Abstracts
This work aimed to know the tolerance mechanisms through the salinity variation by Na+ K+ - ATPase and Mg++ - ATPase and enzymes encountered in the gills of Poecilia vivipara. In field, the presence of this species was observed in salinities of 0 and 28‰. In laboratory, these fish were maintained in aquarium with mean salinity of 30‰ and positive responses were obtained. Some adult specimens, collected in a lagoon of the Coqueiros Beach, were utilized as matrixes. In the experiments the specimens used were those born in the test aquarium. For each salinity studied three replicates were made with three specimens for each one. The alevins were maintained in salinities of 5, 10, 15, 20, 25, 30 and 35‰ during a month for adaptation. Gills were extracted in appropriate buffer for isolation of plasma membrane and used for specific dosage of the total enzymatic activity of Na+ K+ - ATPase and Mg++ - ATPase. The relation of alevins to their adaptation towards the salinity variation was also studied. The activity of the two enzymes showed a different result. The major expression of Na+ K+ - ATPase was observed in 20‰ (35 µmoles Pi.mg protein.h-1), the best salinity to cultivate P. vivipara.
Poecilia vivipara; Na+ K+ ATPase; Mg++ ATPase; osmoregulation
Este trabalho teve como objetivo conhecer os mecanismos de tolerância às variações de salinidade, pelas enzimas Mg++ - ATPase e Na+ K+ - ATPase, encontrada nas brânquias de Poecilia vivipara. No campo, foi observada a presença desta espécie em salinidades entre 0 e 28‰. No laboratório, os indivíduos foram mantidos em salinidade de 30‰ e responderam positivamente. Os indivíduos adultos, coletados em uma lagoa na praia dos Coqueiros, foram utilizados como matrizes. Nos experimentos foram usados alevinos que nasceram nos aquários testes. Para cada salinidade estudada foram feitas três réplicas com três espécimens em cada uma. Os alevinos foram mantidos em salinidades de 5, 10, 15, 20, 25, 30 e 35‰, durante um mês para total adaptação. As brânquias foram extraídas em tampão apropriado para isolar a membrana plasmática e usada dosagem específica para a atividade enzimática total de Na+ K+ ATPase e Mg++ - ATPase e sua relação a adaptação da variação da salinidade. A atividade das duas enzimas mostrou diferentes resultados. A maior expressão de Na+ K+ - ATPase foi observada em 20‰ (35 mimoles Pi/mg proteína.h), sendo a melhor salinidade para se manter o cultivo de P. vivipara.
Activity Determination of Na+ K+ - ATPase and Mg++ - ATPase Enzymes in the Gill of Poecilia vivipara (Osteichthyes, Cyprinodontiformes) in Different Salinities
Marcelo da Cunha Amaral1, Ana Cristina Teixeira Bonecker 1* * Author for correspondence and Cláudio H. D. Ortiz2
1Departamento de Zoologia, Instituto de Biologia, Universidade Federal do Rio de Janeiro, CCS, Bloco A, 21491-590, Rio de Janeiro, RJ.
2 Departamento de Bioquímica, Instituto de Química, Universidade Federal do Rio de Janeiro, CT, Bloco A, 21910-240, Rio de Janeiro, RJ.
ABSTRACT
This work aimed to know the tolerance mechanisms through the salinity variation by Na+ K+ - ATPase and Mg++ - ATPase and enzymes encountered in the gills of Poecilia vivipara. In field, the presence of this species was observed in salinities of 0 and 28. In laboratory, these fish were maintained in aquarium with mean salinity of 30 and positive responses were obtained. Some adult specimens, collected in a lagoon of the Coqueiros Beach, were utilized as matrixes. In the experiments the specimens used were those born in the test aquarium. For each salinity studied three replicates were made with three specimens for each one. The alevins were maintained in salinities of 5, 10, 15, 20, 25, 30 and 35 during a month for adaptation. Gills were extracted in appropriate buffer for isolation of plasma membrane and used for specific dosage of the total enzymatic activity of Na+ K+ - ATPase and Mg++ - ATPase. The relation of alevins to their adaptation towards the salinity variation was also studied. The activity of the two enzymes showed a different result. The major expression of Na+ K+ - ATPase was observed in 20 (35 µmoles Pi.mg protein.h-1), the best salinity to cultivate P. vivipara.
Key words:Poecilia vivipara, Na+ K+ ATPase, Mg++ ATPase, osmoregulation
INTRODUCTION
Poecilia vivipara (Bloch & Schneider, 1801) belongs to the family Poeciliidae and presents wide geographical distribution, being found in the Americas, from Illinois and New Jersey (EUA) to Argentina (Nascimento, 1984). They are commonly found in salt and brackish waters, mainly in the exits of ponds and river mouths (Suzuki, 1983). The individuals of this species are viviparous with internal fertilization. They present sex dimorphism, where the males possess an organ formed by the transformation of the anal fin in aid of the sexual intercourse (Nomura, 1984). The rays of the anal fin, particularly the third, fourth and fifth ones, are longer than the others. They are independent in the beginning of the development, coming unfastened later in the whole length, creating the gonopodium. The reproduction is continuous throughout the year. The females begin reproducing from 3 cm of total length, in continuous gestations. When they reach approximately 7 cm of length, they present an average of 51 embryos per gestation (Novaes & Andreata, 1994) The females have the ventral region permanently dilated by the continuous gestations. Thus, they are vulgarly called "Barrigudinho" (Pereira, 1979). These fish are omnivorous and they participate in the trophic chain, serving as food for carnivorous fish (Koblitz & Andreata, 1994; Novaes & Andreata, 1994).
P. vivipara was used in tests of thermal tolerance, lethal effects of cadmium and tests with derivatives of petroleum in Venezuela (Chunk, 1983; Chunk & Mendez, 1993) and in China (Xu et al., 1991; Xu et al., 1992). Damato (1995) used three species of fish, one of them belonging to P. vivipara, in toxicity tests with potassium dichromate. P. vivipara presented high sensitivity, suggesting its employment as biological indicator. The species Poecilia reticula and P. sphenops are cultivated for use in tests of aquatic effluents in Venezuela (Grau & Esclapés, 1995). Kraus et al. (1998) tested the sensitivity of P. vivipara to four reference substances (potassium dichromate, sodium dodecyl sulfate, copper and zinc).
In natural conditions P. vivipara is found in environments that differ from its geological and physiographical characteristics, such as salt concentrations and they are under different degrees of anthropogenetic impact (Caniçali & Lima, 1996). In the Coqueiros Beach, where the individuals were collected, the salinity varies from 0 to 28 (Amaral, 1997). The aim of this study was to dose Na+ K+ - ATPase and Mg++ - ATPase enzymes that are responsible for the individuals osmoregulation.
MATERIAL AND METHODS
Adult specimens were captured with nets in a lagoon in Coqueiros Beach at Fundão Island, in contact with the waters of Guanabara Bay. The fish were transported to the laboratory in sea water and conditioned in an acclimatized aquarium. The proportion of two females for a male was maintained. After the birth, the alevins were placed in test aquariums with the capacity of 1,000 ml, being standardized in 300 ml of sea water for each individual with a salinity of 30. For seven consecutive days the salinity was gradually reduced and later they were maintained in 5, 10, 15, 20, 25, 30 and 35. For each salinity studied, three replicates were made with three specimens for each one during a month for total adaptation. The aquariums were aerated and maintained in an incubator with controlled temperature at 25±2oC and a 12-hour photoperiod. The alevins were fed twice a day with ration in flakes (Tetra Min) and nauplius of Artemia sp. "ad libitum".
For enzyme extraction, the gills were removed, dried with absorbent paper, and weighed in analytical balance. The membranes cells of the gills of P. vivipara were isolated for dosage of the Na+ K+ - ATPase and Mg++ - ATPase activities (Bouef et al., 1978; Harache et al., 1980). Stages of the membrane isolation for determination of the ATPase activity: (1) Homogenization in the Ultra Turrax of 0.2 g of tissue in 0.5 ml of Tris buffer 5 mM, Sucrose solution 0.25 mM, EGTA (Ethylene-Glycol bis (b aminoethylether) N-N Tetra-acetic Acid) 0.2 mM, pH 7.4. Time of homogenization 1.5 min per 7,000 rpm at 4oC. (2) Centrifugation at 8,714 xg for 10 min and removed the supernatant. (3) Centrifugation at 61,000 xg for 45 min. (4) Ressuspension in 1.5 ml of homogenization buffer in the Potter and preparation of a gradient 1:1 with the Tris buffer 5 mM, Sucrose solution 1.4 M, EGTA 0.2 mM pH 7.4 and centrifugation at 61,000 xg for 45 min. (5) Remotion of the membrane fraction and dilution of 5 times with Tris-EGTA buffer 0.2 mM pH 7.4 and centrifugation at 61,000 xg for 45 min. (6) Ressuspension in 1.5 ml with imidazole buffer 25 mM, Sucrose 0.25 mM, EGTA 0.2 mM pH 7.4. (7) Evaluation of the ATPase activity.
Dosage of Na+, K+ - ATPase and Mg++ - ATPase: The total enzymatic activity of the ATPase was determined by the incubation of the enzyme at 37oC, pH 7.5 in a solution (final volume equal to 0.5 ml) containing ATP.Na2-TRIS 5 mM, NaCl 150 mM (final concentration of Na+ 155 mM), KCl 15 mM, MgCl 15 mM in histidine HCl-TRIS 30 mM. The dosage of Mg++ - ATPase was made under identical conditions. However, oubain was added in the final concentration of 1.5 mM. The Na+, K+ - ATPase activity is obtained by the difference between the total activity and the Mg++ - ATPase activity. For each sample, a reference solution was prepared in which the enzyme was added after the paralyzation of the reaction. The tubes containing the enzyme were pre-incubated for 5 min at 37oC. The reaction was started with the addition of ATP and paralyzed with the addition of 1 ml of reagent for dosage of inorganic phosphates (Pi) after 10 min of incubation at 37oC. The tubes rested for 15 to 20 min at room temperature and each one of the tubes (total ATPase, Mg++ - ATPase and "reference solution") was read at 700 nm by the modified method of Fiske & Subbarow (1925). Patterns of sodium phosphate were prepared concomitantly.
Na+, K+ - ATPase and Mg++ - ATPase activities were expressed in m moles of Pi released per hour per milligram of protein (mmoles Pi.mg protein.h-1) of the preparation adapted for Ortiz (1975).
Dosage of Protein: Protein concentration was measured by the modified method of Lowry et al. (1951). The band of protein was 1 to 100 mg, read at 700 nm for 1.1 ml (microassay) at room temperature after 5 min. Serum of bovine albumin was used as a pattern.
RESULTS AND DISCUSSION
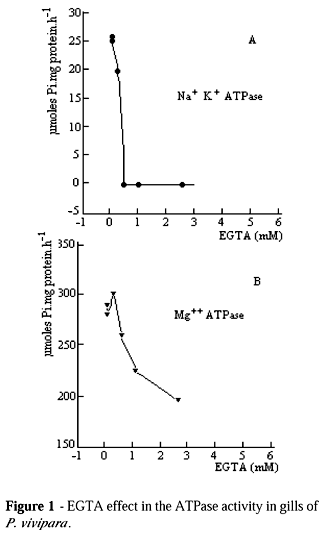
The Na+ K+ - ATPase activity of P. vivipara was of 33.6 mmoles Pi per hour per mg of protein, presenting the same order of magnitude as Na+ K+ - ATPase of Oncorhynchus kisutch (salmon) (Harache et al., 1980). In relation to this enzyme of Tilapia rendalli, the activity was about ten times higher (Arbex, 1983). This study demonstrated that Na+ K+ - ATPase activity of P. vivipara was very similar to fishes, which were adapted to salinity variations. The concentration effect of EGTA was tested in the homogenization buffer and it was found that the ATPase activity in the extract free from cell was sensitive from 0.2 mM onwards (Fig. 1).
The results for Na+ K+ - ATPase and Mg++ - ATPase demonstrated that they were enzymes totally different in relation to their kinetics parameters. These enzymes presented inhibition kinetics for substratum excess (type accompetitive) and Michaeliana, respectively (Fig. 2).
Their kinetic parameters were determined by non lineal programs in microcomputer by the algorithm of the Marquardt of Enzifit. The curves are theoretical and the points are experimental. The Vmax for enzyme Na+K+ATPase was 153.62±41.22 mM and the Km was 3.57±1.10 mM. This enzyme presented a Kis of 1.86±0.75 mM. The Vmax of enzyme Mg++ATPase was 280.79±11.69 mM and the Km was 0.36±0.03 mM. The parameters determined presented quite different values from Na+, K+ - ATPase of the Tilapia rendalli in relation to the Km for ATP.Mg++ that is 0.53 mM. They presented a much higher value (3.618±0.3 mM) and this enzyme presented a Kis of 1.795 mM showing an inhibition for substract not reported for the enzyme of Tilapia rendalli (Arbex, 1983). The adjustment of the experimental data of this kinetics to the Michaeliana equation did not fit better than the equation for inhibition for substract.
The value of Km for Mg++ - ATPase was in accordance with the values found for this enzyme in the literature. These values were for enzymes of gills of P. vivipara adapted to the salinity of 30. Na+ K+ - ATPase was an integral protein of membrane found in cells of superior eucaryotes and it was responsible for translocate ions of sodium and potassium through the membrane of cells using ATP as a driving force. For every three ions of sodium pumped off the cell, two potassium ions were pumped in. Such protein was specifically inhibited by cardiac glycosides, as, for example, ouabain and digoxin (Lingrel & Kuntzweiler, 1994).
Na+ K+ - ATPase presented inhibition for 1.5 mM of oubain and its activity for Na+ and K+, was optimum in the relationship of 150 mM and 15 mM, respectively. These concentrations were in accordance with the literature for Tilapia rendalli (Arbex, 1983) and Oncorhynchus kisutch (Harache et al., 1980). The activity of Na+ K+ - ATPase presented a maximum of the expression of the enzyme in 20. Higher concentrations presented a decrease in the activity (Fig. 3). This result was not expected once it should have been around 35 mmoles Pi.mg protein.h1. The difficulty of maintaining the high levels could be related to the stress accumulated from this value of 20 and linked to a factor that modified the activity Na+, K+ - ATPase "in vivo". Na+ K+ - ATPase increased its activity 7 times in the adaptation to the salinity. Its basal activity was of 5.4 mmoles Pi.mg protein.h-1. All the activities found in the salinity variation were in accordance with the expected for O. kisutch (Harache et al., 1980). Mg++ - ATPase activity during the adaptation to the salinity was different from the one found for this fish, once it followed the Na+ K+ - ATPase profile, presenting a maximum in 20.
ACKNOWLEDGEMENTS
We thank to Prof. Dr. Sérgio L. C. Bonecker for his help in the preparation of the manuscript and the Council of Teaching for Graduated from UFRJ for the financial support.
RESUMO
Este trabalho teve como objetivo conhecer os mecanismos de tolerância às variações de salinidade, pelas enzimas Mg++ - ATPase e Na+ K+ - ATPase, encontrada nas brânquias de Poecilia vivipara. No campo, foi observada a presença desta espécie em salinidades entre 0 e 28. No laboratório, os indivíduos foram mantidos em salinidade de 30 e responderam positivamente. Os indivíduos adultos, coletados em uma lagoa na praia dos Coqueiros, foram utilizados como matrizes. Nos experimentos foram usados alevinos que nasceram nos aquários testes. Para cada salinidade estudada foram feitas três réplicas com três espécimens em cada uma. Os alevinos foram mantidos em salinidades de 5, 10, 15, 20, 25, 30 e 35, durante um mês para total adaptação. As brânquias foram extraídas em tampão apropriado para isolar a membrana plasmática e usada dosagem específica para a atividade enzimática total de Na+ K+ ATPase e Mg++ - ATPase e sua relação a adaptação da variação da salinidade. A atividade das duas enzimas mostrou diferentes resultados. A maior expressão de Na+ K+ - ATPase foi observada em 20 (35 mmoles Pi/mg proteína.h), sendo a melhor salinidade para se manter o cultivo de P. vivipara.
Received: August 13, 1998;
Revised: September 27, 1999;
Accepted: April 07, 2000.
- Amaral, M. da C. (1997), Avaliaçăo da espécie Poecilia vivipara (Bloch & Schneider, 1801) para utilizaçăo em experimentos de laboratório. Monografia. Instituto de Biologia, Universidade Federal do Rio de Janeiro. 44p.
- Arbex, O.F. (1983), Na+ K+ ATPase em brânquias e intestino de Tilapia rendalli: uma comparaçăo cinética Dissertaçăo de Mestrado. Instituto de Química, Universidade Federal do Rio de Janeiro. 67p.
- Boeuf, G.; Lasserre, P. & Harache, Y. (1978), Osmotic adaptation of Oncorhychus kisutch Walbaum. II. Plasma osmotic and ionic variations, and gill Na+-K+ ATPase activity of yearling coho salmon transferred to sea water. Aquaculture 15(1):35-52.
- Caniçali, M.R. & Lima, N.R.W. (1996), Plasticidade fenotípica em quatro populaçőes de Poecilia vivipara (Teleostei: Poeciliidae) do norte fluminense. I Jornada de Ictiologia do Rio de Janeiro, Rio de Janeiro, p. 20 (em resumo).
- Chunk, K.S. (1983), Lethal effects of cadmium in tropical fishes. Bull. Jap. Sci. Fish. Nissuishi 49 (10):1565-1568.
- Chunk, K.S. & Mendez, S. (1993), Comparative thermal tolerance of some tropical fishes of Venezuela. Ciencia-Maracaibo 1(1):1-7.
- Damato, M. (1995), Determinaçăo da toxicidade aguda de dicromato de potássio para Phalloceros caudimaculatus, Poecilia vivipara e Cyprinus carpio XI Encontro Brasileiro de Ictiologia, Campinas, p.E7-E8 (em resumo).
- Fiske, E.H. & Subbarow, Y.V. (1925), The colorimetric determination of phosphorus. Biol. Chem., 66:375-400.
- Grau, J.R. & Esclapés, M.M. (1995), Protocolos estándares para bioensayos de toxicidad com especies acuáticas. Gerencia General de Tecnologia. Departamento de Ecología y Ambiente. Versión 1.0. 109p.
- Harache, Y., Boeuf, G. & Lasserre, P. (1980), Osmotic adaptation of Oncorhychus kisutch Walbaum. III. Survival and growth of juvenile coho salmon transferred to sea water at various times of the year. Aquaculture 19:253-273.
- Kraus, L.A. da S.; Bonecker, A.C.T. & Vital, N. de A.A. (1998), Acute Toxicity of Potassium dichromate, Sodium Dodecyl Sulfate, Copper and Zinc to Poecilia vivipara (Osteichthyes, Cyprinodontiformes). Fresenius Envir. Bull. 7:654-658.
- Koblitz, J.L. & Andreata, J.V. (1994), Hábitos alimentares de Poeciliidae e Jenynsiidae da Lagoa Rodrigo de Freitas, Rio de Janeiro, Brasil Resultados preliminares. XX Congresso Brasileiro de Zoologia, Rio de Janeiro, p. 163 (em resumo).
- Lingrel, J.B. & Kuntzweiler, T. (1994), Na+, K+ - ATPase. J. Biol. Chem. 269(31):19.659-19.662.
- Lowry, O.H.; Rosebrough, N.I; Farr, A.L. & Randall, R.J. (1951), Protein measurement with the phenol reagent. J. Biol. Chem 193:265-275.
- Nascimento, E.P. (1984), Aspectos quantitativos da biologia do desenvolvimento de Phalloceros caudimaculatus e Poecilia reticulata (Pisces, Poeciliidae). Dissertaçăo (Mestrado), Instituto de Biocięncias, Universidade de Săo Paulo, 120p.
- Novaes, J.L.C & Andreata, J.V. (1994), Aspectos reprodutivos de Poecilia vivipara (Bloch & Schneider, 1801) e Jenynsia lineata (Jenyns, 1842) da Lagoa Rodrigo de Freitas, Rio de Janeiro Resultados preliminares. XX Congresso Brasileiro de Zoologia, Rio de Janeiro, p. 162 (em resumo).
- Nomura, H. (1984), Dicionário de Peixes do Brasil Brasília, Ed. Editerra. 482p.
- Ortiz, C.H.D. (1975), Dosagem da Na+K+ ATPase nas etapas de purificaçăo de membrana plasmática de fígado de rato. Dissertaçăo de Monografia, Instituto de Biologia, Universidade do Estado do Rio de Janeiro. 9p.
- Pereira, R. (1979), Peixes da Nossa Terra Săo Paulo, Ed. Nobel. 495p.
- Suzuki, C.R. (1986), Guia de Peixes do Litoral Brasileiro Ediçőes Marítimas Ltda. 394p.
- Xu, X.; Cai, C. & Li, H. (1991), Quantum-chemical study on toxicities of clorobenzenes to some aquatic species. J. Zhanjiang Fish. Coll. Zhanjiang Shuichan Xueyuan Xuebao 11(2):64-66.
- Xu, X.; Lu, Y. & Li, H. (1992), Quantum chemical study on quantitative relationships between structure and toxicities of chlorophenols to some aquatic species. J. Zhanjiang Fish. Coll. Zhanjiang Shuichan Xueyuan Xuebao 12(2):60-64.
Publication Dates
-
Publication in this collection
25 Oct 2001 -
Date of issue
Mar 2001
History
-
Accepted
07 Apr 2000 -
Reviewed
27 Sept 1999 -
Received
13 Aug 1998