Abstracts
This work simulated numerically the n-pentane separation of a mixture of iso-pentane, n-pentane and nitrogen, using an adsorption column with zeolite 5A. The mathematical model equations of the mass and heat transfer in the adsorption column are presented, as well as the boundary and initials conditions, beyond some hypotheses and considerations. The Volume Finite Method was used in the discretization of the equations to get the system of algebraic equations and posterior development of the computational algorithm. The numerical results using the Differencing Central (CDS) and Upwind (UDS) interpolations were compared with experimental results found in the literature. The influence of the partial pressure in the adsorption column performance was also analyzed.
Adsorption column; mass transfer; finite volume method
Neste trabalho é simulada numericamente a separação de n-pentano de uma mistura contendo iso-pentano, n-pentano em uma corrente de nitrogênio (inerte), em uma coluna de adsorção de leito fixo empacotada com zeólita 5A. São apresentadas as equações do modelo matemático da transferência de calor e massa na coluna de adsorção, bem como suas condições de contorno e iniciais, além de algumas hipóteses e considerações. Utiliza-se o Método de Volumes Finitos para discretizar as equações e obter um sistema de equações algébricas aproximadas e posterior desenvolvimento do algoritmo computacional. Os resultados numéricos utilizando as interpolações Diferença Central (CDS) e Upwind (UDS) são comparados com resultados experimentais obtidos na separação do n-pentano encontrados na literatura. A influência da pressão parcial no desempenho da coluna de adsorção de leito fixo também é analisada.
ENGINEERING AND TECHNOLOGY
Numerical study of n-pentane separation using adsorption column
Adriano da SilvaI, * * Author for correspondence ; Viviana Cocco MarianiII; Antônio Augusto Ulson de SouzaIII; Selene Maria de Arruda Guelli Ulson SouzaIII
ICentro de Ciências Agro-Ambientais e de Alimentos; Universidade Comunitária Regional de Chapecó (UNOCHAPECÓ); Rua Senador Attílio Fontana, 591 E; 89809-000; adriano@unochapeco.edu.br; Chapecó - SC - Brazil
IIPrograma de Pós-Graduação em Engenharia Mecânica; Pontifícia Universidade Católica do Paraná (PUCPR); Curitiba - PR - Brasil
IIILaboratório de Simulação Numérica de Sistemas Químicos (LABSIN); Departamento de Engenharia Química e Engenharia de Alimentos; Universidade Federal de Santa Catarina (UFSC); C. P.476; 88.040-900; Florianópolis - SC - Brasil
ABSTRACT
This work simulated numerically the n-pentane separation of a mixture of iso-pentane, n-pentane and nitrogen, using an adsorption column with zeolite 5A. The mathematical model equations of the mass and heat transfer in the adsorption column are presented, as well as the boundary and initials conditions, beyond some hypotheses and considerations. The Volume Finite Method was used in the discretization of the equations to get the system of algebraic equations and posterior development of the computational algorithm. The numerical results using the Differencing Central (CDS) and Upwind (UDS) interpolations were compared with experimental results found in the literature. The influence of the partial pressure in the adsorption column performance was also analyzed.
Key words: Adsorption column, mass transfer, finite volume method
RESUMO
Neste trabalho é simulada numericamente a separação de n-pentano de uma mistura contendo iso-pentano, n-pentano em uma corrente de nitrogênio (inerte), em uma coluna de adsorção de leito fixo empacotada com zeólita 5A. São apresentadas as equações do modelo matemático da transferência de calor e massa na coluna de adsorção, bem como suas condições de contorno e iniciais, além de algumas hipóteses e considerações. Utiliza-se o Método de Volumes Finitos para discretizar as equações e obter um sistema de equações algébricas aproximadas e posterior desenvolvimento do algoritmo computacional. Os resultados numéricos utilizando as interpolações Diferença Central (CDS) e Upwind (UDS) são comparados com resultados experimentais obtidos na separação do n-pentano encontrados na literatura. A influência da pressão parcial no desempenho da coluna de adsorção de leito fixo também é analisada.
INTRODUCTION
In recent years, there has been considerable increase in studies on separation processes using adsorption technique for isolation and purification of products.
This is due to advances in the theoretical and experimental studies that contribute in the prediction of the equipments functionality at large scale in the development of synthetic adsorbent such as zeolite, besides conciliating the necessity of continuous functional of the adsorption unit and desorption - simulated moving bed. The advantage of the fixed bed is its simplicity and low cost of construction besides causing a small abrasion in the adsorbent. The facility of operation and construction cause some disadvantage, such as:
i) Due the necessity of residence time in the bed it has more adsorbent restrained in the equipment.
ii) Difficulty of fast heating and cooling of a large adsorbent volume due to its low thermal conductivity. This factor increases the bed temperature, reducing the relative saturation in the gaseous phase and the concentration of adsorbed phase.
iii) The regeneration heat in general is lost in each cycle.
Petrochemical industry utilizes the adsorption process at industrial scale, in special fixed bed column. The catalytic isomerization of light naphtha stream that leaves refinery the containing more or less 40% of n-pentane and iso-pentane produces an elevated quantity of isoparaffins.
For the selective separation of isoparaffins, e.g. zeolite 5A, the process is optimized by putting adsorption unit with the reactor outlet.
In the petrochemical industry, in paraffin separation, the main objective is the production of high-octane fuel.
Numerical simulations could be useful tools in identifying the suitable condition to operate an industrial plant for separation of gas. Numerical simulation is utilized because it frequently confers time and investment economies. For the numerical simulation to represent the reality, the mathematical models could be used. Ruthven (1984) presented the models that described the mass transfer process in the adsorption column of fixed bed. The models considered different devices that controlled this transfer process, such as inter and intra-particles diffusion, external fluid film resistance, macropore and micropore resistances.
In this work, the modeling the adsorption column of fixed bed is presented. The model resulted in a set of nonlinear partial differential equations, where the analytical solution was impossible. In this case, the partial differential equations were solved by Finite Volume Method (Patankar, 1980).
The main objective was to investigate and simulate the performance of an adsorption unit of fixed bed used in iso-pentane and n-pentane separations.
This paper is based on Silveira (2001). However, in this work the Differencing Central (CDS) and Upwind (UDS) interpolations were adopted, while in Silveira et al. (2002) only the CDS was applied.
MATHEMATICAL FORMULATION
The differential equations that govern the processes of mass and heat transfer of the chemical species in the non isothermal and non adiabatic adsorption column of fixed bed, are the conservation equations of mass, energy, and chemical species in the gas and solid phases. These equations involve an approximation that does not affect the results. The ideal gas is considered, while in the adsorption process the constant pressure gradient is maintained and the mass flux in gaseous phase for axial dispersion model is described. In this case, the mass transfer includes the extern resistance in the fluid film and macropore diffusion, as mentioned in Silva and Rodrigues (1997a).
The resistance for the heat transfer occurs in the external fluid film in turn of the adsorbent. The conservation equations that describe the mass transfer process are the conservation equations chemical species in the gaseous phase, 'ya'
For solution of the equation (1), following boundary conditions (Danckwerts) are used: for z = 0 and t > 0,  , and for z = L and t > 0,
, and for z = L and t > 0,  .
.
The conservation equation of mass is:
where the boundary condition associated to equation (2) is for z = 0 and t > 0 , F = Ff = Cfuf
The conservation equation of chemical species 'qa' for the solid phase, considering a system controlled by diffusion in the macropore (Mazzotti and Morbidelli, 1996) is given by
where Kgl is described by  .
.
In this work, the adsorption equilibrium isotherm described by Nitta et al. (1984) and presented by Silva and Rodrigues (1997a) is used.
where n = 5, P0 = 1,  and
and 
The energy conservation equation in the gaseous phase is given by:
where ac=2/Rc. The boundary conditions for the energy conservation equation are: for z = 0 and t > 0,  , and for z = L and t > 0 ,
, and for z = L and t > 0 , .
.
The energy conservation equation in solid phase is:
where Cps is the specific heat of the adsorbent. The initial conditions for solve the equations are: for
t = 0 and " z,F = Ff ,ya = áyañ = áqañ= 0, C = Cf, T = Ts = Tw=Tw(z). The parabolic temperature profile in the wall, Tw=Tw(z), in the initial instant is adopted.
For the numerical solution of the differential equations describing the problem, the control volume method is employed (Patankar, 1980). The basic idea of the control volume formulation is easy to understand and based on direct physical interpretation. The calculation domain is divided into a number of non-overlapping control volumes such that there is a control volume surrounding each grid point. The differential equation is integrated over each control volume. The structured grid for points storage is used. The grid points are placed at the centers of the control volumes, arrangement collocated, and the one-dimensional structured grid is adopted. For the one-dimensional problem under consideration, the interpolation functions of Differencing Central (CDS) and Upwind (UDS), evaluating the variables and derivatives in the control volume faces (Patankar, 1980) are used.
RESULTS AND DISCUSSION
In this work, the separation process of one paraffin using a packed bed with zeolite 5A was simulated. The results obtained by numerical simulation compared favorably with experimental results found in the literature (Silva and Rodrigues, 1997b; Silveira et al., 2002). With these results it was possible to evaluate the partial pressure influence of n-pentane on the column behavior.
The adsorption column used to obtain the experiments data had length 0.2 m and internal diameter of 0.0335 m. The system had apparent specific mass of solid ra = 1130 Kg/m3; bed porosity of eb = 0.32; solid porosity ep = 0.35; solid adsorbent diameter dp = 0.0016 m, and column length L = 0.006 m. The equilibrium parameters of the adsorption isotherm of Nitta et al. (1984) for zeolite 5A, are: DHads,= 13.2 Kcal/gmol; n = 5; qmax = 13.0 g/100g; K0 = 2.04 x 10-5 atm-1. Table 1 presents the operation conditions for n-pentane separation.
The dispersion coefficient, DL, is determined by correlation of Hsu and Haynes (1981),
where Pe is the Peclet number, Re is the Reynolds number and Sc is the Schmidt number.
The parameters utilized in the mathematical model previously described are presented in the Table 2.
The effective thermal conductivity of the bed, KL, is regarded for the correlation of Wakao (1975). The internal coefficient of mass transfer, Ki, is calculated by Glueckauf (1955), Ki = 5Dp/Rp . The external mass transfer coefficient , Ke, is regarded for the correlation of Wakao and Funazkri (1978), Sh = 2.0 + 1.1Re0.6Sc0.33, where Sh is the Sherwood number. The global coefficient mass transfer, Kgl, was defined in the present work.
The heat transfer coefficient in the film between the fluid and the solid, hp, and the heat transfer coefficient between the fluid and the wall of the column, hw, are obtained as described by Silva and Rodrigues (1997a), utilizing adjusts of experimental data. The boundary conditions are in the south face - symmetry condition, in the north face - wall impermeable condition, in the west face - Danckwerts entrance condition and in the east face - Danckwerts outlet condition.
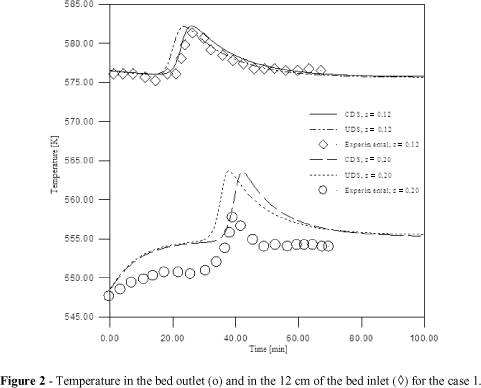
The experimental concentration and temperature profiles obtained by Silva and Rodrigues (1997a) and numerically obtained in the present work are shown in Figs. 1 to 6. Figs. 1, 3 and 5 present the experimental and numerical results of n-pentane molar fraction in the column outlet (breakthrough line). Figs. 2, 4 and 6 show the variation of the temperature in the column outlet and in the position of 12 cm of the entrance, with the time, for 1, 2 and 3 experiments. In these experiments the outflow supply was maintained at 140 mL/min to the 298 K, entrance temperature was maintained 548 K and n-pentane partial pressure was varied as, yaf = 0.19, 0.09 and 0.04, respectively.
The results illustrated in Figs. 1, 3 and 5 showed that when n-pentane partial pressure was increased, the column saturated faster. Analyzing the curves inclination it was possible to conclude that when the partial pressure increased the inclination was larger. Figs. 2, 4 and 6 illustrate the results obtained using the same feed temperature for the three different partial pressures of the n-pentane 0.19; 0.09 and 0.04, respectively. Temperature peaks on the 12 cm of the inlet as on the outlet were larger when the partial pressure was higher. This was justified because the adsorption was favored for the increase of partial pressure, generating an increase in the temperature inside the column. The CDS and UDS interpolation functions had similar performance.
For the breakthrough line illustrated in Figs. 1, 3 and 5 , one could observe that in the case 1 the CDS interpolation function approximated more of the experimental results than that the Upwind scheme. For the case 2, the Upwind interpolation function reproduced the experimental results of the more adequate form. For the case 3, both numerical interpolations obtained a line with the same inclination of the experimental results, but not with the same values. For the temperature profile, the numerical interpolation obtained had good approximation with the experimental results, except for the case 1, that the concordance was not so good, as observed in Figs. 2, 4 and 6 of this work.
The numerical results presented in this work had good approximation with the experimental temperature profile obtained by Silva and Rodrigues (1997a). The maximum error presented in the numerical temperature values for 12 cm of the inlet column were 0.39%, 0.52% and 0.43% and in the outlet of 1.86%, 1.17% and 1.37%, comparatively at the experimental results obtained by Silva and Rodrigues (1997a).
CONCLUSIONS
In this work, the modeling of an adsorption column of fixed bed for the n-pentane separation of a mixture of n-pentane, iso-pentane and nitrogen, considering the system not isothermal and not adiabatic, was presented. The partial pressure influence of the n-pentane (0.19, 0.09 and 0.04) was analyzed in the breakthrough line and in the temperature profile along adsorption column. For each analyzed case, the maximum error obtained in the numerical temperature values for 12 cm of the inlet comparatively at the experimental data was of 0.52%, while that for the outlet temperature in the column was of 1.86%. The developed methodology predicted with good precision the concentration profile of the temperature and chemical species of interest inside of the adsorption column of fixed bed.
Received: September 29, 2004;
Revised: February 25, 2005;
Accepted: March 25, 2005.
- Glueckauf, E. J. (1955), Chem. Soc., 51, 1540.
- Hsu, L. K. P. and Haynes, H. M. (1981), A. I. Ch. E. Journal, 27, 81.
- Mazzotti, G. S. e Morbidelli, M. R., A. (1996), A. I. Ch. E. Journal, 42, 2784.
- Nitta, T., Shigetomi, T., Kuro-Oka, M., Katayama, T. (1984), J. Chem. Eng. Jpn., 17, 39.
- Patankar, S. V. (1980), Numerical Heat Transfer and Fluid Flow Washington, DC : Hemisphere.
- Ruthven, D. M. (1984), Principles of Adsorption and Adsorption Processes, A Wiley - Interscience Publication. New York : John Wiley and Sons, Inc.
- Silva, J. A. C. and Rodrigues A. E. (1997b), Equilibrium and Kinetics of n-Hexane Sorption in Pellets of 5A Zeolite, A.I.Ch.E. Journal, 43, 2524.
- Silva, J. A C. and Rodrigues A E. (1997a), Ind. Eng. Chem. Res., 36, 3769.
- Silveira, S. V. (2001), Remoção de Poluentes Gasosos por Adsorção - Tratamento Numérico. Dissertação de Mestrado, UFSC.
- Silveira, S. V.; Brandão, H. L.; Silva, A.; Silva, E. A. B.; Souza, A. A. U. and Souza, S. M. A. G. (2002), Separação de n-Pentano utilizando uma Coluna de Adsorção. In: Encontro Brasileiro sobre Adsorção, Rio de Janeiro. Anais... Rio de Janeiro.
- Wakao, N. and Funazkri, T. (1978), Chem. Eng. Sci., 33, 1375.
- Wakao, N. (1975), Particle-to-fluid transfer coefficients and fluid diffusivities at low flow rate in packed beds, Chem. Eng. Sci., 31, 1115.
Publication Dates
-
Publication in this collection
15 Aug 2005 -
Date of issue
June 2005
History
-
Accepted
25 Mar 2005 -
Reviewed
25 Feb 2005 -
Received
29 Sept 2004