Abstracts
The present work focuse on the impact of O2, CO2 and ethylene concentrations on ripening rate control of bananas as a contribution for the development of domestic equipments that could allow the user to drive the fruit shelf live. It represented the adjustment of metabolic activity rates in order to manage the maturity process. Ripening variables such as ethylene and CO2 concentrations and temperature were adjusted to accelerate or slow down the process, while the maturity degree was monitored through the physical and chemical parameters and sensorial analysis. Therefore, the objective of this work was to evaluate the influence of these parameters to manage the banana ripening. The optimum temperature was at 25 ºC of storage. The presence of oxygen, CO2 withdraws and ethylene injection were relevant for the ripening process. The "ready-to-eat" quality was achieved in 6 days in confined system. The use of ethylene as trigger was adequate to accelerate the ripening process with advantages in fruit color.
banana; ripening; trigger; ethylene; CO2
O presente trabalho foca no impacto da concentração de O2, CO2 e etileno no controle da taxa de amadurecimento de bananas, como contribuição para o desenvolvimento de equipamentos domésticos que permitam o controle pelo próprio usuário da vida de prateleira de frutas. Isto representa o ajuste das atividades metabólicas para garantir o controle do amadurecimento. Variáveis como concentração de etileno e CO2 e temperatura foram ajustadas para acelerar ou reduzir o processo, enquanto que o grau de maturação foi monitorado através de parâmetros físico-químicos e sensoriais. Desta forma, o objetivo deste trabalho foi avaliar a influência destes parâmetros para controlar o amadurecimento de banana. A temperatura ótima de amadurecimento foi 25ºC. A presença de O2, a retirada de CO2 e a injeção de etileno foram relevantes no processo. A qualidade "pronto-para-consumo" foi obtida em 6 dias em sistema confinado. O uso de etileno como gatilho é adequado para acelerar o amadurecimento, com vantagens para a cor do produto.
FOOD SCIENCE AND TECNHOLOGY
Study of banana (Musa aaa Cavendish cv Nanica) trigger ripening for small scale process
Fábio Donato Soares LarotondaI; Aziza Kamal GenenaI; Daniela DantelaII; Hugo Moreira SoaresI; João Borges LaurindoI; Regina Fátima Peralta Muniz MoreiraI; Sandra Regina Salvador FerreiraI,* * Author for correspondence
IDepartamento de Engenharia Química e Alimentos; Universidade Federal de Santa Catarina; C. P.: 476; sandra@enq.ufsc.br; 88040-900; Florianópolis - SC - Brasil
IIMultibrás S. A. Eletrodomésticos; Joinville - SC - Brasil
ABSTRACT
The present work focuse on the impact of O2, CO2 and ethylene concentrations on ripening rate control of bananas as a contribution for the development of domestic equipments that could allow the user to drive the fruit shelf live. It represented the adjustment of metabolic activity rates in order to manage the maturity process. Ripening variables such as ethylene and CO2 concentrations and temperature were adjusted to accelerate or slow down the process, while the maturity degree was monitored through the physical and chemical parameters and sensorial analysis. Therefore, the objective of this work was to evaluate the influence of these parameters to manage the banana ripening. The optimum temperature was at 25 ºC of storage. The presence of oxygen, CO2 withdraws and ethylene injection were relevant for the ripening process. The "ready-to-eat" quality was achieved in 6 days in confined system. The use of ethylene as trigger was adequate to accelerate the ripening process with advantages in fruit color.
Key-words: banana, ripening, trigger, ethylene, CO2
RESUMO
O presente trabalho foca no impacto da concentração de O2, CO2 e etileno no controle da taxa de amadurecimento de bananas, como contribuição para o desenvolvimento de equipamentos domésticos que permitam o controle pelo próprio usuário da vida de prateleira de frutas. Isto representa o ajuste das atividades metabólicas para garantir o controle do amadurecimento. Variáveis como concentração de etileno e CO2 e temperatura foram ajustadas para acelerar ou reduzir o processo, enquanto que o grau de maturação foi monitorado através de parâmetros físico-químicos e sensoriais. Desta forma, o objetivo deste trabalho foi avaliar a influência destes parâmetros para controlar o amadurecimento de banana. A temperatura ótima de amadurecimento foi 25ºC. A presença de O2, a retirada de CO2 e a injeção de etileno foram relevantes no processo. A qualidade "pronto-para-consumo" foi obtida em 6 dias em sistema confinado. O uso de etileno como gatilho é adequado para acelerar o amadurecimento, com vantagens para a cor do produto.
INTRODUCTION
Banana (Musa acuminata cv. Cavendish) is a commercially significant tropical fruit with innumerable varieties (Prabha and Bhagyalakshmi, 1998). Ten of the major banana producing countries accounted for about 75% of world production in 2003, whereas India, Ecuador, Brazil and China provided almost 50% of the total production in that year (Zhang et al., 2005).
For more than 70 years, the presence or absence of a climacteric increase in respiration during ripening has been used to classify the fruits. However, it has been known for some time that certain features of the ripening process can be separated from the climacteric. Particularly, the timing of color development in relation to the climacteric peak has been altered for several fruits (Laties, 1995; Bower et al, 2002). In climacteric fruits, ethylene (C2H4) is produced at different rates based on the stage of fruit development. The fruits are characterized by a low ethylene production during the pre-climacteric period (unripe or green fruit), followed by a climacteric phase where a sudden increase in the ethylene production takes place during ripening, a phenomenon called autocatalytic C2H4 production (Abeles, 1973). This rise in ethylene concentration is considered the main factor for the ripening of fruits such as banana, avocado, tomato and melon (Bower et al., 2002). After this step, the C2H4 production decreases considerably in the post-climacteric phase (Hoffman and Yang, 1980). Thus, the post-harvest physiology is characterized by the pre-climacteric phase, followed by a sudden increase in the ethylene production, signaling the beginning of ripening, and it is represented by a strong rise in the respiration activity (Palomer et al., 2005). Some modifications during ripening include changes in peel color and pulp texture, conversion of starch into sugar, reduction of polyphenols and synthesis of aromatic compounds and others (Clendennen and May, 1997; Chen and Ramaswamy, 2002).
Although banana fruit can be harvested at a wide range of physiological ages, it still achieves high quality after ripening process with ethylene application. The C2H4 performs an important role during the ripening by stimulating the development of color, texture, aroma and flavor and by reducing the ripening variability (Moya-León and Herrera, 2004; Adkins et al., 2005; Palomer et al., 2005). Treatments using as low as 0.1 mg l-1 of ethylene induces the climacteric stage in banana (Liu, 1976), but in commercial treatments, the use of 100 to 1000 mg l-1 is common (Inaba and Nakamura, 1986; Ke and Tsai, 1988; Stover and Simmonds, 1993).
Because the storage atmosphere can affect the fruits durability (Palomer et al., 2005; Burdon et al., 2005), the ideal conditions of controlled and modified atmosphere are related to the species, degree of ripening, temperature and period of exposure (Kader et al., 1989; Cameron et al., 1995; Pesis, 2005). Generally, a combination of low O2, high CO2 concentration and low temperature is applied (Henig, 1975; Kader et al., 1989; Riquelme et al., 1994). High levels of CO2 can inhibit ethylene-dependent processes, such as autocatalytic ethylene production, and therefore, the ripening of climacteric fruits. The CO2 levels necessary for this inhibition are much higher than the atmospheric level (Mathooko, 1996; Wild et al., 2005), and several authors have shown that the exposure of banana to an atmosphere with high CO2 concentration can suppress the climacteric respiration (Young et al., 1962; Liu et al., 2004; Wild et al., 2005). Besides the control of CO2 concentration, the maintenance of low O2 concentration delays the respiration increase, peel de-greening and starch conversion into sugar (Marriot, 1980; Kanellis et al., 1989; Marchal, 1998).
Although the banana ripening process with ethylene is well known and established, this paper focuses on the evaluation of banana ripening in order to contribute to development of new equipments able to manage and improve fruit shelf-life at small scales. Therefore, the influence of parameters such as CO2 and C2H4 concentration and temperature were evaluated during banana ripening. Furthermore, a set of "start up assays" were performed to evaluate the effect of the ethylene as a trigger factor for the ripening process of confined bananas. Therefore, this work evaluated the ripening control process at domestic scale, where costs analysis would be a further investigation.
MATERIALS AND METHODS
Material
Banana (Musa AAA Cavendish cv Naninca) was purchased from local wholesale market in an unripe stage (greenish) defined by Munsell® color chart (GretagMacbeth, USA) and without previous ethylene treatment.
Ripening cells.
The ripening cells (Fig. 1) were assembled in 2.5 liter acrylic flasks (A), where some accessories were adapted: a fan (B), for homogenization; a septum (C), to collect the gas samples; and inlet and outlet valves (D), to flush the flask and inject the gas mixture for the controlled atmosphere. Initially, a vacuum pump was used to remove the air inside the flasks. Then, the flasks were flushed for 5 minutes with the pre-established atmosphere to create the gas composition inside the ripening cells and to maintain the atmospheric pressure ensured by a hydraulic seal. This procedure was applied for all the experiments that used the injection of ethylene gas inside the ripening cells.
Experimental conditions
The study of the banana ripening control was developed by three classes of experiments: (1) standard assays, (2) ripening control assays, with different process parameters, and (3) start up or trigger assays:
1. The standard experiments were conducted placing the fruits inside a controlled temperature chamber (Brastemp, Brazil) without external supply of ethylene, oxygen and carbon dioxide (no atmosphere control). Fruit samples were collected daily from the chamber to perform sensorial and characterization analysis.
2. Three parameters were evaluated in ripening control assays: ethylene treatment, carbon dioxide treatment and ripening temperature. For the ethylene treatment, the concentration range of ethylene injected in the ripening cells was defined according to literature values considering the goal of this work to evaluate the ethylene influence in banana ripening process (Inaba and Nakamura, 1986; Ke and Tsai, 1988; Stover and Simmonds, 1993; Domínguez and Vendrell, 1994). The levels were established as: without ethylene (0 mg l-1), medium (100 mg l-1) and high (200 mg l-1) concentration with air in balance. Although the conditions of 100 mg l-1 in air and 200 mg l-1 in air were above the concentration to saturate the ethylene response, the levels were defined to observe the extreme effect of ethylene, for further use as trigger. The mixtures were acquired from White Martins (Air products, Brazil). Carbon dioxide treatments were conducted placing a CO2 absorbent material  baking soda with saturation indicator (brand) (Wilson, Brazil)  inside the cells. The CO2 absorption by baking soda was cited by Ohm et al. (1991) and consisted essentially of NaOH in controlled humidity to maximize its absorption capacity. Ethyl violet was used as indicator, measuring the absorption capacity by color modification. The influence of temperature on the ripening process was evaluated at 15, 25 and 32 ºC.
3. Finally, the "start up" assays were performed to observe the trigger effect of the ethylene during the ripening process. The experiments were conducted with or without C2H4 injection to adjust the initial atmosphere inside the cells and to evaluate its trigger effect. In the early 48 h of "closed ripening", O2 was injected daily, controlled with gas chromatography analysis, to ensure the supply for fruit respiration. The cells were then opened and remained inside the temperature controlled chamber but without atmosphere adjustment.
All the experiments were carried out for 7 days to analyze the ripening development over time in relation to changes in the process parameters. The ripening cells were supplied with oxygen injection to ensure the amount necessary for banana ripening. Table 1 shows the experimental conditions used in this study.
Analysis for product characterization
Weight loss
The weight loss of the fruit was evaluated by weight the fruit submitted to controlled conditions. Weight loss mainly indicates a water content reduction, related to the juiciness, flavor, aroma and other important economic characteristics.
The fruit weight was determined using a scale with a precision of ± 0.01g (Gehaka, Model BG-2000 - Brazil). Flesh Firmness: The fruit firmness was analyzed using a manual penetrometer (Mark TR Turoni  FT 327, Italy) and, in order to avoid the influence of the fruit peel, the firmness measurements were taken after the removal of a 2 cm peel disc. Color: The Munsell® color chart (GretagMacbeth, USA) was used to define the color standards during the subjective color test evaluation. The color was observed daily for each fruit for all ripening procedures during the tests. The analysis of fruit pH was carried out using a digital pH meter (Analion Mark, Model AN2000 - Brazil), with a special probe for the solid and semisolid materials. The probe was placed inside the fruit until the stabilization of the pH measurement value.
Gas chromatography analysis
The chromatographic analysis was performed using a CG35  CG Scientific Instruments (Brazil) with a thermal conductivity detector (TCD), equipped with Porapak Q (to analyze CO2 and C2H4), and Molecular sieve 5 A (to analyze O2) columns. Helium was used as the carrier gas at a flow rate of 30 mL/min. The column was held at 70 ºC and the detector at 100 ºC. Standard gas mixtures (White Martins, Brazil) containing 100 mg l-1 and 200 mg l-1 C2H4 (synthetic air in balance) were used, as well as CO2 (3%) (He in balance).
Sensorial analyses
Sensorial analyses of the fruit were performed in order to evaluate the efficiency of the ripening experiments in terms of quality. The "Characteristics Profile Test" was the methodology used for the sensorial analysis. The taste panel consisted of 10 trained individuals who were requested to evaluate the overall color, aroma and flavor of representative samples after storage. The attributes were rated using an Hedonic scale from 1 to 5, where 1 was equal to very bad, 2 was poor, 3 was fair, 4 was good and 5 was excellent. For comparison, Cavendish bananas were obtained daily from a retail outlet, without ripening induction by modified atmosphere and at a maturity stage indicated by the Munsell® chart as "ready-to-eat". These were included in the test as hidden reference. Each sample was served on a white plate, which reduced the likelihood of the background color affecting the perception of color. Presentation of samples was randomized among the panelists and sessions. Evaluations were conducted in isolated sensorial booths, illuminated under the standard conditions.
RESULTS
Table 2 shows the results for weight loss, firmness and pH values for the samples analyzed in the ripening assays. Table 3 gives the results for the gas composition inside the ripening cells. Table 4 shows the sensorial analysis results, with values for color, aroma and flavor. All Tables presents the average values obtained with their standard deviation.
Influence of ethylene concentration
Experiments 2, 5, 7 and 8
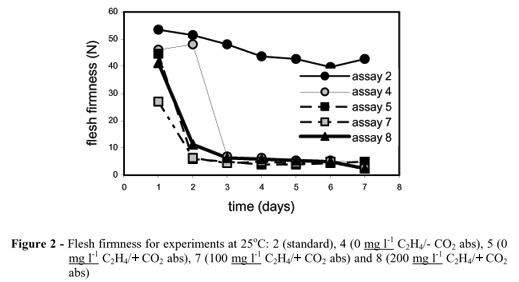
The influence of ethylene concentration on banana ripening process was evaluated comparing assays 2, 5, 7 and 8: standard, 0, 100 and 200 mg l-1, respectively. The tests were carried out at 25 ºC using CO2 absorbent (assays 5, 7 and 8).
For 200 mg l-1 ethylene atmosphere (assay 8: Table 3), the saturation of the CO2 absorbent was detected between day 3 and day 4 when the CO2 concentration in the cells increased from 0.15 to 19.39% due to increase in ripening rate caused by the rising of the ethylene concentration, from 795 mg l-1 (day 2) to 3069 mg l-1 (day 3). For 0 mg l-1 ethylene injection (assay 5), the saturation of the CO2 absorbent was observed on day 6 (11.17%). These results required the increase in the amount of CO2 absorbent material in the cells, procedure followed in the following assays. However, additional studies would be necessary to define the minimal mass of absorbent necessary to avoid the interference of CO2 during the ripening process.
The results for product characterization analysis (Table 2) indicated similar behavior between the ripening evolution of bananas in the cells with and without ethylene, except for the firmness parameter, as shown in Figure 2 for assays 2, 5, 7 and 8. The results indicated a poor ripening degree for the standard samples that gave on day 7 a flesh firmness of 42.66 N, while the average for the other three assays was 3.27 ± 0.14 N.
The results for the sensorial analyses (Fig.s 3 and 4) showed that the confined samples (assays 5, 7 and 8) had a behavior close to the ready-to-eat samples in terms of flavor and aroma, if compared with the standard samples (assay 2). Besides, based on the behavior of assay 2, far from ready-to-eat samples, the sensorial and physicochemical analyses confirmed the importance of ethylene for fruit quality, either the one produced by the fruitduring ripening, or the one injected in the cells for atmosphere adjustment (assays 5, 7 and 8).
Sensorial analysis indicated that the samples collected at day 6 were at better ripening stage. The flavor and aroma development were not affected by the C2H4 concentration injected in the during ripening, or the one injected in the cells for atmosphere adjustment (assays 5, 7 and 8).
Sensorial analysis indicated that the samples collected at day 6 were at better ripening stage. The flavor and aroma development were not affected by the C2H4 concentration injected in the cells and the results were related to the high ethylene production by the fruits, when compared with the quantities injected.
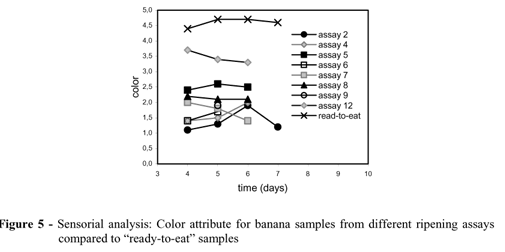
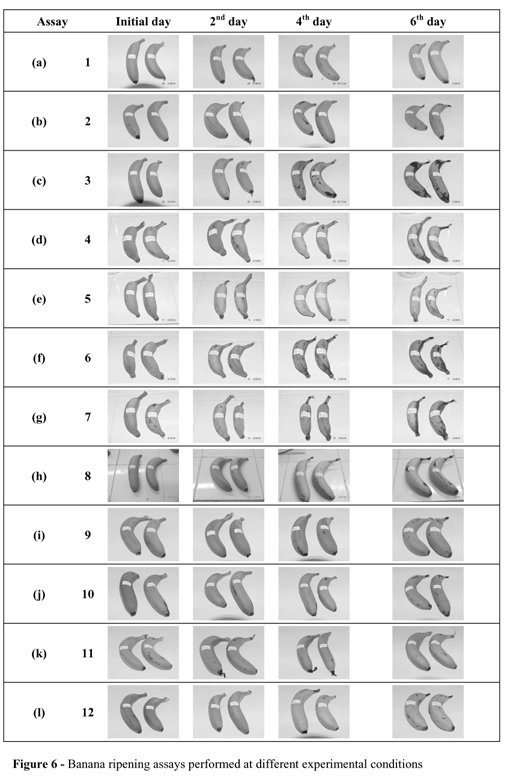
The peel color, shown in Figures 5 and 6 (b, e, g and h), for the samples collected in days 2, 4 and 6, presented better development in assay 5, compared to assays 2, 7 and 8, indicating the low influence of ethylene concentration in peel color. The behavior of the color characteristic, apart from other quality attributes, has also been observed for several fruits (Laties, 1995; Bower et al, 2002).
Experiments 4 and 6
For the injection of 100 mg l-1 of ethylene inside the ripening cells, the maximum production of CO2 associated with the respiration rate occurred at day 2 (24.94% CO2), while for the test without ethylene injection, the peak of respiration was observed at day 5 (38.05% CO2) (Fig. 7), indicating an anticipation of respiration peak due to ethylene injection. It was also observed that pH variation presented similar behavior for assays 4 and 6, indicating low influence of ethylene concentration in this parameter; otherwise, the firmness was more sensitive because the use of ethylene (assay 6) indicated firmness of 2.94 N in day 7, whereas in assay 4, it was 4.41 N (Table 2).
Thus, there was an acceleration of the ripening in the confined samples in comparison to the standard samples, which indicated that ethylene could act as a trigger to the ripening of climacteric fruit.
The sensorial analysis showed that the confined samples presented characteristics similar to ready-to-eat fruits, in aroma and flavor during the ripening, except for the color attribute. On day 5 of ripening, the samples treated with 100 mg l-1 C2H4 (assay 6) showed better sensorial parameters (color, aroma and flavor) than those fruits without ethylene treatment (assay 4) (Table 4).
Influence of CO2
Experiments 2, 4 and 5
The influence of CO2 concentration on the ripening process was evaluated comparing assays 2 (standard), 4 and 5 (with and without CO2 absorbent) (Table 1).
The CO2 concentration did not affect the physical and chemical characteristics nor do the O2 and C2H4 content (Tables 2 and 3). The exception was the flesh firmness attribute, which reduced faster in the presence of CO2 absorbent (Fig. 4) because CO2 suppressed the climacteric respiration of bananas (Liu et al., 2004). This behavior was confirmed in assays with CO2 absorption, where the ethylene production was higher and the flesh firmness decreased faster than other experiments without CO2 absorbent, an indication of earlier maturity stage.
The maturity degree of the samples was observed in the sensorial analyses and the results for the attributes flavor, color and aroma were better for the assays with CO2 absorption, which presented a profile closer to the "ready-to-eat" samples, when compared to the assays with no CO2 absorbent (Table 4, Fig.s 3-5). The color development was better for assays with CO2 absorbent, as presented in Figure 6 (b, d and e). This performance indicated that CO2 influenced negatively the color development during banana ripening. Even without systematic appraisal of sensorial, physical and chemical evolution of the standard sample attributes at 25 ºC (assay 2), the samples reached the ripe state in about 30 days. This behavior was observed by color and flavor evolution of the standard samples. Therefore, with the use of a controlled atmosphere with CO2 withdrawal, the time for the fruit ripening apparently decreased from 30 to 6 days.
Experiments 2, 6 and 7
The second part of this study evaluated the influence of CO2 in banana ripening for assays with 100 mg l-1 ethylene injection in order to accelerate the development of quality attributes. The results showed no influence of the CO2 initial concentration on physical and chemical properties or on gas concentration (O2 and C2H4) during ripening. The firmness for the confined samples decreased with time, confirming the maturity degree evolution, while for standard samples, only small reduction on firmness parameter was observed up to day 7 (Table 2).
During the ripening experiments, the samples placed in the flasks moved towards the ready-to-eat samples, in relation to flavor and aroma attributes (Fig.s 3 and 4). The standard samples did not develop the flavor and aroma attributes similar to the confined samples and were considered as a mature fruit only nearly 30 days in a controlled temperature chamber.
Figure 6 (b, f and g) shows the pictures of the banana ripening assay. It was observed that the color development was better for the closed samples with the removal of CO2 produced by respiration (with CO2 absorbent: assay 7).
Start Up
The effect of ethylene as a trigger on the ripening was investigated in this study.
Experiments 5 and 9
Comparing assays 5 and 9 allowed the evaluation of the influence of the ethylene produced by the fruit as a trigger in the ripening of bananas. In assay 9, the samples were maintained confined only in the first 48 h of ripening, while in assay 5, the ripening cells were closed. The "start up" samples (assay 9) showed lower weight loss when compared with the confined samples (assay 5), while the firmness parameter (Table 2) indicated that the ethylene produced by the fruit in the early 48 h ripening was not sufficient to initiate the ripening (assay 9 in day 7: 43.15N).
Therefore, the ethylene produced up to 48 h was not sufficient to accelerate the ripening (firmness had little reduction) in "start up" samples, except on day 5 where probably a ripened fruit was used to initiate the experiment.
Experiments 8 and 12
In sequence, the influence of ethylene as a trigger in the ripening of banana submitted to 200 mg l-1 ethylene injection was evaluated. The "start up" samples (assay 12) presented about the same weight loss as the confined ones (Fig. 8 and Table 2). The samples showed similar results in pH and firmness, indicating that 48 h with 200 mg l-1 was enough as a trigger, considering the physicochemical results (Table 2). The sensorial analysis showed that "start up" samples presented better characteristics in comparison to confined ones, and closer results to ready-to-eat samples for all the attributes (Table 4 and Fig. 6). Also, the O2 limitation and the CO2 presence affected the fruit color development, resulting in unsatisfactory peel color for the previously confined samples (assay 8).
This behavior was not detected in assay 12 (start up), where the color attribute was well developed due to the ethylene supply in the initial part of the ripening process and also after opening the ripening cells, after 48 h confinement. These procedures reduced the negative effect of CO2 and allowed sufficient supply of O2 for the ripening process already triggered by the ethylene supply.
Influence of Temperature
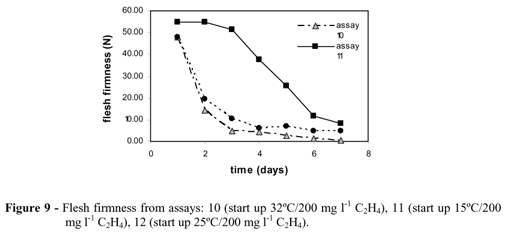
The temperature effect was evaluated during ripening process. The weight loss increased with temperature for standard assays (1, 2 and 3) and assays 10, 11 and 12 (Fig. 8, Table 2), while firmness decreased with increasing temperature, showing the acceleration of ripening with temperature for 15, 25 and 32ºC (Fig. 9). Although an increase in temperature aided the ripening process, the sensorial analysis results (Table 4) showed that 25ºC was the best ripening temperature, when compared to 15 and 32ºC, presenting sensorial grades for the group of quality attributes, closer to "ready-to-eat" fruit, especially for the samples collected on days 5 and 6. Also, start up samples on day 6 using 200 mg l-1 of ethylene (assay 12) overcome the quality grades for "ready to eat" sample on flavor and aroma attributes (Fig. 10).
Results at 15 ºC indicated poor ripening while at 32ºC indicated over-maturation, reducing the quality. The studied temperatures promoted small variation in the fruits pH (Table 2).
CONCLUSIONS
Although several studies have been conducted on banana ripening, no information has focused on ripening tools for small scale uses, such as for the domestic and hotel purposes. In this direction, the present work targeted conditions useful to improve the domestic equipments seeking the increase and decrease in ripening process in a short period of time, as much as 7 days of ripening. This work was a preliminary step for the development of domestic equipments to control the fruit ripening, which must be followed by the cost analysis in a future investigation. Therefore, the results of this work indicated the optimum ripening temperature for banana as 25ºC. The process was strongly influenced by the atmosphere control, where gases involved in the respiration were consumed or produced in the medium. The presence of oxygen and the CO2 absorbent material, and also the injection of ethylene are of relevance. The gas composition inside confined cells can be used to speed up or slow down the ripening process. It was possible to reduce the ripening time from about 30 to 6 days, through the CO2 removal and the O2 injection in confined systems. The initial ethylene concentration tested (100 and 200 mg l-1) did not influence on the ripening velocity, with exception of the color development. A 48 h period of confining in the "start up" assays, without further ethylene injection (atmosphere control) not was sufficient to initiate the fruit ripening. Otherwise, the use of "start up" followed by the atmosphere control with C2H4 presented good results. The alternative of using ethylene as a trigger has shown to be adequate to accelerate the ripening process, also with substantial advantages in terms of the fruit quality, as observed in "start up" assays.
ACKNOWLEDGEMENTS
The authors whish to acknowledge Multibrás S. A., Brazil, for the financial support, especially Eng. Angelo Reck.
Received: September 06, 2006;
Revised: December 28, 2007;
Accepted: June 16, 2008
- Abeles, F. B. (1973), Ethylene in Plant Biology Academic Press. New York, p. 302.
- Adkins, M. F. Hofman, P.J., Stubbings, B.A., Macnish, A.J. (2005), Manipulating avocado fruit ripening with 1-methylcyclopropene. Postharvest Biol. Technol, 35, 33 - 42.
- Bower, J. Holford, P., Latché, A., Pech, J.C. (2002), Culture conditions and detachment of the fruit influence the effect of ethylene on the climacteric respiration of melon. Postharvest Biol. Technol, 26, 135 - 146.
- Burdon, J., Lallu, N., Billing, D., Burmeister, D., Yearsley, C., Wang, M., Gunson, A., Young, H. (2005), Carbon dioxide scrubbing systems alter the ripe fruit volatile profiles in controlled-atmosphere stored ÂHayward kiwifruit. Postharvest Biol. Technol, 35, 133 - 141.
- Cameron, A. C., Talasali, P. C., Joles, D. W. (1995), Predicting film permeability needs for modified atmosphere packaging of lightly processed fruits and vegetables. Hortscience, 30, 25 - 34.
- Chen, C. R., Ramaswamy, H. S. (2002), Color and texture change kinetics in ripening bananas, Lebensm.- Wiss, U.-Technol, 35, 415 - 419.
- Clendennen, S. K., May, G. D. (1997), Differential gene expression in ripening banana fruit. Plant Physiol, 115, 463 - 469.
- Domínguez, M., Vendrell, M. (1994), Effect of ethylene treatment on ethylene production, EFE activity and ACC levels in peel and pulp of banana fruit. Postharvest Biol. Technol, 4, 167 - 177.
- Henig, Y. S. (1975), Storage stability and quality of produce packed in polymeric films. In - Haard, N.F., Salunkhe, D.K. (Eds.), Postharvest Biology and Handling of Fruits and Vegetables, AVI, Westport, CT, pp. 144 - 152.
- Hoffman, N. E., Yang, S. F. (1980), Changes in L-aminocyclo-propane-L-carboxylic acid content in ripening fruits in relation to their ethylene production rates. J. Am. Soc. Hort. Sci, 105, 492Â495.
- Inaba, A., Nakamura, R. (1986), Effect of exogenous ethylene concentration and fruit temperature on the minimum treatment time necessary to induce ripening in banana fruit. J. Jpn. Sot. Hart. Sci, 55, 348 - 354.
- Kader, A. A., Zagory, D., Kerbel, E. L. (1989), Modified atmosphere packaging of fruits and vegetables, Crit. Rev. Food Sci. Nutr, 28, 1 - 30.
- Kanellis, A. K., Solomos, T., Mattoo, A. (1989), Changes in sugar, enzymic activities and acid phosphatase isoenzyme profiles of banana ripened in air or stored in 2.5% O2 with and without ethylene. Plant Physiol, 90, 251 - 258.
- Ke, L. S., Tsai, P. L. (1988), Changes of ACC contents and EFE activity in peel and pulp of banana fruit during ripening in relation to ethylene production. J. Agr. Assoc. Chin., 143, 48 - 60.
- Kubo, Y., Inaba, A., Nakamura, R. (1990), Respiration and C2H4 production in various harvested crops held in CO2-enriched atmospheres. J. Am. Soc. Hort. Sci, 115, 975 - 978.
- Laties, G. G. (1995), Franklin Kidd, Charles West and F.F. Blackman: the start of modern postharvest physiology. Postharvest Biol. Technol, 5, 1 - 10.
- Li, M., Slaughter, D.C., Thompson, J.F. (1997), Optical chlorophyll sensing system for banana ripening, Postharvest Biol. Technol, 12, 273 - 283.
- Liu, F. W., (1976), Banana response to low concentration of ethylene. J. Am. Sot. Hort. Sci 101, 222 - 225.
- Liu, S., Yang, Y., Murayama, H., Taira, S., Fukushima, T. (2004), Effects of CO2 on respiratory metabolism in ripening banana fruit. Postharvest Biol. Technol., 33, 27 - 34.
- Marchal, J. (1998), An overview of post-harvest aspects of banana. Acta Hort, 490, 501 - 509.
- Marriot, J. (1980), Bananas  physiology and biochemistry of storage and ripening for optimum quality. Crit. Rev., Food Sci. Nutr, 13, 41 - 88.
- Mathooko, F. M. (1996), Regulation of ethylene biosynthesis in higher plants by carbon dioxide. Postharvest Biol. Technol, 7, 1 - 16.
- Moya-León, M. A., Moya, M., Herrera, R. (2004), Ripening of mountain papaya (Vasconcellea pubescens) and ethylene dependence of some ripening events. Postharvest Biol. Technol, 34, 211 - 218.
- Ohm, M., Gravenstein, N., Good, M. L. (1991), Duration of carbon dioxide absorption by soda lime at low rates of fresh gas flow. Journal of Clinical Anesthesia, 3, 104 - 107.
- Palomer, X., Roig-Villanova, I., Grima-Calvo, D., Vendrell, M. (2005), Effects of nitrous oxide (N2O) treatment on the postharvest ripening of banana fruit. Postharvest Biol. Technol, 36, 167 - 175.
- Pesis, E. (2005), The role of the anaerobic metabolites, acetaldehyde and ethanol, in fruit ripening, enhancement of fruit quality and fruit deterioration. Postharvest Biol. Technol, 37, 1 - 19.
- Prabha, T. N., Bhagyalakshmi, N., (1998), Carbohydrate metabolism in ripening banana fruit. Phytochemistry 48, 915 - 919.
- Riquelme, F., Pretel, M. T., Martínez, G., Serrano, M., Amorós, A., Romojaro, F. (1994), Packaging of fruits and vegetables: recent results. In: Mathlouthi, M. (Ed.), Food Packaging and Preservation, Blackie, Glasgow, pp. 141 - 158.
- Stover, R. H., Simmonds, N.W. (1993), Bananas. 3rd Edition. Longman Scientific and Technical, Wiley. New York, pp 369-393.
- Wild, H. P. J., Balk, P. A., Fernandes, E. C. A., Peppelenbos, H. W. (2005), The action site of carbon dioxide in relation to inhibition of ethylene production in tomato fruit. Postharvest Biol. Technol, 36, 273 - 280.
- Young, R. E., Romani, R. J., Biale, J. B., (1962), Carbon dioxide effects on fruit respiration. II. Response of avocados, bananas, and lemons. Plant Physiol. 37, 416 - 422.
- Zagory, D., Kader, A. A., 1988. Modified atmosphere packaging of fresh produce. Food Technol, 42, 70Â77.
- Zhang, P., Whistler, R. L., Bemiller, J. N., Bruce, R., (2005), Banana starch: production, physicochemical properties, and digestibility-a review. Carbohydrate Polymers, 59, 443 - 458.
Publication Dates
-
Publication in this collection
29 Oct 2008 -
Date of issue
Oct 2008
History
-
Received
06 Sept 2006 -
Reviewed
28 Dec 2007 -
Accepted
16 June 2008