Abstract
The culture conditions viz. additional carbon and nitrogen content, inoculum size, age, temperature and pH of Lactobacillus acidophilus were optimized using response surface methodology (RSM) and artificial neural network (ANN). Kinetic growth models were fitted to cultivations from a Box-Behnken Design (BBD) design experiments for different variables. This concept of combining the optimization and modeling presented different optimal conditions for L. acidophilus growth from their original optimization study. Through these statistical tools, the product yield (cell mass) of L. acidophilus was increased. Regression coefficients (R²) of both the statistical tools predicted that ANN was better than RSM and the regression equation was solved with the help of genetic algorithms (GA). The normalized percentage mean squared error obtained from the ANN and RSM models were 0.06 and 0.2%, respectively. The results demonstrated a higher prediction accuracy of ANN compared to RSM.
Response surface methodology (RSM); Artificial neural network (ANN); Genetic algorithms (GA); Box-behnken besign (BBD)
AGRICULTURE, AGRIBUSINESS AND BIOTECHNOLOGY
Growth characteristics modeling of Lactobacillus acidophilus using RSM and ANN
Ganga Sahay MeenaI,* * Author for correspondence: gsmndri@gmail.com ; Nitin KumarI; Gautam Chandra MajumdarI; Rintu BanerjeeI; Pankaj Kumar MeenaII; Vijesh YadavII
IMicrobial Biotechnology and Downstream Processing Laboratory; Department of Agricultural and Food Engineering; Indian Institute of Technology; Kharagpur
IIBy Product Laboratory; Dairy Technology Division National Dairy Research Institute; Karnal - Haryana
ABSTRACT
The culture conditions viz. additional carbon and nitrogen content, inoculum size, age, temperature and pH of Lactobacillus acidophilus were optimized using response surface methodology (RSM) and artificial neural network (ANN). Kinetic growth models were fitted to cultivations from a Box-Behnken Design (BBD) design experiments for different variables. This concept of combining the optimization and modeling presented different optimal conditions for L. acidophilus growth from their original optimization study. Through these statistical tools, the product yield (cell mass) of L. acidophilus was increased. Regression coefficients (R2) of both the statistical tools predicted that ANN was better than RSM and the regression equation was solved with the help of genetic algorithms (GA). The normalized percentage mean squared error obtained from the ANN and RSM models were 0.06 and 0.2%, respectively. The results demonstrated a higher prediction accuracy of ANN compared to RSM.
Key words: Response surface methodology (RSM), Artificial neural network (ANN), Genetic algorithms (GA), Box-behnken besign (BBD)
INTRODUCTION
Lactobacillus is the most prominent members of plethora class of bacterial species with probiotic properties. The popularity of these group bacteria is based on the millennia of use in food and feed that are used in probiotic dairy drinks and yoghurts since hundred years (Sanders 1999). Most bacterial species of this class are formally classified as GRAS (generally recognized as safe) organisms. Currently, consumers are very much concerned about the nutritional and functional attributes of food worldwide. A number of health benefits such as anti-mutagenic effects, anti-carcinogenic properties, improvement in lactose metabolism, reduction in serum cholesterol and immune system stimulation have been claimed for probiotic foods, which fall in the category of functional foods (Shah 2007). Consequently, the global functional food market is thriving with recent estimates indicating up to a $50 billion annual share. The world probiotic market is estimated at $15 billions, which included 10% of the lactic bacteria drink market. Today, this market is growing at a pace of 5 to 30% depending on the country and product type. Although the worldwide market for probiotics is growing rapidly, yet, in India, it has just started to gestate for making its first move with leading companies such as Amul, Nestle and Mother Dairy (Bhadoria and Mahapatra 2011). Probiotics have a strong stimulatory effect for both the normal development of microbiota and maturation of gut associated lymphoid tissue (Schezenmeir and De Vrese 2001). Probiotic bacteria such as Lactobacillus and Bifidobacterium sp can play an important role in promoting human health in the gastrointestinal tract (Mitsuoka 1990). They actively contribute in the digestion, immune stimulation and inhibition of pathogens such as Bacteroides, Escherichia, Clostriduim and Proteus, which are potentially harmful bacteria found in the gastrointestinal tract (Ziemer and Gibson 1998).
The primary mechanism for probiotic action is known as competitive colonization, or competitive suppression. It is best described as the proliferation of probiotic bacteria in the human intestine, leaving little space for the growth of any pathogens. Further, they secrete by-products such as lactic acid and acetic acid, which lower the pH, thereby creating a hostile environment for the growth of pathogenic microorganism. The secreted acids also increase the peristalsis, which also help to speed pathogens though the intestines (Ballongue 1992 and Biavati et al. 2000).
To develop the growth model of probiotic bacteria through the traditional method, i.e., one variable at-a-time (OVAT) is time consuming and neglects interactions of different variables which can also affect the yield. Process optimization through statistical method is a technique in which changes or adjustments are made in a process to get better results (Myers and Montgomery 2002). There are several techniques for process optimization such as Response Surface Methodology (RSM), Artificial Neural Networks (ANN), Genetic Algorithms (GA), etc. In these engineering applications, a response of interest is usually influenced by several variables and the objective of the engineering applications is to find the variables that can optimize the response. RSM is a tool to study the optimal process parameters that produce a maximum, or minimum value of the response and represents the direct and interactive effects of the process parameters through two and three-dimensional plots (Gangadharan et al. 2008). ANN is computational models of nervous systems. Natural organisms, however, do not possess only nervous systems but also genetic information stored in the nucleus of their cells (genotype). The nervous system is part of the phenotype, which is derived from this genotype through a process, called development (Rajasekaran and Vijaylakshmi 2004). Using the method of neural networks (NN), the relationship between a set of independent variables X and the dependent variables Y can be obtained. From the given pairs of input X and output Y data, neural network directly learns and develops a relationship between them but does not yield any mathematical equation relating the variables. After the learning, this network is able to predict the correct output from an input data set that has not been previously used during the learning. GA is a tool by which the optimization problems can be accurately solved with in a limited use of computer time (Das 2005). Optimization of various bacterial strains in Erlenmeyer flasks using different optimization tools have been reported by several other authors (Nagarjun et al. 2005; Kumari et al. 2009; Negi and Banergee 2009; Lima et al. 2009; Usmiati and Marwati 2009; Coelho et al. 2011).
This study developed the empirical model to increase the cell growth of L. acidophilus by optimizing growth parameters such as temperature, pH, inoculum volume, incubation period and additional effect of different carbon and nitrogen sources using the RSM, ANN and GA.
MATERIAL AND METHODS
Chemicals
All the solvents and reagents used in the present study were procured from Merck, Germany.
Organism and growth condition
Pure culture of L. acidophilus NCDC 14 was obtained in freeze dried state from National Collection of Dairy Cultures (NCDC), National Dairy Research institute, Karnal, Haryana, India. The culture was maintained at 4°C and sub-cultured on the slants of MRS (De Man Rogosa and Sharpe) growth medium two times in a month. The method used for the microbial culture activation and pellet extraction was same as reported by Sharma et al. (2013).The growth of L. acidophilus was carried out in 250 mL Erlenmeyer flasks each containing 50 mL MRS agar medium (pH 7.0) and maintained at 37°C. The cell biomass was determined by measuring the optical density (OD) of the medium at 600 nm. Before measuring the OD, the liquid containing cells were centrifuged and washed with sterile distilled water for two times to remove the adhering medium constituents.
Experimental Design
Selection of initial parameters
For the selection of initial parameters, 'one variable at a time method' was used. The different variables viz. temperature, pH, volume of inoculum, age of inoculum and additional carbon and nitrogen sources were selected for the growth of L. acidophilus.
Empirical model development
In order to find the effect of different growth parameters on the predicted value of bacterial growth Yp was obtained by conducting the experiments on different combination of independent variables (growth parameters), which was obtained from a standard experimental design. During the experiments, the response, or values of dependent variables obtained from each of the combinations of independent variables was measured. A mathematical relationship between the independent and dependent variables was developed. Using this model, the predicted value of response were find out within the domain of limiting values of independent variables.
For the different growth parameters it was desired to develop a polynomial model between the Lactobacillus growth and growth parameters to develop the following relationship between the coded values x1, x2, x3,x4, x5 and x6 of independent variables and dependent variable Ypas shown below.
Yp=bo+b1x1+b2x2+b3x3+b4x4+b5x5+b6x6+b7x12+b8x22+b9x32+b10x42+b11x52+b12x62+b13x1x2+b14x1x3+bx1x4
+b16x1x5+b17x1x6+b18x2x3+b19x2x4+b20x2x5+b21x2x6+b22x3x4+b23x3x5+b24x3x6+b25x4x5
+b26x4x6+b27x5x6 (Eq. 1)
Where bo,b1,b2.........etc. are the regression constants.
Experimental Modeling
Box-Behnken Design
Optimization process involving OVAT method was only used for the selection of initial parameters while RSM was adopted to optimize the growth conditions for L. acidophilus by Box-Behnken design. MINITAB (version 6.5) software was used for the experimental design.
Optimization
Artificial neural network modeling
In this present investigation, a feed forward back propagation neural network was used to evaluate its capability in cell mass yield prediction of L. acidophilus. In this process, ANN computed the error between the desired (predicted) response and the actual (experimental) response. The number of neurons in input layer, hidden layer and output layer of this neural network were kept as 6, 11 and 1, respectively (Fig. 1). This ANN was first trained with reported data of B. bifidum (Meena et al. 2011). After training, it was able to predict the cell mass yield of L. acidophilus accurately through error minimization that was compared with the predicted value of cell mass yield obtained from RSM.
Genetic Algorithms
In order to maximize the cell mass yield of L. acidophilus, GA was applied to the developed ANN based model (Fig. 2) by monitoring above mentioned six growth parameters. It was posed as the minimization of problem associated with the optimization studies. Genetic optimization was continued till the maximum cell mass yield obtained.
Software used
For the proper execution of ANN and GA, MATLAB 7.0 and MINITAB 6.5 was used to develop the empirical model.
RESULTS AND DISCUSSION
Selection of initial parameters
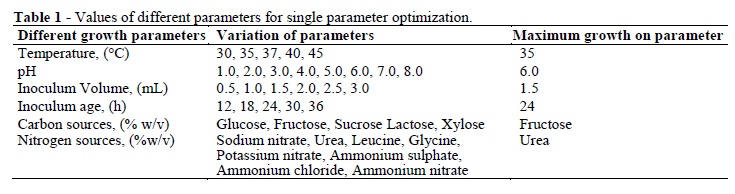
Different growth variables for L. acidophilus were selected by OVAT method and results showed in Figure 3 (A - F). All these parameters, their variation and optimum values are given in Table 1.
Empirical model development
From the initial parameter selection, the maximum and minimum values of six independent parameters for L. acidophilus were fixed as shown in Table 2. It was one of the objectives to develop the model between coded values x1, x2, x3, x4,x5 and x6 of independent variables and dependent variable Yp. For this, experiments were conducted according to Box-Behnken design. All these combinations are given in Table 3 with their corresponding experimental value Ye for the growth of L. acidophilus.
RSM is mainly used for optimization of growth conditions, reaction parameter, or scaling up the L. acidophilus growth conditions (Sen and Babu 2005). Experimental data were fitted to the full quadratic equation and the design matrix and the fitness of each term was analyzed by the means of ANOVA (Kumari et al. 2008). Figure 4 shows the corresponding model coefficients (R2 0.796) together with the regression coefficient of determination, which was a measure of how well the regression model could be made to fit the raw data.
A self-organizing feature map network was used to predict the growth condition parameters. Different factors, viz. temperature, pH, inoculums volume, inoculums age and additional carbon and nitrogen sources were used as each unit of input layer. The output layer was composed of one response variable, the growth of L. acidophilus. A set of factors was used for training and fed into the computer. Several iterations were conducted with different numbers of neurons of hidden layer in order to determine the optimal ANN structure. The optimum number of neurons in the hidden layer was iteratively determined by changing the number of neurons. It started with two neurons and was increased up to six. The least MSE value and a good prediction of the outputs of both training and validation sets were obtained with four neurons in the hidden layer (Dutta et al. 2004). The R2 value between the actual and estimated responses was determined as 0.927 (Fig. 5). In ANN modeling, the replicates at center point did not improve the prediction capability of the network because of the similar inputs.
Using MATLAB 7.0, the constants of regression equation and predicted value of dependent variable (OD) were find out. The obtained model for L. acidophilus was as given below.
Yp = 0.2838 - 0.2319 x1 + 0.2335 x2 + 0.0468x3 + 0.738x4 + 0.0215x5 + 0.133x6 - 0.082x12 - 0.0627x22 + 0.0575x32 + 0.3429x42 + 0.0327x52+ 0.0375x62 - 0.1088x1x2 - 0.1655x1x3 + 0.0244x1x4 + 0.0359x1x5 - 0.1597x1x6 - 0.0689x2x3 - 0.0154x2x4 - 0.1625 x2x5 - 0.0487 x2x6 + 0.603 x3x4 - 0.1381 x3x5 -0.0592x3x6 - 0.0873x4x5 - 0.1972x4x6 - 0.083x5x6 (Eq. 2)
The predicted value of independent variable and corresponding experimental value for L. acidophilus is shown in the Table 4. Genetic algorithms were applied on the data obtained from neural network using MATLAB7.0.
Table 5 showed the optimum value, or combination of different process parameters on which the bacterial growth measured by optical density (OD) was maximum for L. acidophilus.
CONCLUSION
In the present study, RSM and ANN methodologies were used to predict the growth model for L. acidophilus and optimized the growth parameters. Both the models provided similar quality predictions for the above independent variables for the growth conditions of L. acidophilus with ANN showing more accuracy in estimation. Regression coefficient (R2) of ANN and RSM reflected that ANN was better than RSM. RSM was useful in getting insight information (e.g. interactions between different components) of the system directly, but ANN was also equally useful in the sensitivity analysis. ANN showed better modeling technique for data set showing nonlinear relationship over RSM. Thus, ANN could be a very powerful and flexible tool well suited for the development of empirical growth model due to an implicit corrective action arising from the training methodology and the associated estimation procedure. Present results showed that the higher cell mass yield of L. acidophilus was observed at 37.7°C, pH 6.08, inoculum volume and age 0.79 ml and 13.04 h, respectively, carbon content 42.62% (w/v) and nitrogen content (39.92% (w/v). This combination of independent variables could be of good importance to starter culture producing industries in order to scale- up the production of L. acidophilus on commercial scale more economically due to high cell mass yield.
REFERENCES
Ballongue J. Bifidobacteria and probiotic action probiotics, the Scientific Basis. J Dairy Sci. 1992; 7: 357-413.
Bhadoria PBS, Mahapatra SC. Prospects, technological aspects and limitations of probiotics - a worldwide review. Eur J Food Res Review. 2011; 2: 23-42.
Biavati B, Vescovo M, Torriabi S, Bottazzi V. Bifidobacteria: history, ecology, physiology and applications. Anal Microbiol. 2000; 50: 117-131.
Coelho LF, Lima CJBD, Rodovalho CM, Bernardo MP, Contiero J. Lactic acid production by new Lactobacillus plantarum LMISM6 grown in molasses: optimization of medium composition. Braz J Chem Eng. 2011; 28: 27-36.
Das H. Empirical model development. Hand Book of Food Processing Operations Analysis. 1st ed. New Delhi: Asian Book Private Limite. 2005; 357-382.
Dutta JR, Dutta PK, Banerjee R. Optimization of culture parameters for extracellular protease production from a newly isolated Pseudomonas sp. using response surface and artificial neural network models. Process Biochem. 2004; 39: 2193-2198.
Gangadharan D, Sivaramakrishnan S, Nampoothiri KM, Sukumaran RK, Pandey A. Response surface methodology for the optimization of alpha amylase production by Bacillus amyloliquefaciens. Bioresour Technol. 2008; 99: 4597-4602.
Kumari A, Mahapatra P, Banerjee R. Statistical optimization of culture conditions by response surface methodology for synthesis of lipase with Enterobacter aerogenes. Braz Arch Biol Technol. 2009; 52: 1349-1356.
Kumari KS, Babu IS, Rao GH. Process optimization for citric acid production from raw glycerol using response surface methodology, Indian J Biotech. 2008; 7: 496-501.
Lima CJBD, Coelho LF, Blanco KC, Contiero J. Response surface optimization of D (-)-lactic acid production by Lactobacillus SMI8 using corn steep liquor and yeast autolysate as an alternative nitrogen source. Afr J Biotechnol. 2009; 8:5842-5846.
Meena GS, Gupta S, Majumdar GC, Banerjee R. Growth characteristics modeling of Bifidobacterium bifidum Using RSM and ANN. Braz Arch Biol Technol. 2011; 54: 1357-1366.
Mitsuoka T. Bifidobacteria and their role in human health. J Ind Microbiol. 1990; 6: 263-268.
Myers RM, Montgomery DC. Response Surface Methodology: Process and Product Optimization Using Designed Experiments. 2nd ed. Hoboken: Wiley-Interscience; 2002.
Nagarjun PA, Rao RS, Rajesham S, Rao LV. Optimization of lactic acid production in SSF by Lactobacillus amylovorus NRRL B-4542 Using Taguchi Methodology. J Microbiol. 2005; 43: 38-43.
Negi S, Banerjee R. Optimization of extraction and purification of glucoamylase produced by Aspergillus awamori in solid state fermentation. Biotech Bioprocess Eng. 2009; 14:60-66.
Rajasekaran S, Vijaylakshmi PGS. Neural Networks Fuzzy Logic and Genetic Algorithms. 1st ed. New Delhi: Prentice Hall of India; 2004.
Sanders ME. Probiotics. Food Biotechnol. 1999; 53: 67-77.
Schezenmeir J, De Vrese M. Probiotics, Prebiotics, and Symbiotic -Approaching a definition. Am J Clin Nutr. 2001; 73: 361-364.
Sen R, Babu KS. Modeling and optimization of the process conditions for biomass production and sporulation of a probiotic culture. Process Biochem. 2005; 40: 2531-2538.
Shah NP. Functional cultures and health benefits. Int Dairy J. 2007; 17: 1262-1277.
Sharma M, Mridula D, Gupta RK. Development of sprouted wheat based probiotic beverage. J Food Sci Technol. 2013; 1-8.
Usmiati S, Marwati T. Selection and optimization process of bacteriocin production from Lactobacillus sp. Indones J Agric. 2009; 2: 82-92.
Ziemer CJ, Gibson GR. An overview of probiotics, prebiotics and symbiotic in the functional food concept: perspectives and future strategies. Int Dairy J. 1998; 8: 473-479.
Received: August 09, 2012;
Accepted: September12, 2013.
- Ballongue J. Bifidobacteria and probiotic action probiotics, the Scientific Basis. J Dairy Sci 1992; 7: 357-413.
- Bhadoria PBS, Mahapatra SC. Prospects, technological aspects and limitations of probiotics - a worldwide review. Eur J Food Res Review. 2011; 2: 23-42.
- Biavati B, Vescovo M, Torriabi S, Bottazzi V. Bifidobacteria: history, ecology, physiology and applications. Anal Microbiol 2000; 50: 117-131.
- Coelho LF, Lima CJBD, Rodovalho CM, Bernardo MP, Contiero J. Lactic acid production by new Lactobacillus plantarum LMISM6 grown in molasses: optimization of medium composition. Braz J Chem Eng. 2011; 28: 27-36.
- Das H. Empirical model development. Hand Book of Food Processing Operations Analysis. 1st ed. New Delhi: Asian Book Private Limite. 2005; 357-382.
- Dutta JR, Dutta PK, Banerjee R. Optimization of culture parameters for extracellular protease production from a newly isolated Pseudomonas sp. using response surface and artificial neural network models. Process Biochem 2004; 39: 2193-2198.
- Gangadharan D, Sivaramakrishnan S, Nampoothiri KM, Sukumaran RK, Pandey A. Response surface methodology for the optimization of alpha amylase production by Bacillus amyloliquefaciens Bioresour Technol 2008; 99: 4597-4602.
- Kumari A, Mahapatra P, Banerjee R. Statistical optimization of culture conditions by response surface methodology for synthesis of lipase with Enterobacter aerogenes Braz Arch Biol Technol. 2009; 52: 1349-1356.
- Kumari KS, Babu IS, Rao GH. Process optimization for citric acid production from raw glycerol using response surface methodology, Indian J Biotech 2008; 7: 496-501.
- Lima CJBD, Coelho LF, Blanco KC, Contiero J. Response surface optimization of D (-)-lactic acid production by Lactobacillus SMI8 using corn steep liquor and yeast autolysate as an alternative nitrogen source. Afr J Biotechnol. 2009; 8:5842-5846.
- Meena GS, Gupta S, Majumdar GC, Banerjee R. Growth characteristics modeling of Bifidobacterium bifidum Using RSM and ANN. Braz Arch Biol Technol. 2011; 54: 1357-1366.
- Mitsuoka T. Bifidobacteria and their role in human health. J Ind Microbiol 1990; 6: 263-268.
- Myers RM, Montgomery DC. Response Surface Methodology: Process and Product Optimization Using Designed Experiments. 2nd ed. Hoboken: Wiley-Interscience; 2002.
- Nagarjun PA, Rao RS, Rajesham S, Rao LV. Optimization of lactic acid production in SSF by Lactobacillus amylovorus NRRL B-4542 Using Taguchi Methodology. J Microbiol. 2005; 43: 38-43.
- Negi S, Banerjee R. Optimization of extraction and purification of glucoamylase produced by Aspergillus awamori in solid state fermentation. Biotech Bioprocess Eng. 2009; 14:60-66.
- Rajasekaran S, Vijaylakshmi PGS. Neural Networks Fuzzy Logic and Genetic Algorithms. 1st ed. New Delhi: Prentice Hall of India; 2004.
- Sanders ME. Probiotics. Food Biotechnol 1999; 53: 67-77.
- Schezenmeir J, De Vrese M. Probiotics, Prebiotics, and Symbiotic -Approaching a definition. Am J Clin Nutr 2001; 73: 361-364.
- Sen R, Babu KS. Modeling and optimization of the process conditions for biomass production and sporulation of a probiotic culture. Process Biochem 2005; 40: 2531-2538.
- Shah NP. Functional cultures and health benefits. Int Dairy J 2007; 17: 1262-1277.
- Sharma M, Mridula D, Gupta RK. Development of sprouted wheat based probiotic beverage. J Food Sci Technol. 2013; 1-8.
- Usmiati S, Marwati T. Selection and optimization process of bacteriocin production from Lactobacillus sp. Indones J Agric. 2009; 2: 82-92.
- Ziemer CJ, Gibson GR. An overview of probiotics, prebiotics and symbiotic in the functional food concept: perspectives and future strategies. Int Dairy J 1998; 8: 473-479.
Publication Dates
-
Publication in this collection
24 Feb 2014 -
Date of issue
Feb 2014
History
-
Accepted
12 Sept 2013 -
Received
09 Aug 2012