Abstracts
The aim of this study was to evaluate the effect of in vivo treatment with an aqueous cinnamon extract on the labeling of blood constituents with 99mTc and on the morphology of red blood cells from Wistar rats. Animals were treated with cinnamon extract at different doses and for different periods of time. As controls, animals treated with 0.9% NaCl. Labeling of blood constituents with 99mTc was performed. Plasma, blood cells and insoluble fractions were isolated. Radioactivity in each fraction was counted and the percentage of radioactivity (%ATI) was calculated. Also, blood smears were prepared to morphological analysis of red blood cells from. Data showed that in vivo cinnamon extract did not significantly (p>0.05) modify the %ATI of blood constituents and morphology of red blood cells. The results suggest that in vivo aqueous cinnamon could not affect the membrane structures involved in transport of ions or the oxidation state of stannous and pertechnetate ions.
blood constituents; Cinnamomum zeylanicum; technetium-99m
O objetivo deste estudo foi avaliar efeitos do tratamento in vivo com um extrato aquoso de canela na marcação de constituintes sangüíneos com 99mTc e na morfologia de hemácias de ratos Wistar. Os animais foram tratados com diferentes doses ou por diferentes tempos com extrato de canela Como controles, animais tratados com NaCl 0,9%. A marcação de constituintes sangüíneos com 99mTc foi realizada, plasma, células sangüíneas e frações insolúveis foram isoladas. A radioatividade em cada fração foi contada e a porcentagem de radioatividade (%ATI) foi calculada. Distensões sangüíneas foram preparadas para análise morfológica de hemácias. Os dados mostraram que o tratamento in vivo com extrato de canela não modificaria significativamente (p>0,05) a %ATI nos constituintes sangüíneos e a morfologia de hemácias. Os resultados sugerem que o extrato aquoso de canela não afetaria in vivo as estruturas da membrana envolvidas no transporte de íons ou o estado de oxidação dos íons estanoso e pertecnetato.
DRUG INTERACTION AND THE LABELING OF BLOOD CONSTITUENTS
Effects of Cinnamomum zeylanicum treatment on radiolabeling of blood bonstituents and morphology of red blood cells in Wistar rats
Mônica Oliveira BenarrozI; Gabrielle de Souza RochaII; Márcia Oliveira PereiraII; Mauro GellerIII; Adenilson de Souza da FonsecaI,III,* * Author for correspondence ; Giuseppe Antonio PrestaIV; Mario Bernardo-FilhoI
IDepartamento de Biofísica e Biometria; Instituto de Biologia Roberto Alcantara Gomes; Universidade do Rio de Janeiro; Av. 28 de Setembro, 87; 20551-030; Rio de Janeiro - RJ - Brasil
IIUniversidade Federal do Rio Grande do Norte; Av. General Gustavo Cordeiro de Farias, s/n; 59010-180; Natal - RN - Brasil
IIICentro de Ciências da Saúde; Centro Universitário Serra dos Órgãos; Av. Alberto Torres, 111; adenilso@uerj.br; 25964-004; Teresópolis - RJ - Brasil
IVDepartamento de Fisiologia Humana; Instituto Biomédico; Universidade Federal do Estado do Rio de Janeiro; Rua Frei Caneca, 94; 20211040; Rio de Janeiro - RJ - Brasil
ABSTRACT
The aim of this study was to evaluate the effect of in vivo treatment with an aqueous cinnamon extract on the labeling of blood constituents with 99mTc and on the morphology of red blood cells from Wistar rats. Animals were treated with cinnamon extract at different doses and for different periods of time. As controls, animals treated with 0.9% NaCl. Labeling of blood constituents with 99mTc was performed. Plasma, blood cells and insoluble fractions were isolated. Radioactivity in each fraction was counted and the percentage of radioactivity (%ATI) was calculated. Also, blood smears were prepared to morphological analysis of red blood cells from. Data showed that in vivo cinnamon extract did not significantly (p>0.05) modify the %ATI of blood constituents and morphology of red blood cells. The results suggest that in vivo aqueous cinnamon could not affect the membrane structures involved in transport of ions or the oxidation state of stannous and pertechnetate ions.
Keywords: blood constituents, Cinnamomum zeylanicum, technetium-99m
RESUMO
O objetivo deste estudo foi avaliar efeitos do tratamento in vivo com um extrato aquoso de canela na marcação de constituintes sangüíneos com 99mTc e na morfologia de hemácias de ratos Wistar. Os animais foram tratados com diferentes doses ou por diferentes tempos com extrato de canela Como controles, animais tratados com NaCl 0,9%. A marcação de constituintes sangüíneos com 99mTc foi realizada, plasma, células sangüíneas e frações insolúveis foram isoladas. A radioatividade em cada fração foi contada e a porcentagem de radioatividade (%ATI) foi calculada. Distensões sangüíneas foram preparadas para análise morfológica de hemácias. Os dados mostraram que o tratamento in vivo com extrato de canela não modificaria significativamente (p>0,05) a %ATI nos constituintes sangüíneos e a morfologia de hemácias. Os resultados sugerem que o extrato aquoso de canela não afetaria in vivo as estruturas da membrana envolvidas no transporte de íons ou o estado de oxidação dos íons estanoso e pertecnetato.
INTRODUCTION
Cinnamomum zeylanicum (cinnamon) belongs to the family Lauraceae, originates from the Ceylon is an important spice having wide applications in perfumery and beverages (Jayaprakasha et al., 2003). It has been recognized to have medicinal properties and posses beneficial effects on health (Khan et al., 2003). Chemical analysis indicated polyphenolic and benzene compounds and phenolic volatile oils (Shan et al., 2005).
Blood constituents labeling with technetium-99m (99mTc) have been utilized in nuclear medicine (Saha, 2004) and basic scientific research (Fonseca et al., 2007). The labeling process with 99mTc depends on a reducing agent; stannous chloride (SnCl2) is the most used for this purpose (Saha, 2004). The band-3 anion and calcium channels may be the transport systems by which pertechnetate ion and stannous ions pass through of the red blood cell (RBC) membrane, respectively (Callahan and Rabito, 1990; Gutfilen et al., 1996).
Some authors have demonstrated some biological effects of drugs and herbal extracts using the labeling of blood constituents with 99mTc as an in vitro assay (Fonseca et al., 2007; Santos-Filho et al., 2008; Paoli et al., 2008). Alterations on the membrane of RBC could interfere on this radiolabeling procedure (Benarroz al., 2008).
Despite of some biological effects of cinnamon are described, there is few information about the interaction of cinnamon with 99mTc-radiopharmaceuticals (Benarroz et al., 2008). In this study we assessed the effect of the in vivo treatment with an aqueous cinnamon extract on the labeling of blood constituents with 99mTc and on morphology of RBC cells from Wistar rats.
MATERIALS AND METHODS
Plant material and preparation of the extracts
A commercial dried powder cinnamon was purchased from Yoki Alimentos SA, São Paulo, Brazil, lot number 04E05C. To prepare the extract, 2.4 mg of powder were dissolved with 20 mL of saline (0.9% NaCl), centrifuged (1500 rpm, 5 min) and the extract was considered to be 120 mg/mL.
Animals
Wistar rats (3-4 months, 250-300 g) were kept in constant environmental conditions (25±2 ºC, 12 h of light/dark cycle). The experimental procedures were in accordance with the Institutional Committee of Animal Care (Comissão de Ética para o Cuidado e Uso de Animais Experimentais, Instituto de Biologia Roberto Alcantara Gomes, Universidade do Estado do Rio de Janeiro) with the protocol number CEA/134/2006.
In vivo treatments
The animals (n=12, 4 rats each group) were treated by an oral via for 60 minutes with cinnamon extract (1.5, 15.0 and 150.0 mg/kg). Other animals (n=12, 4 rats each group) were treated with the higher dose (150.0 mg/kg) for different periods of time (15, 60 and 120 minutes). Control group (n=8 rats) was treated with 0.9% NaCl. The animals were anesthetized and heparinized whole blood was withdrawn to experimental procedures.
Radiolabeling procedure
Blood samples (n=10, for each dose) were incubated with SnCl2 (1.20 µg/mL, 60 minutes). Afterwards, 99mTc (3.7MBq, Na99mTcO4), recently milked from a 99Mo/99mTc generator (Instituto de Pesquisas Energéticas e Nucleares, Comissão Nacional de Energia Nuclear, São Paulo, Brazil) was added (10 minutes). These samples were centrifuged (1500 rpm, 5 minutes) and aliquots of plasma (P) and blood cells (BC) were isolated. Another aliquots of P and BC were separated and also precipitated with 5% trichloroacetic acid and centrifuged (1500 rpm, 5 minutes) to isolate insoluble fractions (IF). The radioactivity in BC, IF-P and IF-BC was determined in a well counter (Packard, model C5002, Illinois, USA) and the percentage of radioactivity (%ATI) was calculated.
Morphological evaluation of red blood cells
Blood smears were prepared, dried, fixed and staining by May-Grünwald-Giensa method (Junqueira and Carneiro, 2002). Images of RBC were acquired (Optronics, Japan) from blood smears to qualitative morphology analysis under optical microscopy (x1000). To morphometric analysis of RBC the perimeter/area ratio was obtained from images by specific program (Image ProPlus Software).
Statistical analysis
The data are expressed as means ± SD of %ATI and perimeter/area ratio. The values were analyzed by one-way analysis of variance (ANOVA) with a p<0.05 as significant level. Statistical analysis was performed using InStat Graphpad software (GraphPad InStat version 3.00 for Windows 95, GraphPad Software, San Diego, California, USA).
RESULTS
The figure 1 shows the ATI% of BC, IF-P and IF-BC from Wistar rats treated with cinnamon extract. Data indicate that there is no significant (p>0.05) modification on the uptake of radioactivity by BC and on the fixation of 99mTc on the IF-P and IF-BC from animals treated with cinnamon extract.
Figure 2 shows the %ATI of BC, IF-P and IF-BC from Wistar rats treated with an cinnamon extract (150.0 mg/kg) for different periods of time. The data presented in this figure suggest that the extract has not significantly (p>0.05) altered the 99mTc-labeling of these blood constituents at the different times of treatment studied.
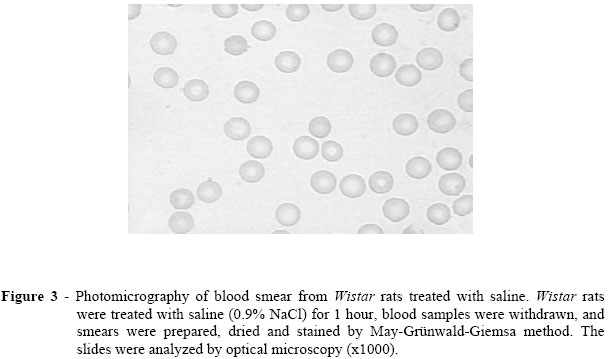
The figures 3 and 4 show the photomicrographs of the blood smears from Wistar rats treated with saline (control) and with an cinnamon extract at the higher concentration used (150.0 mg/kg), respectively. Qualitative morphological analysis suggests that the cinnamon extract has not induced important changes on the shape of the RBC.
The figures 3 and 4 show the photomicrographs of the blood smears from Wistar rats treated with saline (control) and with an cinnamon extract at the higher concentration used (150.0 mg/kg), respectively. Qualitative morphological analysis suggests that the cinnamon extract has not induced important changes on the shape of the RBC.
Figure 7 shows the perimeter/area ratio of RBC from Wistar rats treated with a cinnamon extract at different doses. Data indicate that the aqueous cinnamon extract has not significantly (p>0.05) modified the perimeter/area ratio of RBC.
The figure 5 shows the perimeter/area ratio of RBC from Wistar rats treated with a cinnamon extract (150.0 mg/kg) for different periods of time. These data confirm the absence of effects of the cinnamon extract used on the RBC morphology indicating no significant (p>0.05) modifications on the perimeter/area ratio.
DISCUSSION
The data obtained in this work showed that cinnamon extract at different doses has not effects on the uptake of 99mTc by blood cells and fixation of 99mTc on plasma and blood cells proteins (Fig. 1). Also, no modifications on radiolabeling of blood constituents were obtained when animals were treated for up to 120 minutes with cinnamon extract at the higher dose used (Fig. 2).
It has been reported that in vitro natural or synthetic drugs could alter the labeling of blood constituents with 99mTc (Fonseca et al., 2005; Frydman et al., 2008a). However, other products are not able to interfere on this labeling process, as Pfaffia sp. (Fernandes et al., 2005). Recent data have suggested that in vivo acetylsalicylic acid treatment could alter the labeling of blood constituents with 99mTc (Fonseca et al., 2007).
Pharmacological effects of interest to human health have been reported with 1 up to 6 g per day of cinnamon as crude drug or extracts (Khan et al., 2003). These values are close to commonly-suggested doses (2 up to 4 g) to cinnamon as crude drug (WHO, 1999). In our experiments, animals were treated with cinnamon extract at doses similar to used by human beings as spices or herbal medicine. The absence of effects of the in vivo treatment with cinnamon extract are in disagree with previous data obtained in an in vitro study, whose data have suggested alterations on labeling of BC with 99mTc (Benarroz et al., 2008). This discrepancy could be explained by metabolic process of compounds in cinnamon extract that occurs when an in vivo treatment is performed. Other hypothesis could be the difference in the pharmacokinetic profile to each compound present in cinnamon extract (gastrointestinal absorption and plasma peak concentration) that did not occur in an in vitro treatment.
RBC has been proposed as a prototypical cellular system regarding drug mediated plasma membrane effects (Li et al., 1999). It has been suggested that cinnamon extracts could affect the membrane of eukaryotic cells inhibiting the Na+ -K+-ATPase (Verspohl et al., 2005) and calcium currents (Su et al., 1999). The qualitative and quantitative (area and perimeter) analyses have been used to evaluate the alterations induced by drugs on membrane of RBC (Frydman et al., 2008b). However, aqueous cinnamon extract used could not cause morphological modifications on the membrane of RBC at different doses (Fig. 3, 4 and 5) or different period of treatment (Fig. 6 and 7).
These results are agreed with data obtained in an in vitro study when similar cinnamon extract was used (Benarroz et al., 2008).
In conclusion, our data suggest that in vivo aqueous cinnamon extracts could not alter the labeling of blood constituents with 99mTc and could not affect the membrane structures involved in transport of ions or the oxidation state of stannous and pertecnetate ions.
ACKNOWLEDGEMENTS
This research was supported by Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Universidade do Estado do Rio de Janeiro (UERJ). We are grateful for his technical support and to Mr. Carlos Brown Scavarda (B. A., University of Michigan) for the English language revision.
REFERENCES
Benarroz, M. O.; Fonseca AS; Rocha GS; Frydman JN; Rocha VC; Pereira MO; Bernardo-Filho M. (2008), Cinnamomum zeylanicum extract on the radiolabelling of blood constituents and the morphometry of red blood cells: In vitro assay. Appl Radiat Isot., 66, 139-146.
Callahan RJ; Rabito CA. (1990). Radiolabeling of erythrocytes with technetium-99m: role of band-3 protein in the transport of pertechnetate across the cell membrane. J Nucl Med., 31, 2004-2010.
Fernandes JFO; Brito LC; Frydman JNG; Santos-Filho S; Bernardo-Filho M. (2005). An aqueous extract of Pfaffia sp. Does not alter the labeling of blood constituents with technetium-99m and the morphology of the red blood cells. Braz J Pharmacogn., 126-132.
Fonseca AS; Frydman JN; Rocha VC; Bernardo-Filho M. (2007). Acetylsalicylic acid decreases the labeling of blood constituents with technetium-99m. Acta Biol Hung., 58, 187-198.
Fonseca AS; Frydman JNG; Santos R; Bernardo-Filho M. (2005). Influence of antipyretic drugs on the labeling of blood elements with technetium-99m. Acta Biol Hung., 56, 275-282.
Frydman JNG; Rocha VC; Benarroz MO; Rocha GS; Pereira MO; Fonseca AS; Bernardo-Filho M. (2008a) Assessment of effects of a Cordia salicifolia extract on the radiolabeling of blood constituents and on the morphology of red blood cells. J Med Food, in press.
Frydman JNG; Fonseca AS; Rocha VC; Benarroz MO; Rocha GS; Pereira MO; Pereira MJ; Bernardo-Filho M. (2008b). Acetylsalicylic acid and morphology of red blood cells. Braz Arch Biol Technol., in press.
Gutfilen B; Boasquevisque EM; Bernardo-Filho M. (1992). Calcium channel blockers: interference on red blood cells and plasma proteins labeling with 99mTc. Rev Esp Med Nucl., 11, 195-199.
Jayaprakasha GK; Rao LJ; Sakariah KK. (2002). Chemical composition of volatile oil from Cinnamomum zeylanicum buds. Z Naturforsch, 57, 990-993.
Khan A; Safdar M; Khan MMA; Khattak KN; Anderson RA. (2003). Cinnamom improves glucose and lipids of people with type 2 diabetes. Diabetes Care, 26, 3215-3218.
Li A; Seipelt H; Muller C; Artmann M. (1999). Effects of salicylic acid derivatives on red blood cell membranes. Pharmacol Toxicol., 85, 206-211.
Paoli, S.; Dias, A. P. M.; Capriles, P. V. S. Z.; Costa, T. E. M. M.; Fonseca, A. S.; Bernardo-Filho, M. (2008), Effects of a tomato (Solanum lycopersicum) extract on the labeling of blood constituents with technetium-99m. Rev Bras Farmacogn., 18, 190-196.
Saha GB. (2004). Fundamentals of nuclear pharmacy. Springer-Verlag, New York.
Santos-Filho, S. D.; Maiworm, A. I.; Presta, G. A.; Paoli, S.; Giani, T. S.; Bernardo-Filho, M. (2008), Aqueous extract of the medicinal plant Mentha crispa alters the biodistribution of the radiopharmaceutical sodium pertechnetate in Wistar rats. Med Chem Res., 16, 230-237
Shan B; Cai YZ; Sun M; Corke H. (2005). Antioxidant Capacity of 26 spices extracts and characterization of their phenolic constituents. J Agric Food Chem., 53, 7749-7759.
Su MJ; Chen W P; Lo TY; Wu TS. (1999). Ionic Mechanisms for the antiarrhythmic action of cinnamophilin in rat heart. J Biomed Sci., 6, 376-386.
World Health Organization.(1999). Monographs on selected medicinal plants, 1, 95-103.
Received: August 06, 2008;
Revised: September 12, 2008;
Accepted: September 15, 2008.
- Benarroz, M. O.; Fonseca AS; Rocha GS; Frydman JN; Rocha VC; Pereira MO; Bernardo-Filho M. (2008), Cinnamomum zeylanicum extract on the radiolabelling of blood constituents and the morphometry of red blood cells: In vitro assay. Appl Radiat Isot., 66, 139-146.
- Callahan RJ; Rabito CA. (1990). Radiolabeling of erythrocytes with technetium-99m: role of band-3 protein in the transport of pertechnetate across the cell membrane. J Nucl Med., 31, 2004-2010.
- Fernandes JFO; Brito LC; Frydman JNG; Santos-Filho S; Bernardo-Filho M. (2005). An aqueous extract of Pfaffia sp. Does not alter the labeling of blood constituents with technetium-99m and the morphology of the red blood cells. Braz J Pharmacogn., 126-132.
- Fonseca AS; Frydman JN; Rocha VC; Bernardo-Filho M. (2007). Acetylsalicylic acid decreases the labeling of blood constituents with technetium-99m. Acta Biol Hung, 58, 187-198.
- Fonseca AS; Frydman JNG; Santos R; Bernardo-Filho M. (2005). Influence of antipyretic drugs on the labeling of blood elements with technetium-99m. Acta Biol Hung, 56, 275-282.
- Frydman JNG; Rocha VC; Benarroz MO; Rocha GS; Pereira MO; Fonseca AS; Bernardo-Filho M. (2008a) Assessment of effects of a Cordia salicifolia extract on the radiolabeling of blood constituents and on the morphology of red blood cells. J Med Food, in press
- Frydman JNG; Fonseca AS; Rocha VC; Benarroz MO; Rocha GS; Pereira MO; Pereira MJ; Bernardo-Filho M. (2008b). Acetylsalicylic acid and morphology of red blood cells. Braz Arch Biol Technol., in press.
- Gutfilen B; Boasquevisque EM; Bernardo-Filho M. (1992). Calcium channel blockers: interference on red blood cells and plasma proteins labeling with 99mTc. Rev Esp Med Nucl., 11, 195-199.
- Jayaprakasha GK; Rao LJ; Sakariah KK. (2002). Chemical composition of volatile oil from Cinnamomum zeylanicum buds. Z Naturforsch, 57, 990-993.
- Khan A; Safdar M; Khan MMA; Khattak KN; Anderson RA. (2003). Cinnamom improves glucose and lipids of people with type 2 diabetes. Diabetes Care, 26, 3215-3218.
- Li A; Seipelt H; Muller C; Artmann M. (1999). Effects of salicylic acid derivatives on red blood cell membranes. Pharmacol Toxicol, 85, 206-211.
- Paoli, S.; Dias, A. P. M.; Capriles, P. V. S. Z.; Costa, T. E. M. M.; Fonseca, A. S.; Bernardo-Filho, M. (2008), Effects of a tomato (Solanum lycopersicum) extract on the labeling of blood constituents with technetium-99m. Rev Bras Farmacogn, 18, 190-196.
- Saha GB. (2004). Fundamentals of nuclear pharmacy Springer-Verlag, New York.
- Santos-Filho, S. D.; Maiworm, A. I.; Presta, G. A.; Paoli, S.; Giani, T. S.; Bernardo-Filho, M. (2008), Aqueous extract of the medicinal plant Mentha crispa alters the biodistribution of the radiopharmaceutical sodium pertechnetate in Wistar rats. Med Chem Res., 16, 230-237
- Shan B; Cai YZ; Sun M; Corke H. (2005). Antioxidant Capacity of 26 spices extracts and characterization of their phenolic constituents. J Agric Food Chem, 53, 7749-7759.
- Su MJ; Chen W P; Lo TY; Wu TS. (1999). Ionic Mechanisms for the antiarrhythmic action of cinnamophilin in rat heart. J Biomed Sci, 6, 376-386.
- World Health Organization.(1999). Monographs on selected medicinal plants, 1, 95-103.
Publication Dates
-
Publication in this collection
20 Feb 2009 -
Date of issue
Dec 2008
History
-
Accepted
15 Sept 2008 -
Received
06 Aug 2008 -
Reviewed
12 Sept 2008