Abstracts
The present study was conducted to investigate the influence of initial sucrose concentration, pH and aeration rate on biomass and inulinase production by Kluyveromyces marxianus var. bulgaricus in a stirred batch reactor. Maximum inulinase activity (15.29 UmL-1) was obtained at a sucrose concentration of 10 g L-1, pH 5.0 and aeration rate of 1 vvm. The 20 g L-1 sucrose concentration was suitable for cell growth; however, enzymatic activity at this concentration was inhibited due to catabolic repression. The increase in aeration rate caused a reduction in enzyme activity with no relevant biomass increase.
Kluyveromyces marxianus var. bulgaricus; sucrose; inulinase; biomass; batch fermentation
O estudo foi conduzido para investigar a influência da concentração inicial da sacarose, a taxa da aeração e do pH na biomassa e na produção da inulinase pela Kluyveromyces marxianus var. bulgaricus em um reator em batelada. A máxima atividade de inulinase, 15.29 UmL-1, foi obtida na concentração de 10 g L-1 de sacarose, no pH 5.0 e na taxa da aeração de 1 vvm. A concentração de sacarose de 20g L-1 foi apropriada para o crescimento celular, porém nesta concentração a atividade enzimática foi inibida, devido a repressão catabólica. O aumento na taxa da aeração propiciou redução da atividade enzimática, ao mesmo tempo em que não houve aumento considerável do biomassa.
FOOD/FEED SCIENCE AND TECHNOLOGY
Effects of culture conditions on the production of inulinase by Kluyveromyces marxianus
Marcia Luciana CazettaI; Rubens MontiII; Jonas ContieroI,* * Author for correspondence: jconti@rc.unesp.br
IDepartamento de Bioquímica e Microbiologia; Instituto de Biociências de Rio Claro; Universidade Estadual Paulista; Av. 24 A 1515; 13506-900; Rio Claro - SP - Brasil
IIDepartamento de Alimentos e Nutrição; Faculdade de Ciências Farmacêuticas de Araraquara; Universidade Estadual Paulista; Araraquara - SP - Brasil
ABSTRACT
The present study was conducted to investigate the influence of initial sucrose concentration, pH and aeration rate on biomass and inulinase production by Kluyveromyces marxianus var. bulgaricus in a stirred batch reactor. Maximum inulinase activity (15.29 UmL-1) was obtained at a sucrose concentration of 10 g L-1, pH 5.0 and aeration rate of 1 vvm. The 20 g L-1 sucrose concentration was suitable for cell growth; however, enzymatic activity at this concentration was inhibited due to catabolic repression. The increase in aeration rate caused a reduction in enzyme activity with no relevant biomass increase.
Key words:Kluyveromyces marxianus var. bulgaricus, sucrose, inulinase, biomass, batch fermentation
RESUMO
O estudo foi conduzido para investigar a influência da concentração inicial da sacarose, a taxa da aeração e do pH na biomassa e na produção da inulinase pela Kluyveromyces marxianus var. bulgaricus em um reator em batelada. A máxima atividade de inulinase, 15.29 UmL-1, foi obtida na concentração de 10 g L-1 de sacarose, no pH 5.0 e na taxa da aeração de 1 vvm. A concentração de sacarose de 20g L-1 foi apropriada para o crescimento celular, porém nesta concentração a atividade enzimática foi inibida, devido a repressão catabólica. O aumento na taxa da aeração propiciou redução da atividade enzimática, ao mesmo tempo em que não houve aumento considerável do biomassa.
INTRODUCTION
Inulinase is classified as a hydrolase and is designed as a 2,1-β-D-fructan fructanohydrolase (EC 3.2.1.7). Unlike an invertase, an inulinase is a non-specific b-fructofuranosidase and can hydrolyze 2,1-linked and 2,6-linked b-D-fructofuranose residue in fructan with the release of b-D-fructose. Natural inulinase substrates include inulin, sucrose and levan (Nagem et al., 2004). This enzyme has advantages over the use of invertase in food industries.
Interest in inulinase was aroused by with the discovery that this enzyme has the capability of hydrolyzing the inulin in practically pure fructose. This represents a great advantage over the conventional production of fructose from starch (potato or corn), which requires three enzymatic stages, including the action of a-amylase, amyloglucosidase and glucose isomerase (Vandame and Derycke, 1983). This process produces 45% of the fructose solution due to the thermodynamic equilibrium between fructose and glucose.
Fructose can also be obtained by acid hydrolysis of inulin. However, this is not the method of choice for fructose production, as it results in the formation of difructose anhydrides, which are colored and have no sweetening capacity (Vandame and Derycke, 1983). Fructose synthesis by microbial inulinase, on the other hand, can yield up to 95 percent pure fructose in a single enzymatic step and promises to be a viable alternative (Vandame and Derycke, 1983; Gill et al., 2005; Zhang et al., 2005). Despite providing an adequate tool for overcoming the drawbacks, as the process is carried out under mild pH (4 to 5) and temperatures (35 to 0ºC), compared to the pH 2 and 75ºC used in the chemical process (Kim and Rhee, 1989; Arruda and Vitolo, 1999; Sturm, 1999; Ettalibi and Baratti, 2001), this process has not yet been commercialized. Inulinases is also usually thermostable and commercially available for industrial applications (Chiang et al., 1997; Gupta et al., 1998).
Inulin is described as the most commonly used substrate in inulinase production. Inulin is a fructose polymer that consists of linear chains of b-2,1 linked D-fructofuranose molecules terminated at reduction and by a glucose residue attached through a sucrose-type linkage (Nakamura et al., 1995; Menne et al., 2000). However, due to its non-specificity, other substrates are successfully being used to produce inulinase by K. marxianus. Grootwassink and Flemming (1980) obtained high inulinase synthesis in a batch culture with a glucose, fructose and sucrose medium at low concentrations. In this type of process, Hewitt and Grootwassink (1984) found that maximum activity in sucrose was as high as in inulin. Rownhenrost et al. (1988) also obtained high inulinase production by K. marxianus CBS 6556 in a continuous sucrose-limited culture. The same occurred in experiments performed by Kushi et al. (2000) with K. marxianus var. bulgaricus. Thus, the aim of the present study work was to investigate the influence of culture condition on inulinase production by K. marxianus var. bulgaricus using sucrose as the carbon source in batch fermentation, as Brazil is an important sucrose producer and the industry that uses inverted sugar has a great interest in this research.
MATERIAL AND METHODS
Microorganism
Kluyveromyces. marxianus var. bulgaricus ATCC 16045 was obtained from the Department of Food Engineering of Campinas University UNICAMP. The stock culture was preserved frozen in cryogenic tubes with 20% glycerol. The culture was transferred to Petri dishes and maintained at 4ºC until use
Cell growth
Biomass was determined by measuring the turbidity of the diluted sample at 600 nm using a standard absorbance curve against the dry cell mass.
Enzymatic activity
The enzymatic activity of the supernatant was determined following the procedures described by Suzuki et al. (1988), through the determination of reducing sugars formed by the incubation of 1 mL of enzyme in 2% sucrose, 0.05 M citrate-phosphate buffer in pH 4.0 at 50ºC, using 3,5-dinitrosalisylic acid (Miller, 1959). Glucose (1 g L-1) was used for the standard curve. One unit of inulinase activity is defined as the amount of enzyme that hydrolyses 1 mmol of sucrose per min under the above conditions.
Total reducing sugars (TRS)
Total reducing sugars (TRS) were determined following the hydrolysis of sucrose with 2 M HCl and neutralization with 2 M NaOH through 3,5-dinitrosalisylic acid methods (Miller, 1959).
Batch culturing
Fermentations were carried out in 5-L fermenter containing 2 L of culture medium. The fermentation medium was made up of g/L sucrose (5, 10 and 20 ), 5 yeast extract, 10 peptone, 5 KH2PO4, 1.5 NH4Cl, 1.2 KCl and 0.7 MgSO4.7H2O. The medium was sterilized in an autoclave at 121ºC for 30 min. The sucrose solution was sterilized separately and added aseptically to the culture medium. The pH was adjusted by adding orthophosphoric acid. Temperature was controlled at 30ºC and the agitation rate was 300 rpm. The inoculum was prepared using the fermentation medium with 10 g L-1 of sucrose and maintained under agitation over night at 180 rpm and 30ºC. Ten percent of this was transferred to the fermentation medium.
RESULTS AND DISCUSSION
Influence of initial sucrose concentration
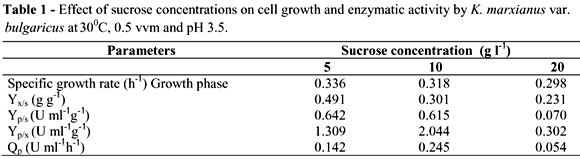
In order to determine the effect of carbon source concentration on biomass and inulinase production, three sucrose concentrations were studied (5, 10 and 20 g L-1) in a 0.5 vvm aerated reactor at 300 rpm of agitation, 30ºC and a working volume of 2 L. The initial pH of the medium was adjusted to 3.5. At 5 g l-1 of initial sucrose concentration, the cell mass yield was 2.76 g l-1. At 10 g l-1 of sugar concentration, the cell growth reached 3.11 g l-1 after 24 h of culture (Fig. 1A). The biomass yield (Yx/s), however was higher in the 5 g l-1 sucrose concentration (0.491 g g-1). In the 10 g l-1 sucrose concentration, biomass yield was 0.318 g g-1 (Table 1).
In cultures with a lower initial sucrose concentration (5 g L-1), enzymatic activity was 3.42 U mL-1 and at 10 g L-1 6.51 U mL-1 after 24 h (Fig. 1B). This signified a higher inulinase yield (Yp/x) for cultures at 10 g L-1 (2.044 U mL-1 g-1 compared to 1.309 U mL g-1 at a sucrose concentration of 5 g L-1). Inulinase yield for sucrose (Yp/s) was similar for both cultures and productivity was also higher in the 10 g L-1 sucrose concentration (Table 1). When the sugar concentrations increase from 10 to 20 g L-1, the cell growth increased to 4.5 g L-1 after 24 h, which represented an increase of 30 %. Nevertheless, the higher initial sucrose substrate concentration, three-fold decrease (1.92 U mL-1) (Fig. 1B). Cazetta et al. (2005) observed the same results using yacon extract as substrate, in which K. marxianus show a decrease of about 14% in enzyme activity at concentrations above 40% (v/v) of the extract. Repression by substrate is common in the metabolism of many microorganisms. Wee et al. (2004) also observed a decrease in lactic acid yield produced by Enterococcus faecalis with an increase in sugar concentration in the medium.
The substrate was completely consumed after 6 h in the 5 and 10 g L-1 sucrose concentrations. At 20 g L-1 of sucrose concentration, substrate was spent after 8 h of culturing (Fig. 2). Inulinase synthesis started together with cell growth and increased after 6 to 8 h, when the amounts of total carbohydrate and reducing sugars were low, suggesting that the inulinase synthesis was suppressed by the high concentration of reducing sugars, as observed by Wei et al. (1998).
According to Parekh and Margaritis (1986), inulinase synthesis is controlled by catabolic repression and higher productions are reached at the end of the growth phase, apparently demonstrating that low concentrations of the carbon source are a prerequisite for inulinase synthesis. Pinheiro et al. (2000) also found that, at low concentrations, K. marxianus ATCC 10022 completely consumed the substrate, producing biomass and ethanol. However, in higher concentrations, this yeast only consumed 30% of the initial substrate, resulting in less productivity.
Similar to the inulinase of Aspergillus niger A 42 (Öngen-Baysal et al., 1994), A. fumigatus (Kauer et al., 1999) and Streptomyces sp. (Gill et al., 2003), K. marxianus var. bulgaricus inulinase also appeared to be regulated by a double mechanism: increase by the substrate and repression by the product (glucose and fructose), as the activity decreased with sucrose concentrations above 10 g L-1, probably suppressed by reducing sugars.
Influence of pH
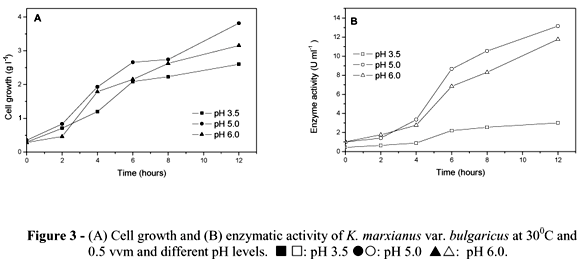
Figures 3A and 3B illustrate the effect of different culture pH values (3.5, 5.0 and 6.0) on cell growth and inulinase activity by K. marxianus var. bulgaricus. The experiments were carried out in a reactor at 30ºC, 0.5 vvm aeration and agitation of 300 rpm. As seen in Fig. 3A, pH did not significantly influence cell growth, achieving 3.13 g L-1 at pH 3.5; 3.82 g L-1 at pH 5.0 and 3.22 at pH 6.0 6.0 However, the specific growth rate (m) increased concomitantly with pH and gave the highest yield at pH 6.0 (0.814h-1). For enzyme activity, however, the best pH was 5.0, reaching 13.14 U ml-1 after 10 h of fermentation, followed by pH 6.0, with 11.75 U ml-1. pH 3.5 was an unfavorable condition, as enzyme activity was 4.27 U mL-1 after 12 h (Fig. 3B). Inulinase yield for sucrose (Yp/s) and inulinase yield for biomass (Yp/x), were similar for pH 5.0 and pH 6.0 (Table 2). Productivity (Qp) was higher at pH 5.0 (1.31 U ml-1 h-1) (Table 2). Pessoa and Vitolo (1999) obtained better results in a batch culture with inulin as the substrate (26 U ml-1) in the pH range from 3.5 to 5.0 for K. marxianus DMS 70106.
Effect of aeration rate
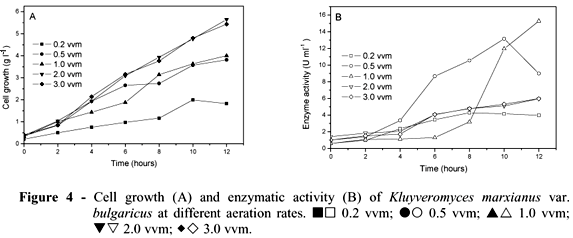
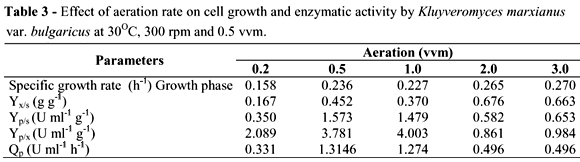
Figures 4A and 4B illustrate the cell growth and enzymatic activity by K. marxianus at different aeration rates (vvm): 0.2, 0.5, 1.0, 2.0 and 3.0. Fermentations were performed at pH 5.0, 30ºC and 300 rpm. Cell growth was generally positively affected by the aeration increase. The lowest growth was observed at 0.2 vvm (1.83 g l-1). Cell growth was similar at both 0.5 vvm and 1.0 vvm, reaching about 4.0 g l-1. Higher cell growth was obtained at 2 and 3 vvm (5.65 and 5.43 g L-1, respectively). The increase in aeration rate was accompanied by increase of specific growth rate (m) from 0.158 to 0.270 h-1. The same occurred for the maximum values of biomass yield (Yx/s) (Table 3). Working with two strains of K. marxianus (K. marxianus ATCC 10022 and CBS 7894), Pinheiro et al. (2000) observed that a small 2-bar increase in air pressure led to a three-fold increase in biomass yield, whereas further increase in air pressure did not lead to a significant increase in biomass yield.
However, enzyme activity decreased with the increase in aeration. The highest enzymatic activity was achieved at 1.0 vvm (15.29 U ml-1) after 12 h, followed by 0.5 vvm (13.15 U ml-1). At 2 and 3 vvm, enzymatic activity decreased significantly, achieving about 6.0 U ml-1 after 12 h (Fig. 4B). The highest values for inulinase yield for sucrose (Yp/s), inulinase yield for biomass (Yp/x) and productivity (Qp) were obtained at 0.5 and 1.0 vvm. The 0.2 vvm aeration was an insufficient rate, showing about 4.0 U mL-1 of enzyme activity (Table 3).
Silva-Santisteban and Maugeri Filho (2005) described a similar behavior in experiments using K. marxianus var. bulgaricus. The authors did not obtain an increase in inulinase yield with an increase in aeration rate from 1 to 2 vvm (89 and 84 IU mL-1, respectively) after 72 h. In fed-batch fermentation with sucrose as the substrate, Cazetta (2005) also observed higher inulinase production at 1 vvm. Cristiani-Urbina et al. (2005) observed cellular metabolism alteration from oxidative to a mix oxidative-fermentative in aerated cultures of some K fragilis strains, resulting in the production of metabolites such as alcohols, aldehydes and esters, which reduced biomass production.
In conclusion, the best condition for inulinase production in batch-reactor fermentation was 10 g l-1 of initial sucrose concentration, pH 5.0 and 1 vvm aeration rate.
NOMENCLATURE
Yx/s - sucrose biomass yield (g g-1)
Yp/s sucrose inulinase yield (U mL-1g-1)
Yp/x inulinase biomass yield (U mL-1g-1)
Qp volumetric enzyme production (U mL-1 h-1)
µ - specific growth rate (h-1)
rpm revolutions per minute (min 1)
ACKNOWLEDGEMENTS
The authors are grateful to CNPq-Brazil Agency and Sao Paulo State University-Unesp for financial support to develop this work.
Received: June 05, 2007; Revised: October 23, 2007; Accepted: July 02, 2009.
- Nagem, RAP, Rojas, AL, Golubev AM, Korneeva OS, Eneyskaya EV, Kulminskaya AA, Neustroev KN, Polikarpov (2004), I. Crystal structure of exo-inulinase from Aspergillus awamori: the enzyme fold and structural determinants of substrate recognition. J Mol Biol; 344, 471-480.
- Vandame E, Derycke D. (1983), Microbial inulinases: Fermentation process, properties and applications. Advances Appl Microbiol; 29, 139-176.
- Gill PK, Manhas RK, Singh P. (2005), Comparative analysis the thermostability of extracellular inulinase activity from Aspergillus fumigatus with commercially available (Novozyme) inulinase. Bioresour Technol. Avaiable on line: <http://www.sciencedirect.com
- Zhang l, Zhao C, Ohta WY, Wang, Y. (2005), Inhibition of glucose on an exoinulinase from Kluyveromyces marxianus expressed in Pichia pastoris Proc Biochem; 40, 1541-1545.
- Kim CH, Rhee SK. (1989), Fructose production from Jerusalem artichoke by inulinase immobilized on chitin. Biotechnol Lett; 11, 201-206.
- Arruda LM, Vitolo M. (1999), Charachterization of invertase entrapped into calcium alginate beads. Appl Biochem Biotechnol; 8, 23-33.
- Sturm A. (1999), Invertases: primary structures, functions and roles in plant development and sucrose partitioning. Plant Physiol; 121, 1-7.
- Ettalibi M, Baratti JC. (2001), Sucrose hydrolysis by thermostable immobilized inulinases from Aspergillus ficuum. Enzyme Microb Technol; 28, 596-601.
- Chiang CJ, Lee WC, Sheu DC, Duan KJ. (1997), Immobilization of beta-fructofuranosidades from Aspergillus on methacrylamide-based polymeric beads for production of fructooligosaccharides. Biotechnol Prog; 13, 577-582.
- Gupta AK, Rathore P, Kaur N. (1998), A HgCl2 insensitive and thermally stable inulinase from Aspergillus oryzae Phytochem; 49 (1), 55-58.
- Nakamura T, Ogata Y, Shitara A, Nakamura A, Ohta K. (1995). Continuous production of fructose syrups from inulin by immobilized inulinase from Aspegillus niger mutant 817. J Ferment. Bioeng; 80 (2), 164-169.
- Menne E, Guggenbuhl N, Roberfroid M. (2000), Fn-type chicory inulin hydrolysate has a prebiotic effect in humans. J Nutr; 130, 1197-1199.
- Grootwassink JWD, Fleming SE. (1980), Non-specific b-frutofuranosidase (inulinase) from Kluyveromyces fragilis: batch and continuous fermentation, simple recovery method and some industrial properties. Enzyme Microb Technol; 2, 45-53.
- Hewitt GM, Grootwassink, WD. (1984), Simultaneous production of inulase and lactase in batch and continuous cultures of Kluyveromyces fragilis Enzyme Microb Technol; 6, 263-270.
- Rouwenhorst RJ, Visser LE, Van der Baan AA, Scheffers WA, van Dijken JP. (1988), Production, distribution and kinetic properties of inulinase in continuous cultures of Kluyveromyces marxianus CBS 6556. Appl Environ Microbiol; 54, 1131-1137.
- Kushi RT, Monti R, Contiero J. (2000), Production, purification and characterization of an extracellular inulinase from Kluyveromyces marxianus var. bulgaricus J Ind Microbiol Biotechnol., 25, 63-69.
- Suzuki H, Ozawa Y, Maeda H. (1988), Studies of water-insoluble yeast invertase. Agric Biol Chem; 30, 807-812.
- Miller GL. (1959), Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal Chem; 31 (3), 426-429.
- Cazetta ML, Martins, PMM, Monti R, Contiero J. (2005), Yacon (Polymnia sanchifolia) extract as a substrate to produce inulinase by Kluyveromyces marxianus var. bulgaricus J Food Eng; 66, 301-305.
- Wee Y J, Kim JN, Yun JS, Ryu HW. (2004), Utilization of sugar molasses for economical L(+)-lactic acid production by batch fermentation of Enterococcus faecalis. Enzyme Microbiol Technol; 35, 568-573.
- Wei W, Zheng Z, Liu Y, Zhu X. (1998), Optimizing the culture conditions for higher inulinase production by Kluyveromyces sp. Y-85 and scaling-up fermentation; 86 (4), 395-399.
- Parekh S, Margaritis A. (1986), Production of inulinase (b- Fructan Fructanohydrolase) by Kluyveromyces marxianus. Agricultural Biol Chem; 50 (4), 1085-1087.
- Pinheiro R, Belo I, Mota M. (2000), Air pressure effects on biomass yield of two different Kluyveromyces strains. Enzyme Microbiol Technol; 26, 756-762.
- Öngen-Baysal G, Sukan SS, Vassilev N. (1994), Production and properties of inulinase from Aspergillus niger. Biotechnology Letters; 16 (3), 275-280.
- Kauer A, Sharma D, Harchand RK, Singh P, Bhullar SS, Kaur A. (1999), Production of a thermostable extracellular inulinase by Aspergillus fumigatus Indian J Microbiol; 39, 99-103.
- Gill P K, Sharma AD, Harchand R K, Singh P. (2003), Effect of media supplements and culture conditions on inulinase production by an actinomycete strain. Bioresource Technol; 87, 359-362.
- Pessoa Jr A, Vitolo M. (1999), Inulinase from Kluyveromyces marxianus: culture medium composition and enzyme extraction. Brazilian J Chem Eng; 16 (6), 1-14.
- Silva-Santisteban BOY, Maugeri Filho F. (2005), Agitation, aeration and shear stress as key factors in inulinase production by Kluyveromyces marxianus Enzyme Microbiol Technol; 36, 717-724.
- Cazetta ML. (2005), Produção de biomassa e inulinase por Kluyveromyces marxianus var. bulgaricus: influência das condições de cultivo. Tese (Doutorado) Depratamento de Bioquímica e Microbiologia, Universidade Estadual Paulista; 2005. Rio Claro Universidade Estadual Paulista;, 113 p.
- Cristiani-Urbina E, Netzahuatl-Muńoz AR, Manriquez-Rojas FJ, Juárez-Ramírez C, Ruiz-Ordaz N, Galínder-Mayer J. (2000), Batch and fed-batch cultures for a treatment of whey with mixed yeast cultures. Proc Biochem; 35, 649-657.
Publication Dates
-
Publication in this collection
11 June 2010 -
Date of issue
June 2010
History
-
Received
05 June 2007 -
Reviewed
23 Oct 2007 -
Accepted
02 July 2009