouraging observation.
Several retrospective studies on evaluating the NIH criteria by assessing outcomes among patients with historically defined chronic GvHD reported conflicting results.(30-35) In a large retrospective study evaluating the NIH consensus criteria in the classification of late acute and chronic GvHD, Vigorito et al.(30) reported that antecedent late acute GvHD was associated with an increased risk of nonrelapse mortality and prolonged treatment in patients with NIH chronic GvHD. In that study, however, there was no statistically significant association between the presence or absence of NIH chronic GvHD criteria with survival, risk factors or non-relapse mortality, recurrent malignancy, or duration of systemic treatment.(30) In two smaller studies, however, Jagasia et al.(31) and Arora et al.(32) reported lower survival among patients with NIH GvHD defined as late, persistent, recurrent and acute compared to patients in overlap syndrome or classic chronic GvHD subcategories. In another study evaluating the NIH GvHD criteria, Cho et al.(33) reported no significant differences in GvHD-specific survival according to the NIH GvHD diagnostic criteria. Pérez-Simón et al.,(34) Kim et al.(35) and other investigators(32,33) evaluated the overall severity of chronic GvHD according to NIH criteria as a risk factor for survival and non-relapsed mortality and reported a lower survival for patients graded as severe.
The discrepant results of the above studies may be explained by differences in the historical criteria used to make the diagnosis of chronic GvHD and by inadequacies in documenting the presence or absence of the diagnostic and distinctive manifestations of chronic GvHD necessary to categorize patients by the NIH consensus criteria. Considering the previously inconsistent results(30-35) and that late acute GvHD by NIH criteria represents a subcategory of the historical chronic GvHD diagnosis,(3,23) with proper stratification, patients with late acute GvHD should be included in clinical trials for chronic GvHD. When analyzing chronic GvHD as an outcome of HSCT, the capture of both late acute and chronic GvHD by the 2005 NIH criteria is important, since failing to do so will likely underestimate the incidence of chronic GvHD and lead to misinterpretation of results when this outcome is compared with prior studies using the historical chronic GvHD criteria.(3,23)
In conclusion, this pilot study demonstrates the feasibility of applying the NIH consensus criteria for the diagnosis and scoring of chronic GvHD in a multicenter prospective study at five Brazilian institutions and, more importantly, in establishing a consortium of investigators interested in new opportunities to collaborate in future intervention, validation, and biomarker clinical trials in chronic GvHD and other areas of common research interest in hematopoietic stem cell transplantation.
Acknowledgment
The authors thank Kate Chilson for her assistance in the study forms and particularly to all patients who participated in the study.
Grant support
M.E.D.F. and S.J.L. were supported, in part, by the National Institutes of Health, Department of Health and Human Services (grants CA 118953).
Submitted: 3/25/2011
Accepted: 6/1/2011
Conflict-of-interest disclosure: The authors declare no competing financial interest
- 1. Wingard JR, Piantadosi S, Vogelsang GB,Farmer ER, Jabs DA, Levin LS, et al. Predictors of death from chronic graft-versushost disease after bone marrow transplantation. Blood. 1989;74:1428-35.
- 2. Ratanatharathorn V, Ayash L, Lazarus HM, Fu J, Uberti JP. Chronic graft-versus-host disease: clinical manifestation and therapy. Bone Marrow Transplant. 2001;28(2):121-9.
- 3. Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9:215-33.
- 4. Higman MA, Vogelsang GB. Chronic graft versus host disease. Br J Haematol. 2004;125:435-54.
- 5. Sullivan KM, Agura E, Anasetti C, Appelbaum F, Badger C, Bearman S, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991;28(3):250-9.
- 6. Rocha V, Wagner JE Jr, Sobocinski KA, Klein JP, Zhang MJ, Horowitz MM, et al. Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an HLA-identical sibling. Eurocord and International Bone Marrow Transplant Registry Working Committee on Alternative Donor and Stem Cell Sources. N Engl J Med. 2000;342:1846-54.
- 7. Remberger M, Aschan J, Lonnqvist B, Carlens S, Gustafsson B, Hentschke P, et al. An ethnic role for chronic, but not acute, graft-versus-host disease after HLA-identical sibling stem cell transplantation. Transplant Proc. 2001;33:1769-70.
- 8. Flowers ME, Inamoto Y, Carpenter PA, Lee SJ, Kiem HP, Petersdorf EW, et al. Comparative analysis of risk factors for acute graftversus- host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood. 2011;117:3214-9.
- 9. Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204-17.
- 10. Sutherland HJ, Fyles GM, Adams G, Hao Y, Lipton JH, Minden MD, et al. Quality of life following bone marrow transplantation: a comparison of patient reports with population norms. Bone Marrow Transplant. 1997;19:1129-36.
- 11. De Souza CA, Duraes MI, Vigorito AC, Penteado Aranha FJ, Oliveira GB, De Brito Eid KA, et al. Quality of life in patients randomized to receive a bone marrow or a peripheral blood allograft. Haematologica. 2002;87(12):1281-85.
- 12. Syrjala KL, Chapko MK, Vitaliano PP, Cummings C, Sullivan KM. Recovery after allogeneic marrow transplantation: prospective study of predictors of long-term physical and psychosocial functioning. Bone Marrow Transplant. 1993;11(4):319-27.
- 13. Duell T, van Lint MT, Ljungman P, Tichelli A, Socié G, Apperley JF, et al. Health and functional status of long-term survivors of bone marrow transplantation. EBMT Working Party on Late Effects and EULEP Study Group on Late Effects. European Group for Blood and Marrow Transplantation. Ann Intern Med. 1997;126:184-92.
- 14. Sullivan KM, Witherspoon RP, Storb R, Deeg HJ, Dahlberg S, Sanders JE, et al. Prednisone and azathioprine compared with prednisone and placebo for treatment of chronic graft-v-host disease: prognostic influence of prolonged thrombocytopenia after allogeneic marrow transplantation. Blood. 1988;72(2):546-54.
- 15. Sullivan KM, Witherspoon RP, Storb R, Deeg HJ, Dahlberg S, Sanders JE, et al. Alternating-day cyclosporine and prednisone for treatment of high-risk chronic graft-v-host disease. Blood. 1988; 72(2):555-61.
- 16. Loughran TP Jr, Sullivan K, Morton T, Beckham C, Schubert M, Witherspoon R, et al. Value of day 100 screening studies for predicting the development of chronic graft-versus-host disease after allogeneic bone marrow transplantation. Blood. 1990;76(1):228-34.
- 17. Socie G, Stone JV, Wingard JR, Weisdorf D, Henslee-Downey PJ, Bredeson C, et al. Long-term survival and late deaths after allogeneic bone marrow transplantation. Late Effects Working Committee of the International Bone Marrow Transplant Registry. N Engl J Med. 1999;341(1):14-21.
- 18. Akpek G, Zahurak ML, Piantadosi S, Margolis J, Doherty J, Davidson R, et al. Development of a prognostic model for grading chronic graft-versus-host disease. Blood. 2001;97(5):1219-26.
- 19. Lee SJ, Klein JP, Barrett AJ, Ringden O, Antin JH, Cahn JY, et al. Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood. 2002;100(2):406-14.
- 20. Pavletic SZ, Lee SJ, Socie G, Vogelsang G. Chronic graft-versushost disease: implications of the National Institutes of Health consensus development project on criteria for clinical trials. 2006;38(10):645-51. Epub 2006 Sep 18
- 21. Lee SJ, Flowers ME. Recognizing and managing chronic graftversus- host disease. Hematology Am Soc Hematol Educ Program. 2008:134-141.
- 22. Farag SS. Chronic graft-versus-host disease: where do we go from here? Bone Marrow Transplant. 2004;33(6):569-77.
- 23. Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945-56.
- 24. Shulman HM, Kleiner D, Lee SJ, Morton T, Pavletic SZ, Farmer E, et al. Histopathologic diagnosis of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: II. Pathology Working Group Report. Biol Blood Marrow Transplant. 2006;12(1):31-47.
- 25. Schultz KR, Miklos DB, Fowler D, Cooke K, Shizuru J, Zorn E, Holler E, Ferrara J, Shulman H, Lee SJ, Martin P, Filipovich AH, Flowers ME, Weisdorf D, Couriel D, Lachenbruch PA, Mittleman B, Vogelsang GB, Pavletic SZ. Toward biomarkers for chronic graft-versus-host disease: National Institutes of Health consensus development project on criteria for clinical trials in chronic graftversus- host disease: III. Biomarker Working Group Report. Biol Blood Marrow Transplant. 2006;12(2):126-37.
- 26. Pavletic SZ, Martin P, Lee SJ, Mitchell S, Jacobsohn D, Cowen EW, Turner ML, Akpek G, Gilman A, McDonald G, Schubert M, Berger A, Bross P, Chien JW, Couriel D, Dunn JP, Fall-Dickson J, Farrell A, Flowers ME, Greinix H, Hirschfeld S, Gerber L, Kim S, Knobler R, Lachenbruch PA, Miller FW, Mittleman B, Papadopoulos E, Parsons SK, Przepiorka D, Robinson M, Ward M, Reeve B, Rider LG, Shulman H, Schultz KR, Weisdorf D, Vogelsang GB. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus- Host Disease: IV. Response Criteria Working Group report. Biol Blood Marrow Transplant. 2006;12(3):252-66.
- 27. Couriel D, Carpenter PA, Cutler C, Bolaños-Meade J, Treister NS, Gea-Banacloche J, Shaughnessy P, Hymes S, Kim S, Wayne AS, Chien JW, Neumann J, Mitchell S, Syrjala K, Moravec CK, Abramovitz L, Liebermann J, Berger A, Gerber L, Schubert M, Filipovich AH, Weisdorf D, Schubert MM, Shulman H, Schultz K, Mittelman B, Pavletic S, Vogelsang GB, Martin PJ, Lee SJ, Flowers ME.. Ancillary therapy and supportive care of chronic graft-versushost disease: national institutes of health consensus development project on criteria for clinical trials in chronic Graft-versus-host disease: V. Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant. 2006;12(4):375-96.
- 28. Martin PJ, Weisdorf D, Przepiorka D, Hirschfeld S, Farrell A, Rizzo JD, Foley R, Socie G, Carter S, Couriel D, Schultz KR, Flowers ME, Filipovich AH, Saliba R, Vogelsang GB, Pavletic SZ, Lee SJ; National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus- Host Disease: VI. Design of Clinical Trials Working Group report. Biol Blood Marrow Transplant. 2006;12(5):491-505.
- 29. Greinix HT, Pohlreich D, Maalouf J, Soukup P, Supper V, Kalhs P, et al. A single-center pilot validation study of a new chronic GVHD skin scoring system. Biol Blood Marrow Transplant. 2007;13(6):715-23. Epub 2007 Apr 23.
- 30. Vigorito AC, Campregher PV, Storer BE, Carpenter PA, Moravec CK, Kiem HP, et al. Evaluation of NIH consensus criteria for classification of late acute and chronic GVHD. Blood. 2009;114(3):702-8.
- 31. Jagasia M, Giglia J, Chinratanalab W, Dixon S, Chen H, Frangoul H, et al. Incidence and outcome of chronic graft-versus-host disease using National Institutes of Health consensus criteria. Biol Blood Marrow Transplant. 2007;13(10):1207-15.
- 32. Arora M, Nagaraj S, Witte J, DeFor TE, MacMillan M, Burns LJ, et al. New classification of chronic GVHD: added clarity from the consensus diagnoses. Bone Marrow Transplant. 2009;43(2):149-53.
- 33. Cho BS, Min CK, Eom KS, Kim YJ, Kim HJ, Lee S, et al. Feasibility of NIH consensus criteria for chronic graft-versus-host disease. Leukemia. 2009;23(1):78-84. Epub 2008 Oct 2.
- 34. Perez-Simon JA, Encinas C, Silva F, Arcos MJ, Díez-Campelo M, Sánchez-Guijo FM, et al. Prognostic factors of chronic graftversus- host disease following allogeneic peripheral blood stem cell transplantation: the national institutes health scale plus the type of onset can predict survival rates and the duration of immunosuppressive therapy. Biol Blood Marrow Transplant. 2008;14(10):1163-71.
- 35. Kim DY, Lee JH, Lee JH, Kim SH, Lim SN, Kim SD, et al. Reevaluation of the National Institutes of Health criteria for classification and scoring of chronic GVHD. Bone Marrow Transplant. 2010;45(7):1174-80.
Corresponding author:
Publication Dates
-
Publication in this collection
20 Sept 2011 -
Date of issue
2011
History
-
Received
25 Mar 2011 -
Accepted
01 June 2011