Abstracts
Water-soluble plant organic compounds have been proposed to be efficient in alleviating soil acidity. Laboratory methods were evaluated to estimate the efficiency of plant extracts to neutralize soil acidity. Plant samples were dried at 65ºC for 48 h and ground to pass 1 mm sieve. Plant extraction procedure was: transfer 3.0 g of plant sample to a becker, add 150 ml of deionized water, shake for 8 h at 175 rpm and filter. Three laboratory methods were evaluated: sigma (Ca+Mg+K) of the plant extracts; electrical conductivity of the plant extracts and titration of plant extracts with NaOH solution between pH 3 to 7. These methods were compared with the effect of the plant extracts on acid soil chemistry. All laboratory methods were related with soil reaction. Increasing sigma (Ca+Mg+K), electrical conductivity and the volume of NaOH solution spent to neutralize H+ ion of the plant extracts were correlated with the effect of plant extract on increasing soil pH and exchangeable Ca and decreasing exchangeable Al. It is proposed the electrical conductivity method for estimating the efficiency of plant extract to neutralize soil acidity because it is easily adapted for routine analysis and uses simple instrumentations and materials.
Plant residue; organic acid; plant alkaline index
Tem sido proposto que os compostos orgânicos de plantas solúveis em água são eficientes na amenização da acidez do solo. Foram avaliados métodos de laboratório para estimar a eficiência dos extratos de plantas na neutralização da acidez do solo. Os materiais de plantas foram secos a 65º C por 48 horas, moídos e passados em peneira de 1mm. Utilizou-se o seguinte procedimento para obtenção do extrato de plantas: transferir 3.0 g da amostra de planta para um becker, adicionar 150 ml de água deionizada, agitar por 8h a 175 rpm e filtrar. Avaliaram-se três métodos de laboratório: sigma (Ca + Mg + K) do extrato de planta, condutividade elétrica (CE) do extrato de planta e titulação do extrato de planta com solução de NaOH entre pH 3 a 7. Os métodos de laboratório foram comparados com o efeito dos extratos de plantas na reação do solo. Todos os métodos de laboratório foram correlacionados com a reação do solo: o aumento de sigma (Ca + Mg + K), Ce e o volume de NaOH gasto para neutralizar os íons H+ do extrato de plantas, foram correlacionados com o efeito nos aumentos de pH e Ca trocável e diminuição de Al trocável do solo. É proposto o método de CE para estimar a eficiência do extrato de plantas na neutralização da acidez do solo por ser facilmente adaptado para análise de rotina e por utilizar instrumentos e materiais simples.
A Laboratory Method to Estimate the Efficiency of Plant Extract to Neutralize Soil Acidity
Marcelo E. Cassiolato1; Mário Miyazawa2; Anderson R. Meda1 and Marcos A. Pavan2* * Author for correspondence 1PIBIC / CNPq / IAPAR scholarship. 2Instituto Agronômico do Paraná (IAPAR); C. P. 481; CEP 86001-970; Londrina - PR - Brasil
ABSTRACT
Water-soluble plant organic compounds have been proposed to be efficient in alleviating soil acidity. Laboratory methods were evaluated to estimate the efficiency of plant extracts to neutralize soil acidity. Plant samples were dried at 65oC for 48 h and ground to pass 1 mm sieve. Plant extraction procedure was: transfer 3.0 g of plant sample to a becker, add 150 ml of deionized water, shake for 8 h at 175 rpm and filter. Three laboratory methods were evaluated: S (Ca+Mg+K) of the plant extracts; electrical conductivity of the plant extracts and titration of plant extracts with NaOH solution between pH 3 to 7. These methods were compared with the effect of the plant extracts on acid soil chemistry. All laboratory methods were related with soil reaction. Increasing S (Ca+Mg+K), electrical conductivity and the volume of NaOH solution spent to neutralize H+ ion of the plant extracts were correlated with the effect of plant extract on increasing soil pH and exchangeable Ca and decreasing exchangeable Al. It is proposed the electrical conductivity method for estimating the efficiency of plant extract to neutralize soil acidity because it is easily adapted for routine analysis and uses simple instrumentations and materials.
Key words: Plant residue, organic acid, plant alkaline index
INTRODUCTION
Plant residues release a range of low molecular weight organic acids such as maleic, citric, oxalic, salicylic, etc, which are involved in many soil processes including lime mobility (Ziglio et al., 1999; Miyazawa et al., 1998), metal organic complexing reactions (Franchini et al., 1999 and 2001), ion sorption (Bolan et al., 1994) and rhizosphere chemistry (Jones, 1998). These organic acids contain carboxyl and phenolic groups, thereby allowing the cation complexation reaction and the anion competition for a soil adsorption sites.
The ability of organic acids for cation complexation in solution is well established (Hue et al., 1986; Miyazawa et al., 1992) and has being attributed to the presence of the negative charges in the functional groups. Complexing formation depends on the number and the position of carboxyl and phenolic functional groups in the organic acids.
The composition of organic acids in plants is well documented (Curl & Trueglove, 1986; Aoyama, 1996). Using high performance size exclusion chromatography (Aoyama, 1996) and semi-quantitative techniques (Curl & Trueglove, 1986), they observed that the composition of organic acids in plants are highly variable and depends on plant species, plant age and physiochemical environment. Despite of this early works, however, the effects of plant organic acids on soil chemistry have only recently started to be elucidated (Miyazawa et al., 1998; Ziglio et al., 1999; Franchini et al., 1999; Meda et al., 1999). In general, these authors concluded that several plant residues are highly efficient in alleviating soil acidity.
The objectives of this work were to develop a laboratory method to evaluate the efficiency of water extracts of plant residues to neutralize soil acidity and to evaluate the most appropriate method for laboratory routine.
MATERIALS AND METHODS
Plant materials: black oats (Avena strigosa), oil seed radish (Raphanus sativus), white lupin (Lupinus albus), gray mucuna (Stizolobium cinereum), wheat straw (Triticum aestivum). The aereal parts of the black oats, oil seed radish, white lupin and gray mucuna were collected at flowering stage and wheat straw after grain's harvest.
Water plant extract: 3 g of dried plant material (aereal parts) were transferred to a plastic flask, added 150 ml of deionized water, shaked for 8 h at 175 rpm and filtrated. Sub samples of plant extracts were used for chemical analysis (Table 1). Basic cations (Ca, Mg, and K) were determined by ICP (Inductively Coupled Plasma).
Experiment with soil: acid soil sample taken from the 0-20 cm horizon was air-dried and passed through a 2 mm sieve. The soil had an original pH CaCl2 value of 4.10; exchangeable Ca, Mg, K, and Al contents of 0.37, 0.17, 0.05, and 1.11 cmolc dm-3, respectively, total acidity (H + Al) of 6.20 cmolc dm-3, and total carbon content of 7.64 g kg-1. The clay content was 27%, silt 2% and sand 71%. Soil sample was transferred to PVC column (30 cm high by 4 cm diameter) and compacted to a homogeneous bulk density (mean 1g cm-3). Plant extract solution (150 ml) in amount equivalent to one porous volume (pV) was added on the soil surface. Then, deionized water was added in amount equivalent to three pV, to a total of 450 ml. Plant extract and water were added at a rate of 1.0 ml min-1. Soil samples were collected at 0-5, 5-10, 10-15, 15-20 and 20-25 cm depth, air-dried, ground to pass 2 mm sieve and analyzed for pH CaCl2 and exchangeable Alex and Caex. Extraction and determination methods were described by Pavan et al. (1992). All treatments had three replicates in a completely randomized block design.
Laboratory methods:
1. Summation of basic cations [S (Ca + Mg + K)]: sum of Ca, Mg, and K contents in the plant extract solution (Table 1).
2. Electrical conductivity (EC): measured with electrode installed into a becker containing a 10% diluted plant extract in deionized water at 25oC.
3. Capacity to reduce H+ ion: transfer 25 ml of plant extract to a becker, set up a H+ ion selective electrode into the becker, adjust to pH 3.0 with 2 mol L-1 HCl solution, titrate with 0.05 mol L-1 NaOH solution to pH 7.0, and register the volume of 0.05 mol L-1 NaOH spent. The pHs 3.0 and 7.0 were selected due to the maximum protonation and deprotonation, respectively, of the organic anions (Young et al., 1981).
RESULTS AND DISCUSSION
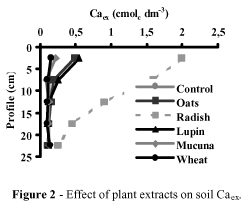
The pH, Caex, and Alex by depth for the control and plant extract treatments are shown in figures 1, 2, and 3 , respectively. Control soil had lower pH and Caex and higher Alex contents. Additions of plant extracts increased soil pH and Caex and decreased Alex, corroborating data presented in early studies with brazilian acid soils (Miyazawa et al., 1993 and 1998; Franchini et al., 1999 and 2000; Meda et al., 1999). These results show that the effect of plant extracts on soil acidity was highly variable and depended on plant species. In general, the effect of plant extract on soil acidity followed the order: oil seed radish > black oats > lupin > mucuna > wheat straw. Oil seed radish, black oats and lupin increased pH up to 20 cm soil depth. Oil seed radish and black oats neutralized totally Alex up to 10 cm soil depth and partially up to 25 cm soil depth. Except for wheat straw, the other plant extracts increased Caex in the soil profile. Black oats and oil seed radish showed the greatest increment on Caex in the soil profile. Oil seed radish was the most efficient to increase the total Caex in the soil column. These results indeed support the view the soluble organic compounds as short chain fatty acids in soil are mobile and efficient in alleviating soil acidity (Franchini et al., 2001).
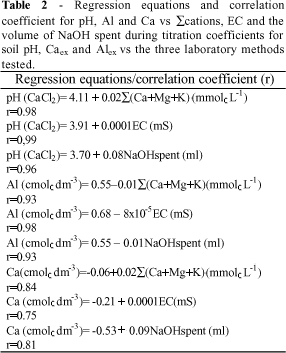
Table 1 shows the S (Ca+Mg+K), the volume of NaOH spent during titration and the electrical conductivity of the plant extracts. The total amount of basic cations, the volume of NaOH spent, and the values of electrical conductivity followed the order: oil seed radish > black oats > mucuna > lupin > wheat straw. This is the same order found for alleviating soil acidity (Figures 1, 2, and 3 ). Table 2 shows the regression equations and the correlation coefficients for soil pH, Alex and Caex vs the three laboratory methods tested. These methods were highly correlated with soil parameters indicating that they can be used to estimate the efficiency of plant extract to neutralize soil acidity
The greater amount of NaOH used, the higher content of basic cations and the higher electrical conductivity of the oil seed radish and black oats than wheat straw extract indicated a higher alkaline capacity of the oil seed radish and black oats. Due to its simplicity (instrumentation and materials) and for being easily adapted for routine analysis it is recommended the electrical conductivity method for estimating the efficiency of the plant extract to neutralize soil acidity.
CONCLUSION
The efficiency of plant extract to neutralize soil acidity can be estimated in the laboratory by sum of Ca, Mg, and K, the volume of NaOH solution spent between pH 3 to 7, and the electrical conductivity of the plant extracts. Due to its simplicity it is recommended the electrical conductivity method.
RESUMO
Tem sido proposto que os compostos orgânicos de plantas solúveis em água são eficientes na amenização da acidez do solo. Foram avaliados métodos de laboratório para estimar a eficiência dos extratos de plantas na neutralização da acidez do solo. Os materiais de plantas foram secos a 65o C por 48 horas, moídos e passados em peneira de 1mm. Utilizou-se o seguinte procedimento para obtenção do extrato de plantas: transferir 3.0 g da amostra de planta para um becker, adicionar 150 ml de água deionizada, agitar por 8h a 175 rpm e filtrar. Avaliaram-se três métodos de laboratório: S (Ca + Mg + K) do extrato de planta, condutividade elétrica (CE) do extrato de planta e titulação do extrato de planta com solução de NaOH entre pH 3 a 7. Os métodos de laboratório foram comparados com o efeito dos extratos de plantas na reação do solo. Todos os métodos de laboratório foram correlacionados com a reação do solo: o aumento de S (Ca + Mg + K), Ce e o volume de NaOH gasto para neutralizar os íons H+ do extrato de plantas, foram correlacionados com o efeito nos aumentos de pH e Ca trocável e diminuição de Al trocável do solo. É proposto o método de CE para estimar a eficiência do extrato de plantas na neutralização da acidez do solo por ser facilmente adaptado para análise de rotina e por utilizar instrumentos e materiais simples.
Received: December 29, 2000;
Revised: March 09, 2001;
Accepted: August 28, 2001.
- Aoyama, M. (1996), Fractionation of water-soluble organic substances formed during plant residue decomposition and high performance size exclusion chromatography of the fractions. Soil Sci. Pl. Nutr, 42, 31-40.
- Boln, N. S.; Naidu, R.; Mahimairaja, S. and Baskaran, S. (1994), Influence of low-molecular-weight organic acids on the solubilization of phosphates. Biol. Fertil. Soils, 18, 311-319.
- Curl, E. and Trueglove, B. (1986), The rhizosphere. Berlin, Springer-Verlag.
- Franchini, J. C.; Malavolta, E.; Miyazawa, M. and Pavan, M. A. (1999), Alterações químicas em solos ácidos após a aplicação de resíduos vegetais. R. bras. Ci. Solo, 23, 533-542.
- Franchini, J. C.; Gonzalez-Vila, F. J.; Cabrera, F.; Miyazawa, M. and Pavan, M. A. (2001), Rapid transformations of plant water-soluble organic compounds in relation to cation mobilization in an acid oxisol. Pl. Soil, 1-9.
- Hue, N. V.; Craddock, G. R. and Adams, F. (1986), Effect of organic acids on aluminum toxicity in subsoils. Soil Sci. Soc. Am. J, 50, 28-34.
- Jones, D. L. (1998), Organic acids in the Rhizosphere - a critical review. Pl. Soil, 205, 25-44.
- Meda, A. R.; Cassiolato, M. E.; Miyazawa, M. and Pavan, M. A. (1999), Plant extracts to improve acid soil chemistry. In - CONGRESSO LATINO AMERICANO DE LA CIENCIA DEL SUELO, 14., Temuco, Universidade de la Fronteira. pp. 360.
- Miyazawa, M.; Pavan, M. A. and Franchini, J. C. (1998), Organic mobility of surface applied lime under no-tillage. In - INTERNATIONAL MEETING OF THE INTERNATIONAL HUMIC SUBSTANCE SOCIETY, 9., Adelaide, IHSS. pp. 166.
- Miyazawa, M.; Chierice, G. O. and Pavan, M. A. (1992), Amenização da toxidade de alumínio às raízes do trigo pela complexação com ácidos orgânicos. R. bras. Ci. Solo, 16, 209-215.
- Miyazawa, M.; Pavan, M. A. and Calegari, A. (1995), Efeito de material vegetal na acidez do solo. Rev Bras. Ci. Solo, 17, 411-416.
- Pavan, M. A.; Bloch, M. F.; Zempulski, H. D.; Miyazawa, M. and Zocoler, D. C. (1992), Manual de análise química do solo e controle de qualidade. Londrina, Instituto Agronômico do Paraná, 40 pp. (IAPAR Circular; 76).
- Young, S. D.; Bache, B. W.; Welch, D. and Anderson, H. A. (1981), Analysis of the potentiometric titration of natural and synthetic poly-carboxylates. Soil Sci, 32, 579-592.
- Ziglio, C. M.; Miyazawa, M. and Pavan, M. A. (1999), Organic and inorganic forms of calcium mobilization in soil. Arq. Biol. Tecnol, 42 : (2), 257-262.
Publication Dates
-
Publication in this collection
27 Sept 2002 -
Date of issue
June 2002
History
-
Accepted
28 Aug 2001 -
Reviewed
09 Mar 2001 -
Received
29 Dec 2000