Abstracts
Low Ca and Mg are serious limitations to crop production in sandy soils of the northwest Paraná, Brazil. Thus soil samples of an Oxisol collected in this region were packed into 30cm long columns. Dolomite lime (2.0, 0.84, 0.30, and < 0.30 mm screen) was added on soil surface, then leached with deionized water. Thereafter, the columns were dismantled and the soil cut into 5cm segments for chemical analysis. Dolomite lime increased pHCaCl2,, KCl-exchangeable Ca and Mg and residual CO3 mostly in the top surface layers. Surface dolomite lime had no effect on pH, Ca, Mg, and CO3 in the leachate, independent on the lime particle size. These results indicated that surface dolomite lime application had no effect on subsoil composition and mostly of the calcium and magnesium carbonates are still unreacted on the soil surface.
Liming; Ca and Mg leaching; subsoil acidity
A deficiência de Ca e Mg são fatores que podem limitar a produção agrícola nos solos arenosos do Noroeste do Paraná, Brasil. Amostras de um Latossolo Vermelho escuro coletado nesta região foram incubadas em colunas de PVC de 30 cm de comprimento, aplicando-se calcário dolomítico (peneiras tamanho 2.0, 0.84, 0.30, e <0.30 mm) na superfície, lixiviando-se com água destilada. Após, as colunas de solo foram fragmentadas de 5 em 5 cm. Cada fragmento da coluna constituiu a amostra de solo que foi analisada quimicamente. O calcário dolomítico aumentou o pHCaCl2, Ca e Mg trocáveis e carbonato residual principalmente na camada superficial (0-5 cm). A aplicação de calcário dolomítico na superfície apresentou pequeno efeito no pH, Ca, Mg e carbonato na água de drenagem, independente do tamanho da partícula do calcário. Estes resultados indicaram que a aplicação de calcário dolomítico na superfície não alterou a composição química das camadas inferiores do solo e grande parte dos carbonatos de cálcio e magnésio aplicados permaneceram sem reação na superfície do solo.
Dolomite Limes Reaction Applied on the Surface of a Sandy Soil of the Northwest Paraná, Brazil
Anderson R. Meda; Marcos A. Pavan* * Author for correspondence ; Marcelo E. Cassiolato and Mário Miyazawa
Instituto Agronômico do Paraná (IAPAR); Caixa Postal 481; CEP 86001-970; Londrina - PR - Brasil
ABSTRACT
Low Ca and Mg are serious limitations to crop production in sandy soils of the northwest Paraná, Brazil. Thus soil samples of an Oxisol collected in this region were packed into 30cm long columns. Dolomite lime (2.0, 0.84, 0.30, and < 0.30 mm screen) was added on soil surface, then leached with deionized water. Thereafter, the columns were dismantled and the soil cut into 5cm segments for chemical analysis. Dolomite lime increased pHCaCl2,, KCl-exchangeable Ca and Mg and residual CO3 mostly in the top surface layers. Surface dolomite lime had no effect on pH, Ca, Mg, and CO3 in the leachate, independent on the lime particle size. These results indicated that surface dolomite lime application had no effect on subsoil composition and mostly of the calcium and magnesium carbonates are still unreacted on the soil surface.
Key words: Liming, Ca and Mg leaching, subsoil acidity
INTRODUCTION
Among the major soil limitations to crop production in northwest Paraná State, Brazil are: extremely deficient levels of Ca, Mg, and K, low cation exchange capacity (CEC) and organic matter contents, and very sandy texture. Fidalski & Auler (1997) and Fidalski et al. (1999) observed that Ca and Mg were the highest nutritional deficiencies in citrus orchards in this region. They also demonstrated that leaf Ca and Mg were positively related with citrus yields. They concluded that dolomite lime is essential for an economic citrus production in this soil.
In long-term pasture and in established perennial crops where deep soil cultivation is not possible, lime may be spread on soil surface and allowed time to leach. Even when cultivation is possible in citrus orchard, lime incorporation is unlikely with conventional machinery and leaching of the ions from the lime is relied upon for amelioration of acidity deeper in the soil. There are some evidences that surface applied lime in this soil has little effect on both surface and subsurface chemical properties (Rêgo, 1997; Fidalski, 1999). These authors reported that soil samples taken after twelve months of dolomite lime application on soil surface showed no effects on pH and KCl-exchangeable Ca, Mg, and Al. Their hypotheses are that lime was either leached bellow the root zone or was still unreacted with the soil acidity. To test these hypotheses, laboratory experiments were conducted with PVC columns with soil samples collected in this region to identify the main chemical reactions involved in the surface applied dolomite lime.
MATERIAL AND METHODS
Soil samples were collected from a cultivated site on the Instituto Agronômico do Paraná (IAPAR) experimental station at Paranavaí, Brazil (23o05'S, 52o26'W, 480m above sea level). This soil was selected based on following characteristics: sandy texture, low basic cations contents, and their potential use for production of citrus and pasture. The soil had an original pH CaCl2 value of 5.0; exchangeable Ca, Mg and K contents of 12.8, 6.6, and 3.0 mmolc dm-3, respectively; total acidity (H + Al) of 29.4 mmolc dm-3, organic carbon content of 10.31 g kg-1 and clay, silt, and sand contents of 11, 1, and 88%, respectively.
Columns (length 30cm; diameter 4cm) of rigid polyvinyl chloride (PVC) were used to conduct the experiment. Soil samples were transferred to PVC column and compacted to a homogeneous bulk density (mean 1.4 g cm-3). Then, the columns were brought to field capacity with distilled water. Approximately 1 kg of agricultural dolomite limestone (27.3% CaO, 20.7% MgO, and 100% neutralizer power) containing both coarse and fine materials was passed through 2.0, 0.84, 0.30, and < 0.30 mm screen. A mechanical shaker was used to separate the lime particle sizes.
Lime was added on the soil surface at a rate of 3.96g per column in order to neutralize two times the total acidity (H + Al) contents. The irrigation program was 330 ml of water per column, equivalent to three porous volume, added at a rate of 2.2 ml min-1.
At each porous volume, the drainage water was collected and analyzed for pH, Ca, Mg and CO3 contents. Soil samples were collected at 0-5, 5-10, 10-15, 15-20 and 20-25 cm depth, air dried, ground to pass 2mm sieve and analyzed for pH CaCl2, KCl-exchangeable Ca and Mg, and total CO3. Ca and Mg were determined by ICP (Inductively Coupled Plasma) and total CO3 content by conductivimetry using flow injection analysis coupled with pervaporation (Kawazaki et al., 2000, Grassi, et al., 2002). All treatments had three replicates in a completely randomized block design.
RESULTS AND DISCUSSION
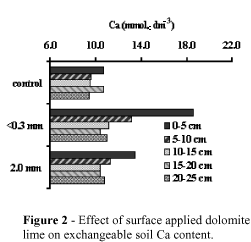
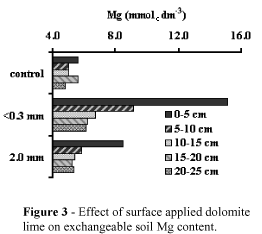
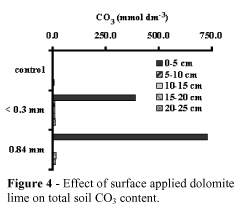
Figures 1, 2, 3 and 4 show soil pH, KCl-exchangeable Ca and Mg and total CO3 content at different depths as a result of surface application of dolomitic lime. For simplicity, data included only the effects of control, the coarsest (2.0 mm) and the finest limestone (<0.3 mm).
Dolomitic lime increased pH, exchangeable Ca, Mg, and CO3 mostly in the top surface layers. The finest the lime the highest the increase in pH, Ca and Mg, corroborating data presented by Verlengia & Gargantini (1972). The lime particle size had not clear effect on the mobility of Ca and Mg with depth. The low Ca and Mg distribution patterns can be explained by the electrical neutrality principles which state that Ca and Mg can not move down in the soil profile unless accompanied by equivalent anions.
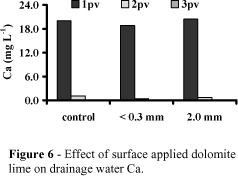
Figures 5, 6, 7 and 8 show the effect of surface applied dolomite lime on drainage water composition. Ca, Mg, CO3 and pH in the leachates were consistent and complementary to their distributions in the soil profile.
The low rate of lime movement into the soil profile was probably a result of the low solubility of the CaMg(CO3)2, weak soil acidity, and the lack of stable soluble anion as a product of the reaction of dolomite with soil acidity. The electrical neutrality principle dictates that Ca and Mg movements must be accompanied by equivalent anions. The dolomite lime reaction is illustrated as following:
CaMg(CO3)2 + 4H+ ® Ca2+ + Mg2+ + 2CO2 + 2H2O
This reaction neutralizes H+ and release Ca2+ and Mg2+, resulting an increase in soil pH and pH-dependent CEC. The Ca2+ and Mg2+ stoichiometrically replace H+ on the existing pH-dependent CEC whose magnitude changes under the influence of the increase alkalinity. As expected, it was found higher exchangeable Ca than Mg after dolomite lime reaction in this soil due to higher selectivity of the colloidal negative sites for Ca2+ than for Mg2+ (Loyola & Pavan, 1989). The above reaction will continue to the right as long as the soil supplies acidity in the form of H+ ions.
The results of this study clearly indicated that surface lime applications in this soil had little effect on the subsoil acidity and most of Ca and Mg-carbonates are still unreacted on the soil surface. Seldom would lime be applied to a topsoil with the objective of improving the subsoil fertility without any acid inputs. For this soil, without acid inputs, transfer of alkalinity to the subsoil is unexpected.
CONCLUSION
Agriculturally realistic liming rates are not likely to ameliorating subsurface soil acidity on the Northwest Paraná without acid inputs. The carbonates stay unreacted on the soil surface.
ACKNOWLEDGEMENTS
The authors wish to thank CNPq for the concession of the scientific initiation scholarship.
RESUMO
A deficiência de Ca e Mg são fatores que podem limitar a produção agrícola nos solos arenosos do Noroeste do Paraná, Brasil. Amostras de um Latossolo Vermelho escuro coletado nesta região foram incubadas em colunas de PVC de 30 cm de comprimento, aplicando-se calcário dolomítico (peneiras tamanho 2.0, 0.84, 0.30, e <0.30 mm) na superfície, lixiviando-se com água destilada. Após, as colunas de solo foram fragmentadas de 5 em 5 cm. Cada fragmento da coluna constituiu a amostra de solo que foi analisada quimicamente. O calcário dolomítico aumentou o pHCaCl2, Ca e Mg trocáveis e carbonato residual principalmente na camada superficial (0-5 cm). A aplicação de calcário dolomítico na superfície apresentou pequeno efeito no pH, Ca, Mg e carbonato na água de drenagem, independente do tamanho da partícula do calcário. Estes resultados indicaram que a aplicação de calcário dolomítico na superfície não alterou a composição química das camadas inferiores do solo e grande parte dos carbonatos de cálcio e magnésio aplicados permaneceram sem reação na superfície do solo.
Received: April 10, 2000;
Revised: March 16, 2001;
Accepted: November 20, 2001.
- Fidalski, J. (1999), Respostas da mandioca à adubação NPK e calagem em solos arenosos do noroeste do Paraná. Pesq. Agropec. Bras, 34, 1353-1359.
- Fidalski, J. and Auler, P. A. M. (1997), Levantamento nutricional de pomares de laranja no noroeste do Paraná. Arq. Biol. Tecnol, 40, 443-451.
- Fidalski, J.; Pavan, M. A.; Auler, P. A. M. and Jaocmino, A. P. (1999), Produção de frutos de laranja pêra e teores de nutrientes nas folhas e no solo, em latossolo vermelho-escuro do noroeste do Paraná. R. bras. Ci. Solo, 23, 273-279.
- Grassi, V.; Miyazawa, M.; Pavan, M. A. and Kamogawa, M. Y. (2002), Determinação em fluxo do carbonato residual do solo empregando pervaporação. Química Nova, 25, 149-152.
- Kawazaki, L. I.; Miyazawa, M.; Pavan, M. A. and Franchini, J. C. (2000), Determinação condutivimétrica de carbonato residual do calcário aplicado no solo por análise em fluxo. Química Nova, 23, 560-562.
- Loyola Jr., E. and Pavan, M. A. (1989), Seletividade de troca de cátions em solos ácidos. R. bras. Ci. Solo, 13, 131-138.
- Rêgo, I. C. (1997), Calagem e gessagem em um latossolo vermelho escuro cultivado com laranja pêra sobre limoeiro cravo Piracicaba, CENA-USP. Tese de Doutorado.
- Verlengia, F. and Gargantini, H. (1972), Estudo sobre a eficiência de diferentes frações granulométricas de calcário no solo. Bragantia, 31, 119-128.
Publication Dates
-
Publication in this collection
27 Sept 2002 -
Date of issue
June 2002
History
-
Received
10 Apr 2000 -
Reviewed
16 Mar 2001 -
Accepted
20 Nov 2001