Abstract
This article reports a first contribution for the elucidation of catalytic mechanism of Lipase from porcine pancreas, type VI-s (PPL), in hydrolyzing an ester substrate in aqueous media. The conclusions were based on the pH-profiles of Michaelis-Menten parameters k cat/Km, k cat and Km, as well as on the absolute temperature profile of k cat/Km, obtained during the hydrolysis of p-nitrophenyl laurate by PPL. It was found that (a) PPL performs catalysis by means of ion pairs formed either as Ser152-Ο-/His263-Im+H and/or Carbonyl-Ο-/His263-Im+H, (b) the parameter k cat/Km equals to k1 and thus ES is formed and destroyed in the course of a series of consecutive reactions governed by the dynamic constant K S = k2/k1, and (c) the hydrolysis of substrate is assisted by a hydrogen bond developed between deprotonated Asp176 and the positively charged imidazole of His263 across a pKa-value 3.85, necessary for efficient catalysis.
Porcine Pancreas Lipase; mechanism of hydrolysis; p-nitrophenyl laurate
HUMAN AND ANIMAL HEALTH
Aspects on the catalysis of lipase from porcine pancreas (type VI-s) in aqueous media: development of ion-pairs
Marina Kokkinou; Leonidas G. Theodorou; Emmanuel M. Papamichael* * Author for correspondence: epapamic@cc.uoi.gr
Sector of Organic Chemistry and Biochemistry; Laboratory of Enzymology; Dept. of Chemistry; University of Ioannina; Ioannina 45110 - GREECE
ABSTRACT
This article reports a first contribution for the elucidation of catalytic mechanism of Lipase from porcine pancreas, type VI-s (PPL), in hydrolyzing an ester substrate in aqueous media. The conclusions were based on the pH-profiles of Michaelis-Menten parameters kcat/Km, kcat and Km, as well as on the absolute temperature profile of kcat/Km, obtained during the hydrolysis of p-nitrophenyl laurate by PPL. It was found that (a) PPL performs catalysis by means of ion pairs formed either as Ser152-Ο-/His263-Im +H and/or Carbonyl-Ο-/His263-Im +H, (b) the parameter kcat/Km equals to k1 and thus ES is formed and destroyed in the course of a series of consecutive reactions governed by the dynamic constant KS = k2/k1, and (c) the hydrolysis of substrate is assisted by a hydrogen bond developed between deprotonated Asp176 and the positively charged imidazole of His263 across a pKa-value 3.85, necessary for efficient catalysis.
Key words: Porcine Pancreas Lipase, mechanism of hydrolysis, p-nitrophenyl laurate
INTRODUCTION
Lipases or triacylglycerol acylhydrolases (E.C. 3.1.1.3) catalyze the hydrolysis of various chain-length fatty acids esters and form diacylglyceride or monoacylglyceride, and/or glycerol and free fatty acids. These enzymes have found a variety of industrial applications due to their catalytic properties on a wide spectrum of substrates, as well as due to their high stability towards extreme temperatures and pH-values of the reaction media (Verger 1997; Thomson et al. 1999). The kinetics and mechanisms of lipolysis have been studied to some extend to improve the applications of lipases, as controlled lipolysis is essential for the consistent quality of commercial products (Salleh et al. 2006). The catalytic site of lipases comprises three residues (Asp or Glu, His, Ser) in a straightforward similarity to serine proteases (Derewenda and Sharp, 1993). Generally, the minimum reaction Scheme 1 appears insufficient and ambiguous to explain how Lipase from porcine pancreas type VI-s (PPL) performs catalysis (Jaeger and Eggert, 1994); thus, it seems reasonable to study the catalytic mechanism of PPL.
This manuscript reports a first contribution for the elucidation of catalytic mechanism of the PPL in hydrolyzing the synthetic ester substrate CH3(CH2)10C(=O)-ONP (p-nitrophenyl laurate - L-p-ONP) in aqueous buffers of 0.01 µ ionic strength, containing 0.15% (w/v) Arabic gum and 5% (v/v) DMSO. For this reason, we analyzed the dependencies of Michaelis-Menten parameters in the hydrolysis of L-p-ONP by PPL as functions of pH-value and/or of kcat/Km versus absolute temperature, of the reaction means. We found that catalysis is performed by means of ion pairs formed either as Ser152-Ο-/His263-Im +H and/or Carbonyl-Ο-/His263 -Im+H, whereas kcat/Km is almost equal to k1; furthermore, the hydrolysis of the substrate is assisted by a hydrogen bond developed between  and the positively charged imidazole of His263 (lipase numbering), across a pKa-value 3.85, necessary for efficient catalysis (Theodorou et al. 2007a; 2007b).
and the positively charged imidazole of His263 (lipase numbering), across a pKa-value 3.85, necessary for efficient catalysis (Theodorou et al. 2007a; 2007b).
MATERIALS AND METHODS
Lipase from porcine pancreas type VI-s (PPL), p-nitrophenyl laurate (L-p-ONP), Arabic gum, 2-mercaptoethanol, dimethylsulfoxide (DMSO) and other chemical were purchased from Sigma.
The pH value of the stock phosphate buffers was checked on a radiometer pH-meter model PHM 82. Suitable pH and temperature activity measurements were carried out in aqueous 0.01 M buffers containing 2 mM 2-mercaptoethanol; same buffers were employed for active site titrations achieved by using the irreversible inhibitor PMSF. The working solutions of the substrate were prepared in DMSO (Theodorou et al, 2007a; 2007b).
All kinetic measurements were performed spectrophotometrically by initial velocities at 405 nm for the L-p-ONP substrate, in a Specord 205 UV-VIS spectrophotometer; aqueous lipase solutions, of about 3930 nµ, were prepared containing 5 gr/100 ml Arabic gum. In a typical kinetic run a test-tube was prepared, containing the appropriate quantities of buffer and enzyme solutions, and DMSO at a final total content of 5% (v/v); next, a reference-tube was prepared where Arabic gum solution had replaced the enzyme solution. Then, both tubes are placed in an ultrasonic bath for 5 min at the appropriate temperature and the reaction is initiated by the addition of 10-50 μl of substrate solution (in DMSO) in both test and reference tubes. The substrate concentration varied from 10 μM to 200 μM. Again, both tubes are placed in the ultrasonic bath for 15 min; then 1 ml NaOH 0.1 µ is added in both tubes and the absorbance of test tube was measured versus the reference one at 405 nm due to the release of p-nitro-phenyl anion  .
.
Additional, measurements were performed in the range 5.50 < pH < 10.50, in buffers of 0.01 M ionic strength prepared as previously (Theodorou et al. 2007a; 2007b), in order to obtain appropriate pH-(k) profiles. Similar measurements were performed at different temperatures ranging from 13ΟC to 65ΟC in phosphate buffers of 0.01 M ionic strength at pH 6.50 (Papamichael et al. 2009).
All parameters were estimated from initial velocities measurements, during the hydrolysis of L-p-ONP substrate by PPL, using nonlinear curve fitting of the appropriate equation to the experimental data. In more details, the experimental data of the dependencies of kcat/Km kcat and Km versus pH were best analyzed according to Schemes 2, 3 and 4, and best fitted by different simplified forms of equation (1) comprising three to five hydrogenic forms but only one operative reactive state (Topham et al. 1991). The experimental data of the dependency of kcat/Km versus temperature were best fitted by equation (2), where k1,0, a0, E1 and Ea= E-1 - E2, represent the values of: rate constant k1, the ratio k2/k-1, the activation energies corresponding to the rate constants k1, k-1 and k2, at the reference temperature T0 = 318.15ºK (45ΟC); T and R are the independent variable (temperature in ºK) and the gas constant (8.3144 Jmol-1 K-1), respectively (Theodorou et al. 2007a; 2007b; Valasaki et al, 2008; Papamichael et al. 2009; 2010; Papamichael and Theodorou, 2010).
Alternative fitting procedures of all series of the above mentioned experimental data were performed also by non-parametric curve fitting methods, and/or by suitable reparametrization of all used simplified forms of equations (1) and (2), until become linear in their parameters, as it has been described previously (Theodorou et al. 2001; Papamichael et al. 2000; Papamichael and Theodorou 2009). In most cases, global minima were approached, and reached to the same results.
RESULTS
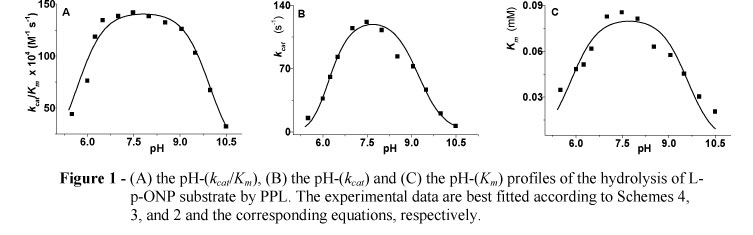
The experimental data from the pH-(kcat/Km) profile are best fitted by equation (kcat/Km)obs = (kcat/Km)lim/(1+10 pKa1+pKa2+pKa3-3pH+10pKa2+p Ka3-2pH +10pKa3-pH+10pH-pK a4) corresponding to Scheme 4; the experimental data from the pH-(kcat) and pH-(Km) profiles are best fitted by equations (kcat)obs= (kcat)lim/(1+10pKa1+p Ka2-2pH+10pKa2-pH+10pH-p Ka3 ) and (Km)obs = (Km)lim/(1+10pKa1-2pH+10 pH-pKa2), corresponding to Scheme 3 and 2, respectively.
All pH-profiles were found as bell-shaped showing maxima at almost neutral pH-values in all cases of Michaelis-Menten parameters (Fig. 1); the pKa-values were estimated with small standard deviations, and are depicted in Table 1.
In the case of absolute Temperature-(kcat/Km ) profile, the experimental data were best fitted by equation (2), and the following parameter values were estimated (Fig. 2):
(a) k1,0 = 1344643.56 ± 4.50 × 10-6 M-1s-1,
which corresponds to the rate constant k1, at 45ΟC,
(b) a0 = k2/k-1 = 21.30 ± (9.80 × 10-10),
(c) Ea = 214477.17 ± (2.50 × 10-6) kJ/mol, and
(d) E1 = 4315.38 ± (1.4 × 10-7) kJ/mol.
DISCUSSION
The pH-profiles obtained during the hydrolysis of L-p-ONP by PPL reflect the ionization corresponding to each single Michaelis-Menten parameter, which maintains enzyme active conformation and/or is directly involved in catalysis (Benjamin and Pandey 2000; Rajendran et al. 2009). Similarly, from the absolute temperature-(kcat/Km ) profile were obtained useful relations. However, we should emphasize that both pH and absolute temperature profiles were achieved by performing kinetic measurements in aqueous media of relatively low ionic strength containing 5% (v/v) DMSO and 0.15% (w/v) Arabic gum, whose usefulness in assays of lipase has been well documented (Mentez and Castro 2005; Salleh et al. 2006).
In all cases, the pKa-values were estimated with small standard errors. The pH-(kcat/Km) profile is affected by three ionizable groups in the acidic and one in the basic limb. A value of pKa1 = 2.21 could be due to the protonic dissociation of Asp176. Likewise, it seems more likely that a catalytic ion-pair (Ser152-Ο-/His263-Im +H) is formed across a pKa3 = 5.68, while it breaks across a pKa4 = 9.96. Furthermore, an estimate of pKa2 = 3.86 seems reasonable to be due to the development of a hydrogen bond connecting deprotonated Asp176 and positively charged His263. In fact, a reasonable explanation for the development of such a hydrogen bond may be based on the fact that the anion Ser152-Ο- (PPL numbering) should be free of hydrogen bonds in order to become more nucleophilic when it is attacking on the substrate (Theodorou et al. 2007a; 2007b; Papamichael et al. 2009; 2010; Papamichael and Theodorou, 2010). The best fit of the experimental data of pH-(kcat) and/or pH-(Km) profiles gave evidence for the estimation of three and/or two pKa-values, respectively. In these latter cases, it seems more likely that a different ion-pair is formed by means of the carbonyl oxygen of the acyl-group, and the positively charged His263 (Carbonyl-Ο-/His263 -Im+H), across almost identical estimates of pKa1 = 5.85 and/or 5.84 respectively. This second ion-pair breaks across a pKa-value 9.60 i.e. the mean value between pKa4 = 9.96, pKa3 = 9.23 and pKa2 = 9.60, estimated from the pH-(kcat/Km), pH-(kcat) and pH-(Km) profiles, respectively, which differ only less than 4% (Papamichael et al. 2004; Theodorou et al. 2007a; 2007b; Papamichael and Theodorou, 2010). Finally, an estimated pKa2 = 6.12 could denote the protonation of the N?2-atom of His263 as a H2O molecule is attacking the acyl-enzyme (Theodorou et al. 2001, 2007a, 2007b; Papamichael et al. 2004, 2009).
From the absolute temperature-(kcat/Km ) profile the ratio α0 = k2/k-1 = 21.30 was obtained, denoting the relation k2 >> k-1 and thus kcat/Km ≈ k1; similarly, the estimated values of activation energies Ea and E1 are in full agreement to the obtained results from the pH profiles of the Michaelis-Menten parameters. Moreover, the relation KS = (k2 + k-1)/k1 = k2/k1 is valid as k2>>k-1. On the basis of all mentioned above, it could be concluded that the ES complex is formed and destroyed in the course of a series of consecutive reactions governed by a dynamic constant, the so-called KS, established according to: 
Additionally, it is not unexpected that under the experimental conditions of the present work it was found that PPL hydrolyses p-nitrophenyl laurate (L-p-ONP) by means of general acid-base catalysis (ionic pairs), and not through a charge-relay-system, as it is the case in serine proteases having similar catalytic site with lipases. Accordingly, a first contribution in the elucidation of the catalytic mechanism of PPL in hydrolyzing the synthetic ester substrate L-p-ONP in aqueous media could be depicted by the below Scheme 5.
Received: July 15, 2011
Revised: November 27, 2011
Accepted: February 15, 2012
- Benjamin S, Pandey A. Isolation and Characterization of Three Distinct Forms of Lipases from Candida rugosa Produced in Solid State Fermentation, Braz Arch Biol Technol. 2000; 43: 453-460.
- Derewenda ZS, Sharp AM. News from the interface: the molecular structures of triacyglyceride lipases. Trends in Biochemical Sciences. 1993; 18: 20-25.
- Jaeger KE, Eggert T. Lipases for biotechnology. Current Opinion In Biotechnology. 2002; 13: 390-397.
- Mentez AA, de Castro HF. Effect on the Enzymatic Hydrolysis of Lipids from Dairy Wastewater by Replacing Gum Arabic Emulsifier for Sodium Chloride, Braz Arch Biol Technol. 2005; 48: 135-145.
- Papamichael EM, Evmiridis NP, Potosis C. Non-parametric Fitting of Nonlinear Equations to Experimental Data without Use of Initial Guessing: A Basic Computer Program. Braz. Arch. Biol. Technol. 2000; 43: 1-9.
- Papamichael EM, Theodorou LG, Bieth JG. Insight Into Catalytic Mechanism of Papain-Like Cysteine Proteinases: the case of D158 Applied Biochemistry and Biotechnology-Part A Enzyme Engineering & Biotechnology 2004; 118: 171-175.
- Papamichael EM, Theodorou LG. Necessary Auxiliary Background for Efficient Use of an Existing Computer Program of Non-parametric Fitting of Nonlinear Equations. Braz Arch Biol Technol 2009; 52: 437-448.
- Papamichael EM, Bieth JG, Theodorou LG, Lymperopoulos K, Valasaki K. The elucidation of the mechanism of action of cysteine proteinases of the Papain-C1 family: possible biotechnological applications, in New Horizons in Biotechnology, Eds: Pandey A., Larroche C.,m Soccol C-R., & Dussap C-G., Asiatech Publishers, INC. New Delhi, India, 2009. p. 104-122.
- Papamichael EM, Theodorou LG. Experimental and theoretical approaches in investigating enzymatic mechanisms: Applications on the thermo-stable extracellular Protease-A-17N-1 from Bacillus sp., with possible biotechnological interest, in Current Topics on Bioprocesses in Food Industry Vol III, Eds: Rao LV, Pandey A, Larroche C, Soccol CR, & Dussap CG. Asiatech Publishers, INC. New Delhi, India. 2010; p. 130-139.
- Papamichael EM, Theodorou LG, Perisynakis A, Drainas C. Purification and Characterization of a novel extracellular protease from a halo-alkalophilic Bacillus sp. 17N-1, active in polar organic solvents. Environmental Technology (Special Issue: Extremophiles) 2010; 31: 1073-1082.
- Rajendran A, Palanisamy A, Thangavelu V. Lipase Catalyzed Ester Synthesis for Food Processing Industries. Braz Arch Biol Technol. 2009; 52: 207-219.
- Salleh AB, Rahman R, Basri M. New Lipases and Proteases. Eds. Salleh A.B., Rahman RNZRA & Basri M., in Nova Science Publishers, Inc. New York; 2006. p. 1-22.
- Theodorou LG, Lymperopoulos K, Bieth JG, Papamichael EM. Insight into the Catalysis of Hydrolysis of four Newly Synthesized Substrates by Papain: A Proton Inventory Study. Biochemistry-US. 2001; 40: 3996-4004.
- Theodorou LG, Bieth JG, Papamichael EM. The catalytic mode of cysteine proteinases of papain (C1) family. Bioresource Technology. 2007a; 98: 1931-1939.
- Theodorou LG, Perisynakis A, Valasaki K, Drainas C, and Papamichael EM. Proton Inventories Constitute Reliable Tools in Investigating Enzymatic Mechanisms: Application on a Novel Thermo-stable Extracellular Protease from a Halo-Alkalophilic Bacillus sp. J. Biochem 2007b; 142: 293-300.
- Thomson CA, Delaquis PJ, Mazza G, Detection and measurement of microbial lipase activity. Crit Rew Food Sci Nutrit1999; 39: 165-187.
- Topham CM, Salih E, Frazao C, Kowlessur D, Overington JP, Thomas M, Brocklehurst SM, Patel M, Thomas EW, Brocklehurst K. Structure-function relationships in the cysteine proteinases actinidin, papain and papaya proteinase. Three-dimensional structure of papaya proteinase omega deduced by knowledge-based modelling and active-centre characteristics determined by two-hydronic-state reactivity probe kinetics and kinetics of catalysis. Biochem. J 1991; 280: 79-92.
- Valasaki K, Staikou A, Theodorou LG, Charamopoulou V, Zacharaki P, Papamichael EM. Purification and kinetics of two novel thermophilic extracellular proteases from Lactobacillus helveticus, from kefir with possible biotechnological interest. Bioresource Technology 2008; 99: 5804-5813.
- Verger R. Interfacial activation of lipases: Facts and artifacts. Trends in Biotechnology. 1997; 15: 32-38.
Publication Dates
-
Publication in this collection
03 May 2012 -
Date of issue
Apr 2012
History
-
Received
15 July 2011 -
Accepted
15 Feb 2012 -
Reviewed
27 Nov 2011