Abstracts
Studies were carried out on anatomic aspects of the stigma and style of yellow passionfruit. The stigmatic style consisted of papillae, many layers of sub-epidermal cells and a central transmitting tissue. The stigma was of dry, unbranched, multicellular multiseriate papillate type and the cells were highly vacuolated with walls that were relatively thin consisting of compactly arranged fibrils. The style was solid with a central core of transmitting tissue that had intercellular spaces containing a matrix that included polysaccharidic and lipidic substances. During development, cells of the transmitting tissue were arranged compactly while the mature pistil showed cells with a little more loosely arranged intercellular spaces. Both the mature stigma and style contained a peripheral cytoplasmic layer showing a cytoplasmic profile with a prominent nucleus containing a nucleolus, evenly distributed mitochondria, extensive RER, ribosomes and amylochloroplasts.
Yellow passionfruit; anatomy; stigma and style; histochemistry; cytochemistry
Foram realizadas observações sobre aspectos anatômicos do estigma e estilo de maracujazeiro amarelo, uma vez que estudos detalhados não têm sido encontrados em literatura recente. O estigma é seco, não-ramificado com papilas multiseriadas e multicelulares, com células altamente vacuoladas e parede celular relativamente fina. O estilo é sólido apresentando no centro o tecido de transmissão com espaços intercelulares contendo uma matriz que inclui polissacarídeos e substâncias lipídicas. Durante o desenvolvimento, células jovens do tecido de transmissão não apresentam espaços intercelulares, ao contrário do pistilo maduro. O estigma e estilo contêm citoplasma periférico apresentando um perfil citoplasmático com núcleo e um nucléolo, mitocôndrias distribuídas espaçadamente, extenso retículo endoplasmático rugoso, ribossomos e amiloplastos.
BIOLOGICAL AND APPLIED SCIENCES
Structural, hystochemical and cytochemical characteristics of the stigma and style in Passiflora edulis f. flavicarpa (Passifloraceae)
Margarete Magalhães SouzaI, * * Author for correspondence ; Telma Nair Santana PereiraII; Angelo José Burla DiasIII; Beatriz Ferreira RibeiroIII; Alexandre Pio VianaII
IDepartamento de Ciências Biológicas; Universidade Estadual de Santa Cruz (UESC); Km. 16 Rodovia Ilhéus-Itabuna; souzamag@ig.com.br; 45650-000; Ilhéus - BA - Brasil
IICentro de Ciências e Tecnologias Agropecuárias; Laboratório de Melhoramento Genético Vegetal
IIICentro de Biociências e Biotecnologias; Laboratório de Biologia Celular e Tecidual; Universidade Estadual do Norte Fluminense Darcy Ribeiro (UENF); Campos dos Goytacazes - RJ - Brasil
ABSTRACT
Studies were carried out on anatomic aspects of the stigma and style of yellow passionfruit. The stigmatic style consisted of papillae, many layers of sub-epidermal cells and a central transmitting tissue. The stigma was of dry, unbranched, multicellular multiseriate papillate type and the cells were highly vacuolated with walls that were relatively thin consisting of compactly arranged fibrils. The style was solid with a central core of transmitting tissue that had intercellular spaces containing a matrix that included polysaccharidic and lipidic substances. During development, cells of the transmitting tissue were arranged compactly while the mature pistil showed cells with a little more loosely arranged intercellular spaces. Both the mature stigma and style contained a peripheral cytoplasmic layer showing a cytoplasmic profile with a prominent nucleus containing a nucleolus, evenly distributed mitochondria, extensive RER, ribosomes and amylochloroplasts.
Key words: Yellow passionfruit, anatomy, stigma and style, histochemistry, cytochemistry
RESUMO
Foram realizadas observações sobre aspectos anatômicos do estigma e estilo de maracujazeiro amarelo, uma vez que estudos detalhados não têm sido encontrados em literatura recente. O estigma é seco, não-ramificado com papilas multiseriadas e multicelulares, com células altamente vacuoladas e parede celular relativamente fina. O estilo é sólido apresentando no centro o tecido de transmissão com espaços intercelulares contendo uma matriz que inclui polissacarídeos e substâncias lipídicas. Durante o desenvolvimento, células jovens do tecido de transmissão não apresentam espaços intercelulares, ao contrário do pistilo maduro. O estigma e estilo contêm citoplasma periférico apresentando um perfil citoplasmático com núcleo e um nucléolo, mitocôndrias distribuídas espaçadamente, extenso retículo endoplasmático rugoso, ribossomos e amiloplastos.
INTRODUCTION
The genus Passiflora L. originated in tropical South America with its major center of geographical distribution in central-north of Brazil (Bruckner, 1994). Yellow passionfruit (Passiflora edulis Sims f. flavicarpa Degener) is an allogamous plant mainly due to its floral morphology and self-incompatibility of sporophytic type (Bruckner et al., 1995) being cultivated for its edible fruits. It occurs naturally in Brazil being a important domestically consumed crop marketed in 2000 amounted 30.992 thousand tons (FNP, 2002) and still is selected for genetic improvement (Meletti et al., 2000; Viana, 2001).
In yellow passion fruit, there are three or four styles that are united towards their base and in the top there are free stigmas (Vanderplank, 1991) that belong to the group of genus with a dry papillate stigma (Heslop-Harrison and Shivanna, 1977). In general, in the style the pollen tubes grow through an extracellular matrix that either lines a hollow channel or is secreted between style cells that make up a solid transmitting tract. The composition of this complex matrix may include lipids, proteins, carbohydrates, and small molecules, which are believed to provide the signals that are critical for directing and supporting pollen tube growth (Wilhelmi and Preuss, 1997). These interactions can be as complex as protein-protein interactions, like ligand-protein kinase signaling, or can involve molecules as simple as water, calcium, lipids and sugar (Gaude and McCormick, 1999).
Although the floral biology of yellow passion fruit has been studied (Ruggiero, 1973), detailed studies have not been carried out on the structure of stigma. This work was part of initial studies on anatomic characteristics of flowers in passionfruit, describing its ultrastructural and some hystochemical and cytochemical characteristics.
MATERIALS AND METHODS
Plants of yellow passion fruit (Passiflora edulis f. flavicarpa Deg.) grown in the Universidade Estadual do Norte Fluminense greenhouse - Brazil - were used. For optical microscopic studies, unpollinated pistils were fixed in 2.5 % glutaraldehyde and 4% formaldehyde in 0.1 M cacodylate buffer pH 7.2 for an hour at room temperature, dehydrated through an ethanol series and embedded in Spurr's resin (Spurr, 1969). Semi-thin sections (2 µm) were cut with diamond knife and used to localize lipoidal material with Sudan black (Jensen, 1962), pectic substances with Ruthenium red (Chamberlain, 1932 modified by Kraus and Arduin, 1997), and total insoluble polysaccharides with periodic acid-Schiff reagent (PAS) (O'Brien and McCully, 1981). PAS is selective for carbohydrate residues with vicinal-hydroxyl groups, which in the first step is oxidized to aldehyde groups by periodic acid, and then reacts with Schiff's reagent to give a pink-colored complex (Hotchkiss, 1948; McManus, 1948).
For transmission electron microscopy, the glutaraldehyde and formaldehyde fixed pistils were post-fixed in 1 % osmium tetroxide, dehydrated through an ethanol series and embedded in Spurr's resin. Sections were cut using a diamond knife and stained with uranyl acetate and lead citrate for 10 min and 3 min, respectively. Other sections were collected on gold grids and treated with periodic acid-thiocarbohydrazide-silver proteinate (Thiéry, 1967) to detect polysaccharides. All sections were cut on the Reichert Ultracut S ultramicrotome. Semi-thin sections were examined with Olympus B202 optical microscope and ultrathin sections were examined with a Zeiss 900 electron microscope at 60 kV.
RESULTS
The style
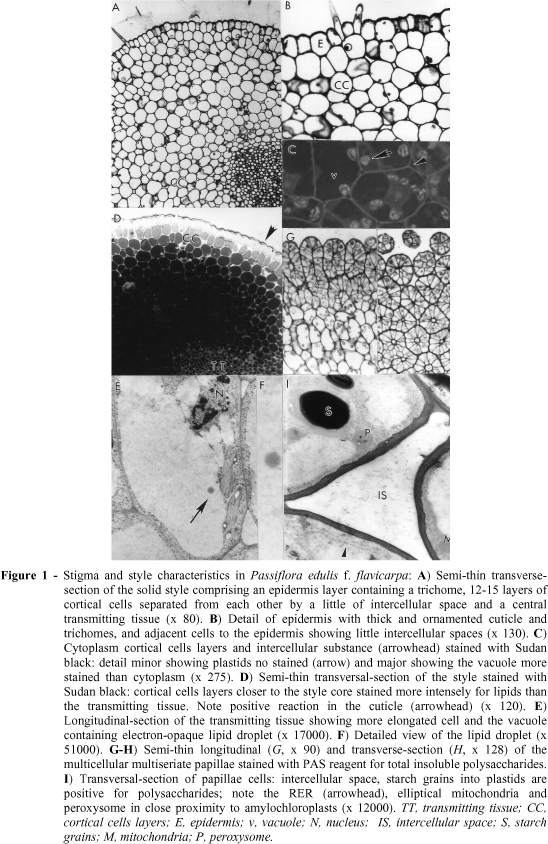
The style was anatomically characterized as solid type. It comprised a single epidermis layer with thick and ornamented cuticule and some trichomes, a parenchymatic tissue (cortical cells layers) and a central transmitting tissue (Fig. 1, A). Adjacent to the epidermis, there were 12-15 layers of cortical cells separated from each other by a little of intercellular space (Fig. 1, B). The volume of the cortical cells was occupied by a large vacuole and the cytoplasm contained amylochloroplasts (Fig. 1, C) with a high quantity of starch grains. The cells of the transmitting tissue with thin walls appeared roundish in transverse sections (Fig. 1, D) and elongated in longitudinal sections (Fig. 1, E) and its walls reacted positively to test for polysaccharides. Positive staining for pectin was notably absent. Cortical cells layers closer to the style core and intercellular spaces stained more intensely for lipids than the transmitting tissue (Fig. 1, D).
The cytoplasm of transmitting tissue cells contained only a very reduced set of RER composed of one type of cistern seen in longer profiles with a narrow lumen, preferentially located in the peripheral cytoplasmic region of cell with amylochloroplasts; dictyosomes and mitochondria were present, but few in number, and ribosomes were observed (not demonstrated). The vacuole showed electron-opaque lipid droplet randomly distributed (Fig. 1, E-F). The defined well nucleus had one nucleolus with a chromatin strongly electron-dense. The young cells of the transmitting tissue were arranged compactly without any intercellular spaces and there was small vacuoles distributed randomly while the mature pistil showed a little more loosely arranged cells, with intercellular spaces, with medium electron-dense secretion products; vacuoles fused to form only one that full the centre of cell. At ultrastructural level the intercellular substance reacted positively to test for polysaccharides. Plasmodesmata were not found.
The stigma
The stigma was dry and papillate. The papillae were unbranched, originated from the epidermis and densely arranged over all heart-shaped stigma. They were relatively short and uniform in diameter, multicellular multiseriate (Figs. 1, G-H). The cell walls were relatively thin consisting of compactly arranged fibrils (Fig. 1, I).
The cytoplasm was confined to the lateral part of the papillae. The major part of the cell cytoplasm was made up of large vacuole that frequently showed some lightly stained contents (Fig. 1, I). Papillae peripheral cytoplasmic layer showed a cytoplasmic profile with a nucleus containing a nucleolus, evenly distributed elliptical mitochondria, extensive rough endoplasmic reticulum (RER), ribosomes, amylochloroplasts with a well developed, thylakoid system containing up to six large starch grains and peroxysomes in close proximity to amylochloroplasts. Dictyosomes were observed in an inactive state, since their cistern-endings were not swollen and associated vesicles were not present. The stigma reacted negatively to test for pectin. The cell walls and starch grains into plastids reacted positively for polysaccharides, so that the reaction was visualized by electron-dense for Thiéry and grey to black intensity for PAS (Fig. 1, E-F).
DISCUSSION
Ultrastructural details of the stigma have not yet been described for Passiflora. Contrary to the classification by Heslop-Harrison and Shivanna (1977), yellow passionfruit had papillae stigma, which was multicellular multiseriate unlike described for the genus Passiflora (unicellular papillae). There was an enormous inter- and intra-specific genetic variability in the genus Passiflora (Ferreira, 1994) and in the family Passifloraceae hence variability reflected in the stigma morphology like the genus Adenia that has non-papillate stigma surface (Heslop-Harrison and Shivanna, 1977). The relationship among stigma characteristics and the type of mechanisms involved in the self-incompatibility system was studied by Heslop-Harrison et al. (1975). In solid-styled sporophytic self-incompatible species, the pollen tubes were arrested on the stigma. In yellow passion fruit, two inhibition sites of pollen-tube growth, stigma and style, were identified (Rêgo et al., 2000).
Dry stigma has an important biological role in the sporophytic self-incompatibility system because it permits an cell-by-cell interaction between pollen and stigma-cell, as occurring in yellow passion fruit (Rêgo et al., 2000), while the papillae has an adaptive significance by increasing the capturing area even though it reduces the opportunity for interaction by ensuring that pollen surface materials cannot be distributed too freely on the stigma surface from pollen grains (Heslop-Harrison and Shivanna, 1977).
In plants, sugar functions as a metabolic resource (Jang and Sheen, 1997), hence information concerning the sugar status of plant cells is of great importance during all stages of the plant life cycle (Graham, 1996), but sugar is also important regulators of many processes involving repression and activation of many genes (Jang and Sheen, 1997). In P. edulis f. flavicarpa, the intercellular substances of stigma and style were rich in polysaccharides. Intercellular substances of the other dry stigma species are generally rich in polysaccharides, pectins (Ciampolini et al., 1990) and protein (Sedgley and Buttrose, 1978). Lipid storage in the wall is a common event in plants. However, in P. edulis f. flavicarpa it was seen mainly in cytoplasm of the parenchymatic and transmitting tissues and intercellular spaces. Lipids are the essential factor needed for pollen tubes to penetrate the stigma directing pollen-tube growth by controlling the flow of water to pollen in species with wet stigmas, while the pollen coat is functionally equivalent to the exudate in species with dry stigmas (Wolters-Arts et al., 1998). The presence of lipids in Passiflora edulis f. flavicarpa is most possibly linked to reserve substance function than in pollen-pistil interaction.
The scanty cytoplasm was observed in the cells of papillae and transmitting tissue where the cytoplasm was occupied by a large vacuole as in Corylus avellana L. (Ciampolini and Cresti, 1998) unlike the cells of the transmitting tissue of other species such as Primula vulgaris (Heslop-Harrison et al., 1981), both solid-styled. Although some dictyosomes were seen in an inactive state, since their cistern-endings were not swollen and associated vesicles were completely absent, the thin cytoplasm was active with many amylochloroplasts containing starch grains, ribosomes and extensive RER assenting the fact that they were associated with the polysaccharides synthesis and secretion. Polysaccharides are necessary for pollen tube growth (Ciampolini and Cresti, 1998). During this process the pistil plays an essential role in the production of compounds necessary for recognition, guidance, protection, and nourishment of the pollen tubes (Kao and McCubbin, 1996).
ACKNOWLEDGEMENTS
We thank Márcia Adriana S.C. Dutra and Arthur Rodrigues, CBB/UENF Photographic Laboratory technicians for help in developing and amplifying the photographs for this article. This research was supported by FENORTE and FAPERJ.
Received: February 27, 2004;
Revised: September 22, 2004;
Accepted: August 05, 2005.
- Bruckner, C. H. (1994), Autoincompatibilidade em maracujazeiro. In: São José, A. R. (Ed.). Maracujá: Produção e Mercado Universidade Estadual da Bahia, Bahia. pp. 6-18.
- Bruckner, C. H.; Casali, V. W. D.; Moraes, C. F.; Regazzi, A. J. and Silva, E. A. M. (1995), Self-incompatibility in passion fruit (Passiflora edulis Sims). Acta Hortic., 370, 45-57.
- Chamberlain, C. J. (1932), Methods in plant histology Chicago: The University of Chicago Press.
- Ciampolini, F. and Cresti, M. (1998), The structure and cytochemistry of the stigma-style complex of Corylus avellana L. "Tonda gentile delle langhe" (Corylaceae). Ann. Bot., 81, 513-518.
- Ciampolini, F.; Shivanna, K. R. and Cresti, M. (1990), The structure and cytochemistry of the pistil of Sternbergia lutea (Amaryllidaceae). Ann. Bot., 66, 703-712.
- Ferreira, F. R. (1994), Germoplasma de Passiflora no Brasil. In: São José, A. R. (Ed.). Maracujá: Produção e Mercado Universidade Estadual da Bahia, Bahia. pp. 24-26.
- FNP Consultoria e Comércio (2002), Agrianual. São Paulo: FAPESP.
- Gaude, T. and McCormick, S. (1999), Signaling in pollen-pistil interactions. Semin. Cell Dev. Biol., 10, 139-147.
- Graham, I. A. (1996), Carbohydrate control of gene expression in higher plants. Res. Microbiol., 147, 572-580.
- Heslop-Harrison, J.; Heslop-Harrison, Y. and Barber, J. (1975), The stigma surface in incompatibility responses. Proc. R.. Soc. Lond. B. Biol. Sci., 188, 287-297.
- Heslop-Harrison, Y. and Shivanna, K. R. (1977), The receptive surface of the angiosperm stigma. Ann. Bot., 41, 1233-1258.
- Heslop-Harrison, Y.; Heslop-Harrison, J. and Shivanna, K. R. (1981), Heterostyly in Primula1. Fine-structural and cytochemical features of the stigma and style in Primula vulgaris Huds. Protoplasma 107, 171-187.
- Hotchkiss, R. D. (1948), A microchemical reaction resulting in the staining of polysaccharide structures in fixed tissue preparation. Arch. Biochem., 16, 131-141.
- Jang, J. C. and Sheen, J. S. (1997), Sugar sensing in higher plants. Trends Plant Sci., 2, 208-214.
- Jensen, W. A. (1962), Botanical histochemistry London: WH Freeman and Co.
- Kao, T. H. and McCubbin, A. G. (1996), How flowering plants discriminate between self and non-self pollen to prevent inbreeding. Proc. Natl. Acad. Sci., 93, 12059-65.
- Kraus, J. E. and Arduin, M. (1997), Manual básico de métodos em morfologia vegetal Editora Universidade Rural, Seropédica.
- McManus, J. F. A. (1948), Histological and histochemical uses of periodic acid. Stain Technol., 23, 99-108.
- Meletti, L. M. M.; Santos, R. R. and Minami, K. (2000), Melhoramento do maracujazeiro-amarelo: obtenção do cultivar 'COMPOSTO IAC-27'. Sci. Agric., 57, 491-498.
- O'Brien, T. P. and McCully, M. E. (1981), The study of plant structure: principles and selected methods Victoria: Termarcarphi.
- Rêgo, M. M.; Rêgo, E. R.; Bruckner, C. H.; Da Silva, E. A. M.; Finger, F. L. and Pereira, K. J. C. (2000), Pollen tube behavior in yellow passion fruit following compatible and incompatible crosses. Theor. Appl. Genet., 101, 685-689.
- Ruggiero, C. (1973), Estudos sobre floração e polinização no maracujá amarelo (Passiflora edulis f. flavicarpa). Doctorate Thesis, Universidade Estadual de São Paulo, Jaboticabal, SP.
- Sedgley, M. and Buttrose, M. S. (1978), Structure of the stigma and style of the avocado. Aust. J. Bot., 26, 667-682.
- Spurr, A. R. (1969), A low viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res., 26, 31-43.
- Thiéry, J. P. (1967), Mise en evidence des polysaccharides sur coupes fines en microscopie éléctronique. J. Microsc., 6, 987-1018.
- Vanderplank, J. (1991), Passion flowers London: Cassel Publishers Limited.
- Viana, A. P. (2001), Correlações e Parâmetros genéticos em populações de maracujazeiro amarelo (Passiflora edulis f. flavicarpa) e diversidade molecular no gênero Passiflora. Doctorate Thesis, Universidade Estadual do Norte Fluminense, Campos dos Goytacazes, RJ.
- Wilhelmi, L. K. and Preuss, D. (1997), Pollen tube guidance in flowering plants. Plant Physiol., 113, 307-312.
- Wolters-Arts, M.; Lush, W. M. and Mariani, C. (1998), Lipids are required for directional pollen-tube growth Nature, 392, 818-821.
Publication Dates
-
Publication in this collection
10 Apr 2006 -
Date of issue
Jan 2006
History
-
Received
27 Feb 2004 -
Reviewed
22 Sept 2004 -
Accepted
05 Aug 2005