Abstracts
This work studied the effects caused by five different formulae of gasoline on the stability of the lysosomes isolated from the liver of the tilapia fish (Oreochromis niloticus). The functional integrity of the lysosomal membranes was evaluated via the acid phosphatase activity. The results showed that there were significant changes in the stability of the lysosomes exposed to the presence of the hydrocarbons in the environment. Therefore, considering the method's simplicity, the sensitivity of the responses and its low cost the assessment of the lysosomal activity could be an important tool for the study of the effects of pollution in the aquatic environments.
Biomarkers; acid phosphatase; lysosomes; aquatic environments; pollution
A procura de biomarcadores de agentes poluidores, mais simples e menos custosos, tem levado ao estudo dos lisossomos, isolados de animais componentes da biota nos ambientes contaminados, principalmente por poluentes com características lipofílicas, a exemplo dos hidrocarbonetos policíclicos e seus derivados. Este trabalho estudou os efeitos provocados por 05 diferentes formulações de gasolina sobre a estabilidade de lisossomos, isolados de fígado de tilápia (Oreochromis niloticus). A integridade funcional das membranas lisossômicas foi avaliada através da atividade da fosfatase ácida, expressa em mU/mg de proteínas totais. Os resultados obtidos mostraram que existem alterações significativas na estabilidade dos lisossomos isolados de fígado de tilápias submetidas aos efeitos de hidrocarbonetos presentes no meio ambiente. Portanto, levando em conta a simplicidade, a sensibilidade de resposta e o baixo custo, os autores recomendam a avaliação da atividade lisossômica, como uma importante ferramenta para o estudo dos efeitos da poluição dos meios aquáticos.
ENVIRONMENTAL SCIENCES
The lysosomal stability as a biomarker for the determination of pollution in aquatic environments
Maria Loreto Nazar I, * * Author for correspondence ; Luiz Erlon Araujo RodriguesII; Iracema NascimentoIII
INúcleo de Pesquisa Transferência Tecnológica e Desenvolvimento Sócio Ambiental; Av. ACM, 2487, s/ 1001; loreto@tecnoceanic.org.br; 40280-000; Salvador - Bahia - Brasil
IIDepartamento de Apoio Diagnóstico e Terapêutico; Faculdade de Medicina, Universidade Federal da Bahia; Salvador - BA Brasil
IIILaboratório de Biologia Marinha e Biomonitoramento; Instituto de Biologia, Universidade Federal da Bahia; Salvador - BA - Brasil
ABSTRACT
This work studied the effects caused by five different formulae of gasoline on the stability of the lysosomes isolated from the liver of the tilapia fish (Oreochromis niloticus). The functional integrity of the lysosomal membranes was evaluated via the acid phosphatase activity. The results showed that there were significant changes in the stability of the lysosomes exposed to the presence of the hydrocarbons in the environment. Therefore, considering the method's simplicity, the sensitivity of the responses and its low cost the assessment of the lysosomal activity could be an important tool for the study of the effects of pollution in the aquatic environments.
Key words: Biomarkers, acid phosphatase, lysosomes, aquatic environments, pollution
RESUMO
A procura de biomarcadores de agentes poluidores, mais simples e menos custosos, tem levado ao estudo dos lisossomos, isolados de animais componentes da biota nos ambientes contaminados, principalmente por poluentes com características lipofílicas, a exemplo dos hidrocarbonetos policíclicos e seus derivados. Este trabalho estudou os efeitos provocados por 05 diferentes formulações de gasolina sobre a estabilidade de lisossomos, isolados de fígado de tilápia (Oreochromis niloticus). A integridade funcional das membranas lisossômicas foi avaliada através da atividade da fosfatase ácida, expressa em mU/mg de proteínas totais. Os resultados obtidos mostraram que existem alterações significativas na estabilidade dos lisossomos isolados de fígado de tilápias submetidas aos efeitos de hidrocarbonetos presentes no meio ambiente. Portanto, levando em conta a simplicidade, a sensibilidade de resposta e o baixo custo, os autores recomendam a avaliação da atividade lisossômica, como uma importante ferramenta para o estudo dos efeitos da poluição dos meios aquáticos.
INTRODUCTION
Increasing pollution of the water environments in the rivers, lakes and seas is becoming a global problem of serious consequences. Sarkar et al. (2006) have described the various types of highly persistent polluting agents, among which are the derivatives of organochlorines such as pesticides like DDT ((dichlorodiphenyltrichloroethane), HCH (hexachlorocyclohexane) aldrin, dieldrin, phthalate esters, derivatives of nitro-aromatic and various toxic metals such as cadmium (Cd), mercury (Hg) and others. Okay et al. (2006) considered the aromatic polycyclic hydrocarbons, natural components of gasoline, which constitute, up to 20% of all the hydrocarbons found in the crude oil, as the most toxic among all its components. In this context, the use of biomarkers which are able to detect the presence as well as the toxic effects caused by those pollutants is increasing in importance and is being increasingly used.
The biomarkers, according to Bodin et al. (2004), can be defined as the measurements carried out in the biological liquids, cells or tissues which indicate, in biological or cellular terms, the presence of contaminants or the magnitude of the response of organisms to their toxic effects. According to Bodin et al. (2004) and Pfeifer et al. (2005), the biomarkers as the enzymatic induction of cytochrome P4501A, inhibition of the activity of acetylcholine esterase, integrity of DNA and induction of metallothioneins have been used in the monitoring of the ecological impact of various pollutants in the aquatic environments.
The search for simple and cheap biomarkers has led to the physicochemical and/or histopathologycal studies of the lysosomes which are capable of detecting the slightest cellular damage resulting from the exposure to toxic polluting agents, mainly those with lipophilic characteristics such as the polycyclic hydrocarbons and their derivatives (Köhler et al. 2002). The components of the lysosomal compartment are primary lysosomes, auto and heterophagic vesicles, secondary lysosomes (phagosomes) and the residual corpuscles are cellular organelles, multifunctional, rich in the hydrolytic enzymes, responsible for the breakdown of all the constituents of the cells and macromolecules derived from the extracellular space via endocytosis. They are also involved in cell defence mechanisms, in the protection against the toxic agents and infection by the viruses and bacteria (Cuervo and Dice, 2000). The physicochemical modifications which lead to the loss of the integrity of the membranes of diverse components of the lysosomes are almost always associated with cellular dysfunction, inflammatory and degenerative diseases as well as apoptosis and cell death (van Nierop et al., 2006).
The aim of this work was to study the effects caused by five gasoline formulations on the stability of the membranes of the lysosomes isolated from the liver cells of young Nile tilapia (Oreochromis niloticus).
MATERIALS AND METHODS
Description of the species studied
Nile tilapia belonging to the class Actinopterygii, order Perciformis, family Cichlidae, subfamily Pseudocrenilabrinae, genus Oreochronis and species niloticus, is part of the set of the cichlidae fish which is bred throughout the world, though originally found in an area from the central South Africa to Syria. According to Bozano et al., (1999), the spread of the tilapia around the world has been a result of subsistence fish breeding programmes in the underdeveloped countries. In Brazil, Nile tilapia was introduced into the North East in 1971 and has since become one of the most cultivated fish.
Gasoline's formulae
Five different formulae of gasoline, (Table 1), varying in composition of the aromatic hydrocarbons, were used in the present experiment. They were prepared from petrochemical sub products. The different formulae were kept separate in sealed darkened glass flasks in a refrigerator at 4ºC until used in the experiments.
The samples were agitated in a homogenizer at 150 rpm for 15 minutes and then diluted 1/10 with the fresh water in Mariotti flasks which were subsequently stirred for 20 hours. After this, they were left to decant for a further hour. The samples of the water soluble part were carefully removed and final dilutions of 1/500 and 1/1000 were made. These solutions were poured into 8 L aquarium each with four young tilapias (13.5 ± 0.7cm). The fishes were not fed for the next 24 h and kept at a constant temperature of 22ºC. In the controlled experiment, the fishes were held in similar aquaria
containing only the fresh water. All the experiments were carried out in triplicate. Some physicochemical parameters such as the amount of dissolved oxygen (OD meter Orion), pH (Procyon pH meter), ammonium (Procyon ion meter) and temperature were evaluated at the beginning and the end of each experiment. Only the experiments carried out with the dilutions of 1/1000 were continued. Almost all the fishes put into the 1/500 solutions did not survive and the mortality rate was extremely high.
Separation of the various components of the lysosomal compartment from the liver of young tilapia
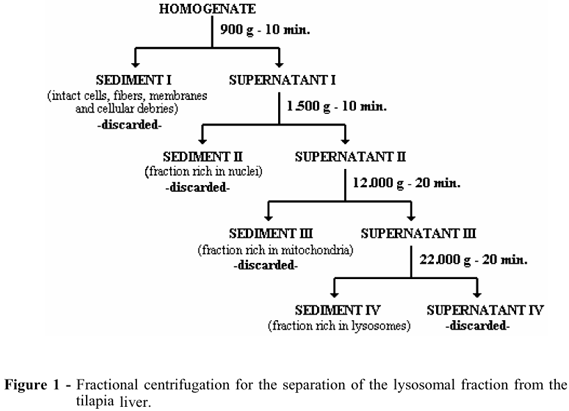
Each fish was killed individually by the oxygen suppression and had its liver immediately removed and put into a labelled beaker containing 50 mL of buffered sucrose/phosphates solution (sucrose 25,10-2 M, disodium phosphate 10-2 M and monopotassium phosphate 10-2 M) pH 7.4, refrigerated at 4ºC, where it was minced by a small scissor and washed twice to remove the blood. The fragments of each liver were suspended in a proportion of 1g of the tissue to 10 mL of another buffered solution containing manitol 25,10-2 M, TRIS 2,10-3 M, EDTA 4,10-4 M, disodium phosphate 10-2 M and monopotassium phosphate 10-2 M, adjusted to pH 7.4, and homogenized in a Potter-Elvejhem homogenizer, at 250 rpm for a maximum of 2 minutes, in a bath of melting ice. The various components of the lysosomal compartment were separated by the fractionated centrifugation and refrigerated at 4 ºC, according to the scheme described in Figure 1.
The 22,000 g sediment, which contained most of the lysosomes, was re-suspended to the same volume as the initial homogenized material with the same buffered solution and divided into 2 tubes. 4,10-3 M of digitonin was added to one of them and both were incubated on ice for 20 minutes. After this, they were spun down, once more, at 22,000 g for 20 minutes with the aim to collect the intact lysosomes (Rodrigues, et al., 1998). The stability of the components of the lysosomal fraction was assessed by the acid phosphatase activity, (Roy et al., (1971), and the total protein content of each experiment was assayed according to the technique described by Komsa-Penkova et al., 1996. The results of the experiments were compared to the controls using ANOVA and the Tukey-Kramer multiple comparison test. The values were considered to be significant if p<0.05 (Smith, 1995). The isolation conditions of the lysosomes was controlled by the digitonin test which, according to Ishisaka et al.(1998), breaks down all the lysosomes.
RESULTS
The functional integrity of the membranes from the lysosomal components, isolated in the sediment at 22,000 g was evaluated by measuring the activity of the acid phosphatase in the floating material which resulted from the last centrifugation. In this material, higher enzyme activity is related to greater fragility of isolated lysosomes. The migration of the enzymes of the lysosomes to the external medium has been used as one of the safest parameters for the evaluation of the physicochemical stability of their membranes (Petrovic et al., 2004). Table 2 shows the activity of the acid phosphatase in the experiments. The digitonin test (42.0 ± 4.1 mU/mg) demonstrated a four time higher activity in the treated lysosomes than the control (10.5 ± 2.7 mU/mg). This difference was highly significant: p<0.002.
The experiments in which the lysosomal activity, treated with the digitonin, were lower than two times the respective activity of the control were ignored and considered that the experimental isolation conditions had interfered with the stability of the lysosomes. Only those in which the activities, after the treatment with digitonin, were at least three times higher were included in the study. Table 2 shows the results of the tests for enzyme activity of the lysosomes from the livers of the tilapias. Only the result for the activity related to the first formula was not statistically different from the control. All the other formulae had significant labilising effects on the hepatic lysosomes of the fishes. Formulae 3, 4 and 5 displayed acid phosphatase activity more than double of that from the control. Formula 1 had the lowest amount of the aromatic hydrocarbons (34.97%, v/v) and formula 4 the highest (98,64%,v/v).
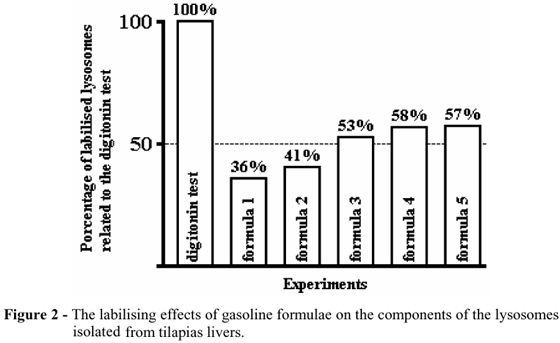
The treatment of the lysosomes with 4,10-3 M concentration of the digitonin was capable of labilising 100% of these corpuscles and allowed to calculate the percentages of the lysosomal components broken down by the treatment with the different formulae. These results are presented in Figure 2.
The formulae 3, 4, and 5 broke down more than half of the lysosomes isolated from the livers of the tilapia. Only the formulae 1 and 2 labilised less than 50% of the lysosomes. The results suggested the possibility of a labilising effect on the lysosomal membranes directly linked to the concentration of the aromatic cyclic hydrocarbons from the different gasoline formulae.
DISCUSSION AND CONCLUSION
The use of the biomarkers capable of detecting the first effects on the biota of the aquatic environments showed to be an important tool for the control, preservation and the monitoring of the changes in these environments. The eco-toxicological effects on the various populations of the ecosystems are a result of the interactions at the molecular, cellular and tissue levels of the polluting agent with the biota (Fent, 2001). According to Winkaler et al., (2001), most of the techniques used to assess the toxic effects of the chemical substances on the aquatic organisms, in recent decades, have been based on the simple laboratory assay of the polluting agents using the samples taken from the ecosystems, or in toxicity tests, generally using one of the species from the threatened environment. However, expensive and often technically complicated, most of these do not take into account the effect of possible interactions between the environment and the different types of the toxic agents, such as the heavy metals, xenobiotic agents of an organic nature and organometallic compounds (Sarkar et al., 2006). The trend, according to Petrovic et al. (2004), was to choose the biomarkers which traced the cytophysiological or histopathological alterations occurring in the aquatic organisms due to their exposure to the contaminants. In this regard, the lysosomes appeared to be sensitive biomarkers and directly linked to the biochemical mechanisms of the response and defence against the aggressors. Furthermore, it involves the use of a simple, low cost technical approach.
According to Köhler et al., (2002), the morphological alterations and/or the stability of the lysosomal membranes determined the loss of the characteristic enzymes of these organelles as a result of the effect of various xenobiotics. Nowadays, the techniques that make use of the various components of the lysosomal compartment are increasingly being used in the monitoring of the effects of the anthropogenic toxic substances in the most diverse of species of the aquatic biota such as molluscs, crustaceans, fish, larva, worms, etc. Among them, the most reliable are those that evaluate the activity of the marker enzymes such as acid phosphatase (EC 3.1.3.2), β-N-acetyl-hexosaminidase (EC 3.2.1.52), cytochrome C oxidase (EC 1.9.3.1), neutral esterase (EC 3.1.1.1), cathepsin B (EC 3.4.22.1) and β-glucuronidase (EC 3.2.1.31) (Strφmhaug et al., 1998, Petrovic et al., 2004, Marigómez et al., 2005 and van Nierop et al., 2006).
According to Koukouzika and Dimitriadis (2005), the assessment of the lysosomal stability, measured by the techniques related to the autophagy or endocytosis of the pigments, such as neutral red and cresyl violet, demonstrated the seasonal changes which depended on the species under the study, as well as on the inherent characteristics of the locations from where the specimens were collected. Furthermore, the formation of the pigment containing heterophagosomes depends, among other things, on the involvement of cellular receptors located in the plasmatic membranes which vary according to the species. These receptors are extremely sensitive to the fluctuations in the extracellular environment (Strömhaug et al. 1998).
These results corroborate the results of Hwang et al., (2004) and Okay et al., (2006), who showed that there were significant alterations in the stability of the lysosomes isolated from the liver of tilapias which was exposed to the hydrocarbons in the environment. According to Martins et al. (2005), the lysosomes are sub cellular particles which are directly linked to the endocytosis, immunoreactions and defense. The physicochemical integrity of their membranes is considered an important biomarker for the environmental modifications. Therefore, given the simplicity of the method, the sensitivity of the response and its relatively low cost, this lysosomal evaluation technique, assayed through the acid phosphatase activity, could be an important tool for the evaluation of the effects of the aquatic pollution by the toxic xenobiotics.
ACKNOWLEDGMENT
The authors thank to the National Council for Scientific and Technological Development (CNPq-Brazil) for the financial support and the Basic Science Researches Laboratory (EBMSP FBDC) for the technical facilities.
Received: May 03, 2007;
Revised: November 19, 2007;
Accepted: June 03, 2008.
- Bodin, N.; Burgeot, T.; Stanisière, J. Y.; Bocquené, G.; Menard, D.; Minier, C.; Boutct, I.; Amat, A.; Chcrcl, Y. and Budzinski, H. (2004), Seasonal variations of a battery of biomarkers and physiological indices for the mussel Mytilus galloprovincialis transplanted into the northwest Mediterranean sea. Comp. Biochem. Physiol. C. Toxicol. Pharmacol, 138(4), 411-427.
- Bozano, G. L. N.; Rodrigues, S. R. M.; Caseiro, A.C. and Cyrino, J. E. P. (1999), Desempenho da tilápia nilótica Oreochromis niloticus (L.) em gaiolas de pequeno volume. Sci, Agric, 56(4), 819-825.
- Cuervo, A. M. and Dice, J. F. (2000), When lysosomes get old. Exp. Gerontol 35, 119-131.
- Fent, K. (2001), Fish cell lines as versatile tools in ecotoxicology: assessment of cytotoxicity, cytochrome P4501A induction potential and estrogenic activity of chemicals and environmental samples. Toxicol. in vitro, 15, 477-488.
- Hwang, H-M.; Wade, T. L. and Sericano, J. L. (2004), Destabilized lysosomes and elimination of polycyclic aromatic hydrocarbons and polychlorinated biphenyls in eastern oysters (Crassotrea virginica). Environ. Toxicol. Chem, 23, 1991-1995.
- Ishisaka, R.; Utsumi, T.; Yabuki, M.; Kanno T.; Furuno, T.; Inoue, M. and Utsumi, K. (1998), Activation of caspase-3-like protease by digitonina-treated lysosomes. FEBS Lett, 435, 233-236.
- Köhler, A.; Wahl, E. and Söffker, K. (2002), Functional and morphological changes of lysosomes as prognostic biomarkers of toxic liver injury in a marine flatfish (Platichthys flesus). Environ. Toxicol. Chem, 21(11), 2434-2444.
- Komsa-Penkova, R.; Spirova, R. and Bechev, B. (1996), Modification of Lowry's method for collagen concentration measurement. J. Biochem. Biophys. Meth., 32,33-43.
- Koukouzika, N. and Dimitriadis, V. K. (2005), Multiple biomarker comparison in Mytilus galloprovincialis from Greece Coast: "Lysosomal membrane stability, neutral red retention, micronucleus frequency and stress on stress". Ecotoxicol, 14, 449-463.
- Marigómez, I.; Lekube, X.; Cajaraville, M. P.; Domouhtsidou, G. and Dimitriadis, V. (2005), Comparison of cytochemical procedures to estimate lysosomal biomarkers in mussel digestive cells. Aquat. Toxicol, 75, 86-95.
- Martins, L. K. P.; Nascimento, I. A.; Fillmann, G.; King, R.; Evangelista, A. J. A.; Readman, J. W. and Depledge, M. H. (2005), Lysosomal responses as a diagnostic tool for the detection of chronic gasoline pollution at Todos os Santos Bay, Brazil. Environ. Res 99, 387-396.
- Okay, O. S.; Tolun, L.; Tüfekçi, V.; Telli-Karakoç, F. and Donkin, P. (2006), Effects of pyrene on mussels in different experimental conditions. Environ. Intern, 32, 538-544.
- Petrovic, S.; Semencic, L.; Ozretic, B. and Ozretic, M. (2004), Seasonal variations of physiological and cellular biomarkers and their use in the biomonitoring of north Adriatic coastal waters (Croatia). Mar. Pollut. Bull, 49, 713-720.
- Pfeifer, S.; Schiedek, D. and Dippner, J. W. (2005), Effect of temperature and salinity on acetylcholinesterase activity, a common pollution biomarker, in Mytilus sp from the south-western Baltic sea. J. Exp. Mar. Biol. Ecol., 320, 93-103.
- Rodrigues, L. E. A.; Paes, I. B. and Jacobina, H. (1998), Role of lysosomes on human ulcerogenic gastropathies. Effect of zinc ion on the lysosomal stability. Arq. Gastroenterol, 35(14), 247-251.
- Roy, A. V.; Brower, M. E. and Hayden, J. E. (1971), Sodium thymolphthalein monophosphate: a new acid phosphatase substrate with greater specificity for the prostatic enzyme in serum. Clin. Chem, 17, 1093-1102.
- Sarkar, A.; Ray, D.; Shrivastava, A. N. and Sarker, S. (2006), Molecular biomarkers: Their significance and application in marine pollution monitoring. Ecotoxicol, 27, 109-107.
- Smith, S. M. (1995), Distribution-free and robust statistical methods: viable alternatives to parametric statistics? Ecology, 76(6), 1997-1998.
- Strömhaug, E.; Berg, T. O.; Fengsrud, M. and Seglen, O. (1998), Purification and characterization of autophagosomes from rat hepatocytes. Biochem. J, 335, 217-224.
- van Nierop, K; Muller, F. J. M.; Stap, J.; van Noorden, C. J. F.; van Eijk, M. and de Groot, C. (2006), Lysosomal destabilization contributes to apoptosis of germinal center B-lymphocytes. J. Histochem. Cytochem, 54(12), 1425-1435.
- Winkaler, E. U.; Silva, A. G.; Galindo, H. C. e Martinez C. B. R. (2001), Biomarcadores histológicos e fisiológicos para o monitoramento da saúde de peixes de ribeirões de Londrina, Estado do Paraná. Acta Scient, 23(2), 507-514.
Publication Dates
-
Publication in this collection
29 Oct 2008 -
Date of issue
Oct 2008
History
-
Accepted
03 June 2008 -
Reviewed
19 Nov 2007 -
Received
03 May 2007