Abstract
OBJECTIVE: The aim of this study was to evaluate the protective effects of quercetin, rutin, hesperidin and myricetin against reactive oxygen species production with the oxidizing action of tert-butylhydroperoxide in erythrocytes from normal subjects and sickle cell anemia carriers treated with hydroxyurea. METHODS: Detection of intracellular reactive oxygen species was carried out using a liposoluble probe, 2',7'-dichlorfluorescein-diacetate (DCFH-DA). A 10% erythrocyte suspension was incubated with flavonoids (quercetin, rutin, hesperidin or myricetin; 30, 50, and 100 µmol/L), and then incubated withtert-butylhydroperoxide (75 µmol/L). Untreated samples were used as controls. RESULTS: Red blood cell exposure to tert-butylhydroperoxide resulted in significant increases in the generation of intracellular reactive oxygen species compared to basal levels. Reactive oxygen species production was significantly inhibited when red blood cells were pre-incubated with flavonoids, both in normal individuals and in patients with sickle cell anemia. Quercetin and rutin had the highest antioxidant activity, followed by myricetin and hesperidin. CONCLUSION: Flavonoids, in particular quercetin and rutin, showed better antioxidant effects against damage caused by excess reactive oxygen species characteristic of sickle cell anemia. Results obtained with patients under treatment with hydroxyurea suggest an additional protective effect when associated with the use of flavonoids.
Flavonoids; Quercetin; Rutin; Hydroxyurea; Anemia, sickle cell; Reactive oxygen species
ORIGINAL ARTICLE
Protective effect of flavonoids against reactive oxygen species production in sickle cell anemia patients treated with hydroxyurea
Railson HennebergI; Michel Fleith OtukiII; Aline Emmer Ferreira FurmanI; Priscila HermannI; Aguinaldo José do NascimentoI Maria Suely Soares LeonartI
IUniversidade Federal do Paraná - UFPR, Curitiba, PR, Brazil
IIUniversidade Estadual de Ponta Grossa - UEPG, Ponta Grossa, PR, Brazil
Corresponding author Corresponding author: Aguinaldo José do Nascimento Post-graduate Program in Pharmaceutical Science, Universidade Federal do Paraná - UFPR Rua Prefeito Lothário Meissner 632, Jardim Botânico 80210-170, Curitiba, PR, Brazil ajnasc@ufpr.br
ABSTRACT
OBJECTIVE: The aim of this study was to evaluate the protective effects of quercetin, rutin, hesperidin and myricetin against reactive oxygen species production with the oxidizing action of tert-butylhydroperoxide in erythrocytes from normal subjects and sickle cell anemia carriers treated with hydroxyurea.
METHODS: Detection of intracellular reactive oxygen species was carried out using a liposoluble probe, 2',7'-dichlorfluorescein-diacetate (DCFH-DA). A 10% erythrocyte suspension was incubated with flavonoids (quercetin, rutin, hesperidin or myricetin; 30, 50, and 100 µmol/L), and then incubated withtert-butylhydroperoxide (75 µmol/L). Untreated samples were used as controls.
RESULTS: Red blood cell exposure to tert-butylhydroperoxide resulted in significant increases in the generation of intracellular reactive oxygen species compared to basal levels. Reactive oxygen species production was significantly inhibited when red blood cells were pre-incubated with flavonoids, both in normal individuals and in patients with sickle cell anemia. Quercetin and rutin had the highest antioxidant activity, followed by myricetin and hesperidin.
CONCLUSION: Flavonoids, in particular quercetin and rutin, showed better antioxidant effects against damage caused by excess reactive oxygen species characteristic of sickle cell anemia. Results obtained with patients under treatment with hydroxyurea suggest an additional protective effect when associated with the use of flavonoids.
Keywords: Flavonoids; Quercetin; Rutin; Hydroxyurea; Anemia, sickle cell; Reactive oxygen species
Introduction
Although the pathophysiology of the disease is related to hemoglobin S polymerization of sickled red blood cells under deoxygenating conditions, increasing evidence shows that sickle cell anemia is an inflammatory condition with increased endothelial activation(1). It is known that chronic oxidative stress, caused by an imbalance between the production of reactive oxygen species (ROS) and antioxidant enzyme activity, occurs in sickle cell disease(2). Endothelial dysfunction has been linked to an inflammatory process, the high production of reactive species of nitrogen (REN) and ROS and increased cell adhesion to tissues(3).
In the body, ROS and REN engage in energy production, phagocytosis, cell growthregulation, intercellular signaling and synthesis of important biological substances. However, their excess gives rise to injurious effects, such as the peroxidation of membrane lipids, aggression to the proteins of tissues and membranes as well as to enzymes, carbohydrates and DNA(4).
The mechanism of the formation of O2- via hemoglobin deoxygenation(5), is approximately 1.7 times higher in sickled erythrocytes, which could explain the susceptibility of these cells to oxidative stress(6). Despite sickle cell anemia having been extensively studied, there is no consensus on the treatment of the disease and, with the clear evidence of the importance of oxidative stress in its pathophysiology, studies on the therapeutic effects of anti-oxidants and anti-inflammatory drugs in the treatment of the disease should be carefully explored(5). A better understanding of the process would allow new discoveries on the mechanisms of action of some drugs already used, and would open room for new therapies.
Hydroxyurea, for example, has a beneficial effect on vascular function, unknown at the onset of drug use; it is considered by many physicians as the treatment of choice for severe sickle cell anemia. It seems that hydroxyurea exerts its activity through several mechanisms such as promoting increases in the fetal hemoglobin levels, alterations in erythrocytes, myelosuppression, and others(6-7).
In addition, the use of compounds able to sequester free radicals seems to be beneficial for sickle cell anemia patients(8).
Several studies have described the antioxidant activity of flavonoids as hydrogen donors and abductors of radicals with their use preventing oxidative changes(9).
The aims of this study were to evaluate i) the production of ROS in erythrocytes of normalindividuals and of sickle cell anemia patients under treatment with hydroxyurea; ii) the cellular response to oxidative stress caused by tert-butylhydroperoxide (t-BHP); and iii) the protective effect against oxidative stress of quercetin, rutin, hesperidin, and myricetin, flavonoids common in fruits and vegetables.
Methods
Dimethyl sulfoxide (DMSO), quercetin, rutin, myricetin, hesperidin, t-TBH, and dichlorodihydrofluorescein-diacetate (DCF-DA) were obtained from Sigma Chemical Co. (St. Louis, MO, USA).
Patients with sickle-cell disease, attended at the Centro de Hematologia e Hemoterapia de Paraná (HEMEPAR), were selected for this study. The 16 male and 12 female participants, aged between 11 and 49 years, had been under treatment with hydroxyurea (0.5 to 1 g/day) as the primary therapeutic agent for at least 2 years. The control group consisted of five male and ten female apparently healthy individuals with ages ranging from 23 to 60 years old. Venous blood samples were collected using K3EDTA Vacutainers. All individuals read and signed an informed consent form in accordance with the approval of the Ethics Committee of the Universidade Federal do Paraná (UFPR).
Blood samples were centrifuged at 1000 xg at 4ºC for 10 minutes. The remaining erythrocytes were washed three times with 154 µmol/L NaCl solution and then resuspended in 10 µmol/L saline phosphate buffer solution (PBS) at pH 7.4 to a 10% globularvolume. The globular volume and hemoglobin concentration were determined using a Coulter T-890 hematology analyzer.
Polyphenols were dissolved in 10 µmol/L phosphate buffer solution (PBS) at pH 7.4 and then diluted 1:2 in DMSO as described by Cesquini et al.(9). Polyphenol solutions were prepared immediately before use.
Detection of intracellular ROS was accomplished by the use of a DCFH-DA liposoluble probe. This probe is hydrolyzed to 2′,7′-dichlorodihydrofluorescein (DCFH2), which is available for oxidation by ROS to produce fluorescent 2′,7′-dichlorofluorescein (DCF) as described by López-Revuelta et al.(10). A 10% erythrocyte suspension (0.95 mL) was incubated with 5 µL of 10 µmol/LDCFH-DA probe for 30 minutes in dark conditions at 37ºC, diluted 1:10 in 10 µmol/L PBS at pH 7.4 and then incubated with quercetin, rutin, hesperidin or myricetin (30, 50 and 100 µmol/L) in 96-well plates for 30 minutes at 37ºC. Untreated samples were used as control. t-BHP (75 µmol/L) was added and, after 15 minutes, fluorescence was measured at 488 nm (excitation) and 528 nm (emission) in a Synergy HT fluorometer (Biotek ®). ROS was expressed in UF/g/dL of hemoglobin.
Statistical significance of the experimental data was analyzed with the non-parametric Mann-Whitney U and Kruskal-WallisANOVA tests using the Statistica statistics package (StatSoft) version 10.0. A p-value < 0.05 was considered to be statistically significant.
Results
Figure 1 shows that, while the action of t-BHP causes a significant increase in the formation of ROS, no significant difference between the two groups, even in the presence of the oxidant, was observed.
This study demonstrated the protective activities of quercetin,rutin, hesperidin and myricetin against the oxidizing action of t-BHP (75 µmol/L) by inhibiting ROS production. Quercetin andrutin gave the best results and a similar behavior, i.e. at 50 µmol/L,and no significant differences occurred when compared to the basal state in normal and hemoglobin SS erythrocytes (Figures 2 & 3).
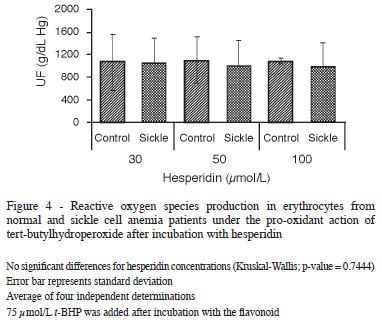
Of the flavonoids tested, hesperidin had the least effect. The production of ROS was statistically higher when compared to the other polyphenols tested (Figure 4). Myricetin, on the other hand, only demonstrated antioxidant activity similar to that of quercetin and rutin at a concentration of 100 µmol/L (Figure 5).
Discussion
Sickle cell anemia is an important and emerging model study of oxidative stress. Oxygen transport to tissues makes erythrocytes highly susceptible to oxidative damage, a predisposition that increases when there are structural abnormalities in hemoglobin molecules(11). Studies have shown that erythrocytes of patients with sickle cell anemia produce O2-, H2O2 and OH-in greater quantities than normal erythrocytes(12,13).
Although cells have mechanisms to protect themselves against toxic agents; some of these systems suffer a decline in their activities in some physiological and environmental conditions that lead to an increased production of ROS. Thus, dietary supplementation with nutrients that contain antioxidants may be important for additional protection against oxidative stress and prevention of diseases such as atherosclerosis, cancer, ischemia, inflammation, and cardiovascular and neurological diseases(10).
In this study, t-BHP was used as the oxidizing agent. The process induced in erythrocytes is rapid oxidation of glutathione(GSH) by glutathione peroxidase (GSH-Px) and by the reaction between t-BHP and hemoglobin with the formation of free radicals and methemoglobin (metHb)(14). Chaves et al.(15) demonstrated increased susceptibility to oxidation of erythrocytes in individuals carrying hemoglobin S (AS and SS), by a significant increase in the formation of Heinz bodies, metHb, and hemolysis, in addition to depletion of GSH when compared to healthy individuals.
The antioxidant action of flavonoids is due to the combination of its chelating activity, via ortho-dihydroxy structures, and its ability to sequester free radicals(16). This occurs in three stages: formation of the superoxide ion and hydroxy radicals by Fenton's reaction, and formation of lipid radicals and mechanisms that decrease lipid peroxidation(17).
Several studies have demonstrated the protective effect of quercetin against oxidative damage in vitro with preservation of the integrity of the erythrocyte membrane being singled out as the main effect, mainly due to the easy penetration into cells(18). The presence of catechol in quercetin and the rutin B-ring are essential to sequester free radicals and for antioxidant activity. However, the OH at position 3 in rutin is blocked with a rutinoside group thus, impairing the chelating action of rutin compared to quercetin(9). Results of this work did not show significant differences between both. Barreca et al.(19) showed that quercetinand rutin have a greater ability to sequester free radicals compared to other flavonoids, such as hesperidin and neohesperidin.
Interest in the use of antioxidants in the treatment of sickle cell anemia is due to the fact that most antioxidants are easily found at a low cost, which is important as often the incidence of sickle cell anemia is high in countries with limited resources(5) and in particular in black individuals(20).
It is important to note that all sickle cell anemia patients who participated in our study were taking hydroxyurea as the primary therapeutic agent. Therefore, we cannot dismiss the effect of this drug in our results. Hydroxyurea administration in patients with sickle cell anemia crises reduces the number of vaso-occlusive events and appears to extend their life-expectancy, which is attributed mainly to its ability to promote the synthesis of fetal hemoglobin, which seems to inhibit hemoglobin S polymerization. Hydroxyurea is oxidized by heme groups to produce nitric oxide, which increases the production of cyclicguanosine monophosphate (cGMP), with consequent gene l transcription(2). Fetal hemoglobin induction by hydroxyurea results in a reduction of hemolysis, limiting ROS production and the sequestration of nitric oxide. Hydroxyurea may also increase antioxidant capacity by increasing the expression of GSH-Px 1(5).
The results of our work may contribute to a better understanding of the mechanisms involved in sickle cell anemiatherapy, suggesting that the use of hydroxyurea together with continuous flavonoid intake may provide an additional beneficial effect on the clinical course of the disease. More studies on the pharmacokinetics of polyphenols are needed to define their action in vivo and the dose required for therapeutic effect.
Conclusion
Thus, studies on these compounds and their introduction in conventional therapies is a promising field however their use depends on a thorough knowledge, mainly of the physiochemical properties of flavonoids, which may help their inclusion in therapeutic regimens.
References
1. Kaul DK, Liu XD, Zhang X, Ma L, Hsia CJ, Nagel RL. Inhibition of sickle red cell adhesion and vasooclusion in the microcirculation byantioxidants. Am J Physiol Heart Circ Physiol. 2006;291(1):H167-75.
2. Cho CS, Kato GJ, Yang SH, Bae SW, Lee JS, Gladwin MT, et al. Hydroxyurea-induced expression of glutathione peroxidase 1 in red blood cells of individuals with sickle cell anemia. Antioxid Redox Signal. 2010;13(1):1-11.
3. Rusanova I, Escames G, Cossio G, de Borace RG, Moreno B, Chahboune M, et al. Oxidative stress status, clinical outcome, and β-globin gene cluster haplotypes in pediatric patients with sickle cell disease. Eur J Haematol. 2010;85(6):529-37.
4. Barreiros AL, David JM, David JP. Oxidative stress: relations between the formation of reactive species and the organism's defense. Química Nova. 2006;29(1):113-23.
5. Nur E, Biemond BJ, Otten HM, Brandjes DP, Schnog JJ; CURAMA Study Group. Oxidative stress in sickle cell disease; pathophysiology and potential implications for management. Am J Hematol. 2011;86(6):484-9.
6. Končić MZ, Barbarić M, Perković I, Zorc B. Antiradical, chelating and antioxidant activities of hydroxamic acids and hydroxyureas. Molecules. 2011;16(8):6232-42.
7. Davies SC, Gilmore A. The role of hydroxyurea in the management of sickle cell disease. Blood Rev. 2003;17(2):99-109.
8. Aslan M, Thornley-Brown D, Freeman BA. Reactive species in sickle cell disease. Ann N Y Acad Sci. 2000;899:375-91.
9. Cesquini M, Torsoni MA, Stoppa GR, Ogo SH. t-BOOH-induced oxidative damage in sickle red blood cells and the role of flavonoids. Biomed Pharmacother. 2003;57(3-4):124-9.
10. López-Revuelta A, Sánchez-Gallego JI, Hernández-Hernández A, Sánchez-Yagüe J, Llanillo M. Membrane cholesterol contents influence the protective effects of quercetin and rutin in erythrocytes damaged by oxidative stress. Chem Biol Interact. 2006;161(1):79-91.
11. Ray D, Deshmukh P, Goswami K, Garg N. Antioxidant vitamin levels in sickle cell disorders. Natl Med J India. 2007;20(1):11-3.
12. Hebbel RP, Eaton JW, Balasingam M, Steinberg MH. Spontaneous oxygen radical generation by sickle erythrocytes. J Clin Invest. 1982;70:1253-1259.
13. Repka T, Hebbel RP. Hydroxyl radical formation by sickle erythrocyte membrane: role of pathologic iron deposits and cytoplasmic reducing agents. Blood. 1991;78(10):2753-8.
14. Domanski AV, Lapshina EA, Zavodnik IB. Oxidative processes induced by tert-butyl hydroperoxide in human red blood cells:chemiluminescence studies. Biochemistry (Mosc). 2005;70(7):761-9.
15. Chaves MA, Leonart MS, do Nascimento AJ. Oxidative process in erythrocytes of individuals with hemoglobin S. Hematology. 2008;13(3):187-92.
16. Moridani MY, Pourahmad J, Bui H, Siraki A, O'Brien PJ. Dietary flavonoid iron complexes as cytoprotective superoxide radical scanvengers. Free Radic Biol Med. 2003;34(2):243-53.
17. Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free RadicBiol Med. 1996;20(7):933-56. Erratum in: Free Radic Biol Med.1996;21(3):417.
18. Hapner CD, Deuster P, Chen Y. Inhibition of oxidative hemolysis by quercetin, but not other antioxidants. Chem Biol Interact. 2010;186(3):275-9.
19. Barreca D, Laganà G, Tellone E, Ficarra S, Leuzzi U, Galtieri A, et al. Influences of flavonoids on erythrocyte membrane and metabolic implication through anionic exchange modulation. J Membr Biol. 2009;230(3):163-71.
20. Watanabe AM, Pianovski MA, Zanis Neto J, Lichtvan LC, Chautard Freire-Maia EA, Domingos MT, et al. Prevalência da hemoglobina S no Estado do Paraná, Brasil, obtida por triagem neonatal. Cad Saude Publica. 2008;24(5):993-1000.
Submitted: 6/5/2012
Accepted: 9/3/2012
Conflict-of-interest disclosure: The authors declare no competing financial interest
- 1. Kaul DK, Liu XD, Zhang X, Ma L, Hsia CJ, Nagel RL. Inhibition of sickle red cell adhesion and vasooclusion in the microcirculation byantioxidants. Am J Physiol Heart Circ Physiol. 2006;291(1):H167-75.
- 2. Cho CS, Kato GJ, Yang SH, Bae SW, Lee JS, Gladwin MT, et al. Hydroxyurea-induced expression of glutathione peroxidase 1 in red blood cells of individuals with sickle cell anemia. Antioxid Redox Signal. 2010;13(1):1-11.
- 3. Rusanova I, Escames G, Cossio G, de Borace RG, Moreno B, Chahboune M, et al. Oxidative stress status, clinical outcome, and β-globin gene cluster haplotypes in pediatric patients with sickle cell disease. Eur J Haematol. 2010;85(6):529-37.
- 4. Barreiros AL, David JM, David JP. Oxidative stress: relations between the formation of reactive species and the organism's defense. Química Nova. 2006;29(1):113-23.
- 5. Nur E, Biemond BJ, Otten HM, Brandjes DP, Schnog JJ; CURAMA Study Group. Oxidative stress in sickle cell disease; pathophysiology and potential implications for management. Am J Hematol. 2011;86(6):484-9.
- 6. Končić MZ, Barbarić M, Perković I, Zorc B. Antiradical, chelating and antioxidant activities of hydroxamic acids and hydroxyureas. Molecules. 2011;16(8):6232-42.
- 7. Davies SC, Gilmore A. The role of hydroxyurea in the management of sickle cell disease. Blood Rev. 2003;17(2):99-109.
- 8. Aslan M, Thornley-Brown D, Freeman BA. Reactive species in sickle cell disease. Ann N Y Acad Sci. 2000;899:375-91.
- 9. Cesquini M, Torsoni MA, Stoppa GR, Ogo SH. t-BOOH-induced oxidative damage in sickle red blood cells and the role of flavonoids. Biomed Pharmacother. 2003;57(3-4):124-9.
- 10. López-Revuelta A, Sánchez-Gallego JI, Hernández-Hernández A, Sánchez-Yagüe J, Llanillo M. Membrane cholesterol contents influence the protective effects of quercetin and rutin in erythrocytes damaged by oxidative stress. Chem Biol Interact. 2006;161(1):79-91.
- 11. Ray D, Deshmukh P, Goswami K, Garg N. Antioxidant vitamin levels in sickle cell disorders. Natl Med J India. 2007;20(1):11-3.
- 12. Hebbel RP, Eaton JW, Balasingam M, Steinberg MH. Spontaneous oxygen radical generation by sickle erythrocytes. J Clin Invest. 1982;70:1253-1259.
- 13. Repka T, Hebbel RP. Hydroxyl radical formation by sickle erythrocyte membrane: role of pathologic iron deposits and cytoplasmic reducing agents. Blood. 1991;78(10):2753-8.
- 14. Domanski AV, Lapshina EA, Zavodnik IB. Oxidative processes induced by tert-butyl hydroperoxide in human red blood cells:chemiluminescence studies. Biochemistry (Mosc). 2005;70(7):761-9.
- 15. Chaves MA, Leonart MS, do Nascimento AJ. Oxidative process in erythrocytes of individuals with hemoglobin S. Hematology. 2008;13(3):187-92.
- 16. Moridani MY, Pourahmad J, Bui H, Siraki A, O'Brien PJ. Dietary flavonoid iron complexes as cytoprotective superoxide radical scanvengers. Free Radic Biol Med. 2003;34(2):243-53.
- 17. Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free RadicBiol Med. 1996;20(7):933-56.
- Erratum in: Free Radic Biol Med.1996;21(3):417.
- 18. Hapner CD, Deuster P, Chen Y. Inhibition of oxidative hemolysis by quercetin, but not other antioxidants. Chem Biol Interact. 2010;186(3):275-9.
- 19. Barreca D, Laganà G, Tellone E, Ficarra S, Leuzzi U, Galtieri A, et al. Influences of flavonoids on erythrocyte membrane and metabolic implication through anionic exchange modulation. J Membr Biol. 2009;230(3):163-71.
- 20. Watanabe AM, Pianovski MA, Zanis Neto J, Lichtvan LC, Chautard Freire-Maia EA, Domingos MT, et al. Prevalência da hemoglobina S no Estado do Paraná, Brasil, obtida por triagem neonatal. Cad Saude Publica. 2008;24(5):993-1000.
Corresponding author:
Publication Dates
-
Publication in this collection
05 Apr 2013 -
Date of issue
2013
History
-
Received
05 June 2012 -
Accepted
03 Sept 2012