Abstract
The cyanobacterium Oscillatoria agardhii was isolated from the fresh water Mawatha lake, Jaipur and was grown in Zarrouk's medium at 25 ± 2°C, illuminated with white fluorescent light at the intensity of 2 500 lux with 12:12 h light and dark photoperiod. The effects of photoperiod and temperature on the growth and protein expression by Oscillatoria agardhii were studied under different controlled culture conditions (ALR, ALC, CLR, CLC, and NDL), measuring optical density, cell count and dry weight. Protein content was measured quantitatively by Bradford assay and qualitatively by SDS-PAGE. The densitometric analysis was also carried out for the measurement of the expression level of different proteins/peptides under different culture conditions. Maximum growth and protein content were observed in ALR condition while minimum was in the CLC. Alternate light and dark periods proved efficient as contrasting banding patterns were observed with many new unique polypeptides such as 32, 36.3, 47.9, 60.8, and 67.0 kDa, whereas, expressions of three polypeptides of 57.2, 110.1, and 117.3 kDa were inhibited in constant light cultures.
Bradford assay; Densitometer; Oscillatoria agardhii; Photoperiod; SDS-PAGE
INTRODUCTION
Cyanobacteria are ecologically and economically important group of living organisms in the world, that possess a wide range of coloured compounds, including chlorophylls, carotenoids, and phycobiliproteins. Phycobiliproteins are naturally occurring water-soluble fluorescent pigments which are widely used in cosmetic industry, clinical research and immunological studies (Luning and Dring 1979Luning K, Dring MJ. Continuous under water tight measurements near Helgoland (North Sea) and its significance for characteristic light limits in the sub littoral region. Heg Wis Meentrs. 1979; 32:403-424.; Telford et al. 2001Telford WG, Moss MW, Morseman JP, Allnutt FCT. Cryptomonad algal phycobilliproteins as fluorochromes for extracellular and intracellular antigen detection by flow cytometry. Cytometry. 2001; 44: 16-23.). Cyanobacteria are exposed to various types of environmental stresses, such as temperature, pH, light intensity and light quality that can change the cellular and metabolic activities of the organism. Reynolds (1984)Reynolds CS. The ecology of freshwater phytoplankton. Cambridge University Press, London. 1984. demonstrated that growth rate of phytoplankton changes exponentially with increasing temperature and the maximum values occur between 25-40°C. Growth rate, photosynthetic rate and biochemical composition of microalgae vary when cells are grown with either a short L/D cycle (Foy 1983Foy RH. Interaction of temperature and light on the growth rates of two planktonic Oscillatoria species under a short photoperiod regime. Bri Phycol J. 1983; 18, 267-273.), a long L/D cycle (Post et al. 1985aPost AF, De Wit R, Mur LR. Interactions between temperature and light intensity on growth and photosynthesis of the cyanobacterium Oscillatoria agardhii. J. Plank Res. 1985a; 7: 487-49.), or with continuous light (Van Liere and Mur 1980Van Liere L and Mur LR. Occurrence of Oscillatoria agardhii and some related species, a survey. Hypertrophic Ecosystems, 1980; 67-77.).Lorenzen (1980) demonstrated that under constant illumination, the algae can perform only a reduced growth, but changes in light (duration and quality) modify the quantity as well as the quality of differences the pigment and biochemical composition of microalgae (Renaud et al. 1991Renaud SM, Parry DL, Luong VT, Kuo C, Padovan A, Sammy N. Effect of light intensity on the proximate biochemical and fatty acid composition of Isochrysis sp. and Nannochloropsis oculata for use in tropical aquaculture. J Appl Phycol. 1991; 3: 43-53.; Fabregas et al. 2002Fabregas J, Maseda A, Domınguez A, Ferreira M, Otero A. Changes in the cell composition of the marine microalga, Nannochloropsis gaditana, during a light dark cycle. Biotechnol Lett. 2002; 24:1699-1703.; Sanchez-Saavedra and Voltolina 2002Sanchez-Saavedra MP, Voltolina D. Effect of photon fluence rates of white and blue-green light on growth efficiency and pigment content of three diatom species in batch cultures. Cienc Mar. 2002; 28: 273-279.).
Overall, the growth and biochemical composition of microalgae depend on three abiotic factors i.e. quantity and quality of light, temperature, and level of nutrients. Falkowski et al. (1985)Falkowski PG, Dubinsky Z and Wyman K. Growth irradiance relationships in phytoplankton. Limnol Oceanogr. 1985; 30: 311-321. suggested that the light directly influence the photosynthetic mechanism. Pandey et al. (2000)Pandey R, Chauhan S, Singhal GS, Kale RK. Spectral Light Quality Affects Protein Profile of Synechococcus sp.PCC 7942: A Comparative 2-Dimensional Gel Electrophoresis (2-DGE) Analysis, Curr Microbiol. 2000; 40: 297-301. reported that exposure to various light treatments resulted in a qualitative and quantitative regulation of individual proteins in Synechococcus. Similarly, other studies have shown that different environmental conditions are responsible for the induction or inhibition of expression of certain peptides and also increase or decrease in the level of protein expression (Weber and Jung 2002Weber A, Jung K. Profiling Early Osmostress dependent gene expression in Escherichia coli using DNA Macroarrays." J Bacteriol. 2002; 184, 5502-5507.; Chan et al. 2004Chan LL, Hodgkiss IJ, Wan JMF, Lum JHK, Mak ASC, Sit WH, Lo SCL. Proteomic study of a model causative agent of harmful algal blooms, Prorocentrum triestinum II, The use of differentially expressed protein profiles under different growth phases and growth conditions for bloom prediction. Proteomics. 2004; 4,3214-3226.). The aim of this work was to study the effects of different photoperiods and temperatures on the growth and protein contents of Oscillatoria agardhii.
MATERIALS AND METHODS
Organism
The experimental organism Oscillatoria agardhii was isolated from Mawatha lake, Jaipur, Rajasthan. The cells were grown in modified Zarrouk's medium (Zarrouk 1966Zarrouk C. Contribution a retude du cyanophyceae. Influence dedivers facteurs physiques et chimiques sur la croissance et la photosynthse de Spirulina maxima (Setch et Gardner) Geitl. PhD, Paris. 1966.) at 25 ± 2°C; pH 9.5. To remove the inhibitory substances and curb any possible mineral deficiency, the entire growth medium was replaced with fresh medium from time to time.
Culture conditions
In order to find out the impact of different culture conditions, cultures were subjected to five different combinations of temperature range and illumination as follows:
-
Alternate light and dark periods (12:12 h) at 27-36oC (ALR)
-
Constant light at 27-36oC (CLR)
-
Alternate light and dark periods (12:12 h) at 20-25oC (ALC)
-
Constant light at 20-25oC (CLC)
-
Natural day light period at 27-36oC (NDL)
The five sets each with three test tubes were taken. Each test tube contained 2.0 mL of freshly growing culture and 10 mL of Zarrouk's medium. Such five sets of growing cultures in test tube were subjected to different conditions mentioned above. Subsequently, the cell culture growth in each test tube was measured by optical density (OD), cell (filament) count (CC) and dry weight (DW). Simultaneously conical flasks, each containing 30 mL O. agardhii cultures and 70 mL Zarrouk's medium were subjected to these culture conditions for protein estimation. Cultures were shaken gently three times a day to avoid clumping and to accelerate growth. Each conical flask was then subjected to OD recording using colorimeter at 670 nm and cell count using compound microscope (Nicone SE, Japan). Cells were counted in at least 10 slides from each sample using a homogeneous culture suspension. Five flasks, each with 50 mL medium each were taken and inoculated by O. agardhii culture at 0.1 OD. Dry weight determination was done by filtering 50 mL algal sample of suspension through a Whatman GF/C Filter of 47 mm diameter. The retentate was dried in an oven at 70°C for optimum duration, and then kept in the dessicator to be subsequently weighed.
Protein profiling
The effect of different temperatures and photoperiods on polypeptide profile was determined by SDS-PAGE (Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis). The soluble proteins were extracted by homogenizing 0.5 g of algal tissues with extraction buffer (pH 6.8) consisting of 20 mL solution of 0.5 M Tris (pH 7.0) 2.5 mL, urea (4.0 g), SDS (0.5 g), glycerol (4.0 mL), β mercaptoethanol (500 μL), dH2O (10.0 mL) and the suspension was then centrifuged at 4°C for 20 min at 15 000 rpm. The supernatant was used as crude protein extract. The protein quantity in it was measured following the method of Bradford (1976)Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976; 72, 248-254.. The 50 μL of sample extracted from the alga was mixed with equal quantity of sample buffer [0.5 M Tris (pH 7.0) 2.5 mL, urea (9.6 g), SDS (1.0 g), glycerol (4.0 mL), β mercaptoethanol (500 μL), dH2O (5.0 mL)]. The mixture was subjected to thermal denaturation in water bath, subsequently was put on ice till loading. Protein extracts from all the treatments were resolved on 12 % SDS-PAGE (Sambrook et al. 1989Sambrook J, Fritsch EF, Maniatis T. (1989). Molecular Cloning: A Laboratory Manual. 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.) and stained with 0.1% coomassie brilliant blue (R250) dye. In order to score and preserve the banding pattern, the gel was subjected to image scanning using BIO-RAD GS -700 Imaging Densitometer (USA) and the protein profiles were obtained for each variety. The bands were designated on the basis of their molecular weight, for which molecular weight marker loaded simultaneously with the samples. The distance travelled from the well by the amplified fragment was translated to the molecular weight with reference to protein molecular weight marker. The presence of each band was scored as (+) plus and the absence as minus (-) (Table 1).
RESULTS AND DISCUSSION
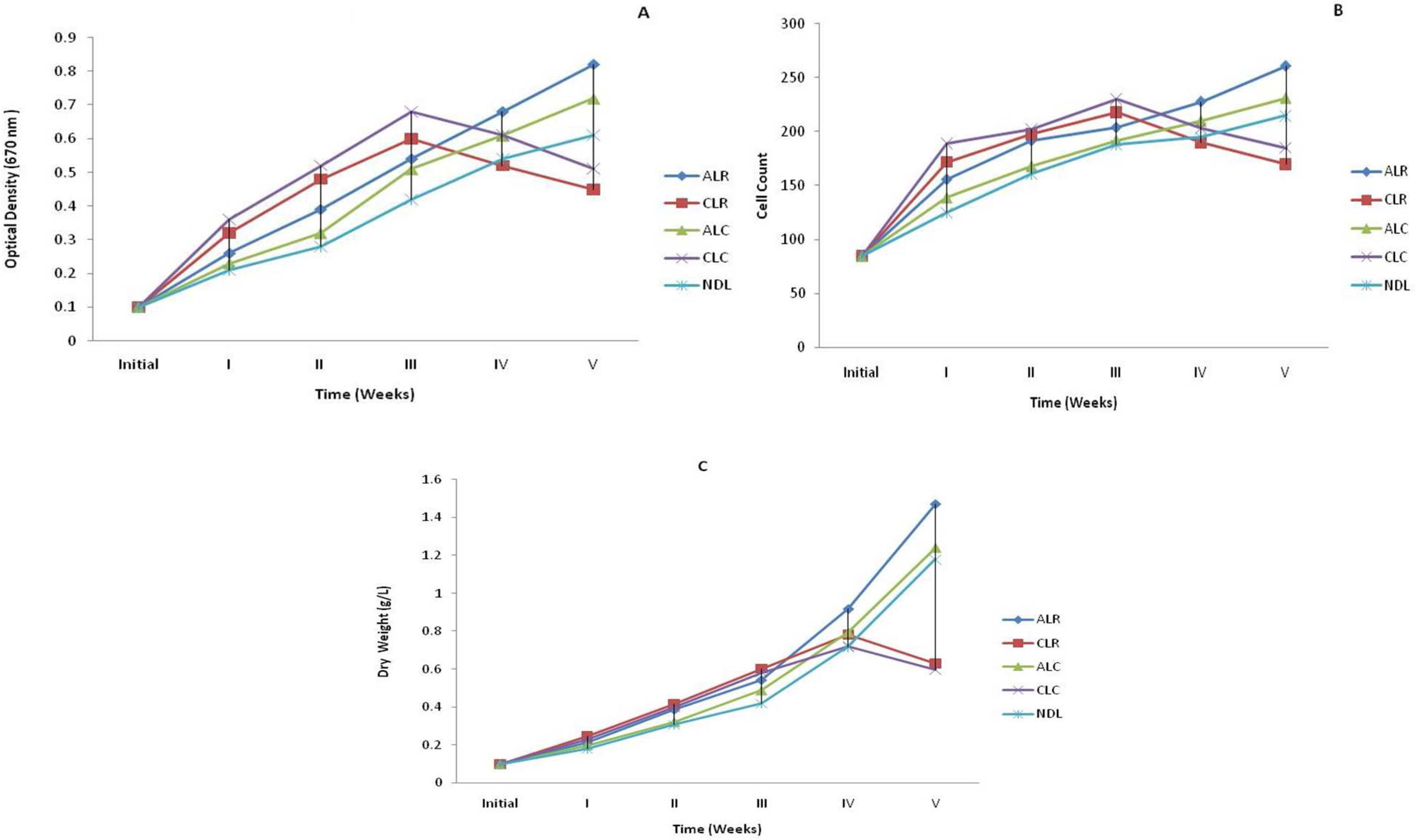
When algal cultures were exposed to different temperatures and photoperiods, a characteristic set of cellular and metabolic responses triggered. Amongst these conditions, the best growth was observed in the alternate light and dark periods at 27-36ºC (ALR) followed by alternate light and dark period at 20-25ºC (ALC), natural day light at 27-36ºC (NDL), constant light at 27-36ºC (CLR) and minimum growth was observed in constant light at 20-25ºC (CLC) (Fig. 1A, 1B, 1C). Shuter (1979)Shuter B. A model of physiological adaptation in unicellular algae. Theor Biol. 1979; 78: 519-552. observed the harmful effect of exposure to the continuous light on chloroplast complex. The observations of the Shuter's experiment supported the evidence of Healey (1985)Healey FP. Interacting effects of light and nutrient limitation on the growth rate of Synechococcus linearis (Cynanophyceae). Phycol. 1985; 21: 34- 46. and Millie et al. (1990)Millie DF, lngram DA, Dionigi CP. Pigment and photosynthetic responses of Oscillatoria agardhii (Cyanophyta) to photon flux density and spectral quality. Phycol. 1990; 26: 660-666.. High light intensity in some algal cultures led to photoinhibition, decreasing the cellular growth rate and photo-oxidation with severe cell damage. In extreme cases it was made total loss of culture (Jensen and Knutsen 1993Jensen S and Knusten G. Influence of light and temperature on photoinhibition of photosynthesis in Spirulina plantensis. J App Phycol. 1993; 5: 495-504.; Vonshak et al. 1994Vonshak A, Torzillo G, Tomaseli L. Use of chlorophyll fluorescence to estimate the effect of photoinhibition in outdoor cultures of Spirulina platensis. 1994; J Appl Phycol., 6: 31-34.). Richmond (1988)Richmond A. Spirulina, Cambridge University Press, Cambridge, Micro-algal Biotec. 1988; 85-121. demonstrated that optimal growth temperature was between 30-37ºC with 40ºC being definitely injurious for different strains of Spirulina. Earlier reports of Srivastava (1967)Srivastava P. Studies on the experimental cultures of certain Chlorococcales, Ph.D. Thesis Osmania University, Hyderabad, 1967., Singh and Srivastava (1991)Singh GP, Srivastava P. Impact of varied culture conditions on growth and morphology of Ankistrodesmus Fusiformis. J lndian bot Soc. l99l; 71: 341-345. and Kumar et al. (2008)Kumar M, Rawat S, Singh GP. Impact of different culture conditions on growth and pigment contents of spirulina platensis (jal mahal isolate). J Indian bot Soc. 2008; 87 (3 & 4): 267-271.confirmed that alternate light and dark periods and 30-35ºC temperature were the optimal culture conditions for cellular and metabolic activity of Cyanobacteria.
Effect of photoperiod and temperature on growth (A) optical density (B) Cell count (C) Dry weight (g/L) of Oscillatoria agardhii. (ALR- Alternate Light at 27-36ºC, ALC- Alternate Light at 20-25ºC, CLR- Continuous Light at 27-36 ºC , CLCContinuous Light at 20-25ºC, NDL- Natural Day Light at 27-36ºC.)
In alternate light and dark periods 12: l2h for both the ranges of temperatures i.e. 27-36ºC and 20-25ºC growth was higher which supported the results of Kumar et al. (2008)Kumar M, Rawat S, Singh GP. Impact of different culture conditions on growth and pigment contents of spirulina platensis (jal mahal isolate). J Indian bot Soc. 2008; 87 (3 & 4): 267-271.. Despite having started with similar initial inoculum, after 18 days of cultivation, the growth on alternate light and dark periods at 27-36ºC (ALR) was most amongst all culture conditions. The difference became more evident on day 28, when cell concentration reached 2.68 times of the initial growth. After 35 days of the initial growth the OD was enhanced 8.2 times at 27-36ºC (ALR) and was only 7.2 times at 20-25ºC (ALC) (Fig. 1A). Cell counts also supported the above results. Maximum cell number was recorded at 27-36ºC (ALR) and showed an increase of about 3.7 times the initial number. At 20-25ºC (ALC) the number of cells increased only about 2.7 times (Fig. 1B). The biomass (dry weight) measurement also favored the OD and cell count results that increased 8.14 times (ALR) and 6.2 times (ALC) of the initial record (Fig. 1C). The growth also increased in natural day light at 27-36ºC (NDL) up to fifth week i.e. 6.1 times (optical density), 2.5 times (cell count), 5.9 times (dry weight) of the initial record. Under continuous illumination out of the two sets of temperature i.e. 27-36ºC (CLR) and 20-25ºC (CLC) condition, algae grew rapidly up to third week but the growth gradually decreased due to photoinhibition and photo-oxidation. Photoinhibition and photo-oxidation are responsible for decreasing the cellular growth rate and cell damage and gradually algal culture became unhealthy and degraded (Jensen and Knutsen 1993Jensen S and Knusten G. Influence of light and temperature on photoinhibition of photosynthesis in Spirulina plantensis. J App Phycol. 1993; 5: 495-504.; Vonshak et al. 1994Vonshak A, Torzillo G, Tomaseli L. Use of chlorophyll fluorescence to estimate the effect of photoinhibition in outdoor cultures of Spirulina platensis. 1994; J Appl Phycol., 6: 31-34.). The CLR condition optical density, cell count and dry weight increased 5.1 times, 2.1 times and 4.0 times respectively of the initial level. Similarly CLC condition growth increased for OD (4.5 times) CC (2.1 times) and DW (3.8 times) of the initial recorded values.
The Bradford results showed that the maximum protein content was present in ALR condition i.e. 3.94 mg/mL that is gradually decreased in the ALC (3.72 mg/mL), NDL (3.65 mg/mL), CLR (3.12 mg/mL) and minimum was in the CLC (3.02 mg/mL) condition as compared to the control (Fig. 2). Berges et al. (1992) recommended the use of the Bradford assay for quantification of protein. There are only a few reports on the quantification of cyanobacterial proteins by Bradford method (Tandeau De Marsac 2003Tandeau De Marsac NT. Phycobiliproteins and phycobilisomes: the early observations. Photosynth Res. 2003; 6: 197-205.; Vijaya and Anand 2009Vijaya V, Anand N. Blue light enhance the pigment synthesis in cyanobacterium Anabaena ambigua rao (nostacales), J Agrl Bio Sci. 2009; 4: 3.).
Total measured protein content of Oscillatoria agardhii subjected to different culture conditions. Each bar is an average of three replicates. (ALR- Alternate Light at 27-36ºC, ALCAlternate Light at 20-25ºC, CLR- Continuous Light at 27-36ºC, CLC- Continuous Light at 20-25ºC, NDL- Natural Day Light at 27-36ºC).
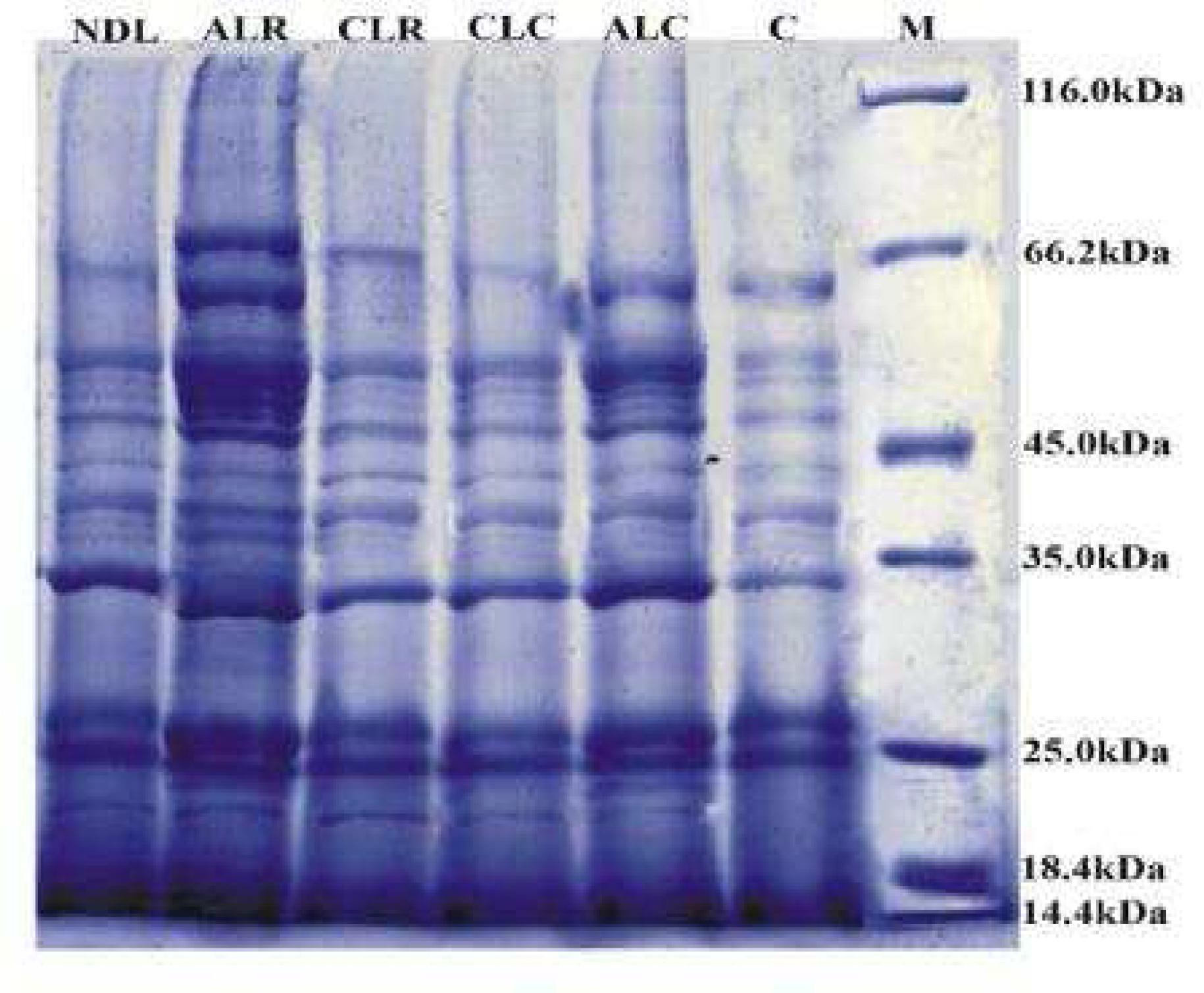
The qualitative estimation of the extracted proteins from the algal culture grown at different photoperiods and temperatures were subjected to 12% polyacrylamide gel electrophoresis along with PMW-B. Total 24 polypeptide species were observed in SDS-PAGE profile of the control culture (Fig. 3). The molecular weights of these polypeptides were ranging from 14.0 to 117.3 kDa. The major polypeptide bands were around 14.0, 18.0, 26.0, 50.0 and 65.0 kDa (Fig. 3). Similar to the control, samples of all the treatment were subjected to gel analysis. Comparative study in all the culture conditions (ALR, ALC, CLR, CLC and NDL) with the control sample indicated increased thickening and sharpening of bands. Similar polypeptide profiles were found in all the lanes of control and different culture condition samples irrespective of varietal differences (Table 1). Chan et al. (2004)Chan LL, Hodgkiss IJ, Wan JMF, Lum JHK, Mak ASC, Sit WH, Lo SCL. Proteomic study of a model causative agent of harmful algal blooms, Prorocentrum triestinum II, The use of differentially expressed protein profiles under different growth phases and growth conditions for bloom prediction. Proteomics. 2004; 4,3214-3226. observed different phase specific and stress responsive proteins in 2D-PAGE, expressed in O. agardhii grown under different culture conditions. The proteomic study shows both the constancy of protein profiles and variability of the relative abundance of some peptides under different culture conditions in the O. agardhii strain. Weber and Jung (2002)Weber A, Jung K. Profiling Early Osmostress dependent gene expression in Escherichia coli using DNA Macroarrays." J Bacteriol. 2002; 184, 5502-5507. demonstrated that the changes in protein profiling and newly formed proteins might be helping cyanobacteria to tolerate the stress conditions. The light dark photoperiod may be more beneficial than other regimes, as cell number is sustained longer in exponential phase (Seyfabadi et al. 2010). In the present study alternate light and dark periods at 27-36ºC temperature was proved efficient as contrasting banding pattern was observed with many new unique polypeptides such as 32, 36.3, 47.9, 60.8, and 67.0 kDa (Table 2). However, two novel bands of 21.0 and 49.8 kDa appeared over control in all the samples (Fig. 3).
Coomassie blue stained polypeptide profiles of extracted protein from Oscillatoria agardhii separated by SDS-PAGE. (C- Control, ALR- Alternate Light at 27-36ºC, ALC- Alternate Light at 20-25ºC, CLRContinuous Light at 27-36ºC, CLCContinuous Light at 20-25ºC, NDL- Natural Day Light at 27-36ºC.)
Another novel band of 23.0 kDa was observed in alternate light and dark periods at 20-25ºC. A novel protein of 65.9 kDa was observed in constant light at 27-36ºC that was probably responsible for the survival of the algae in continuous light. The 35.7 and 37.0 kDa bands almost degraded in the genotypes at alternate light and dark periods at 27-36ºC while were consistently present in other four conditions. Three polypeptides such as 57.2, 110.1, and 117.3 kDa were observed in alternate light and dark periods at both the temperatures but were absent in constant light. The gene expression for these peptides was inhibited in the constant light probably due to photoinhibition (Jensen and Knutsen 1993Jensen S and Knusten G. Influence of light and temperature on photoinhibition of photosynthesis in Spirulina plantensis. J App Phycol. 1993; 5: 495-504.; Vonshak et al. 1994Vonshak A, Torzillo G, Tomaseli L. Use of chlorophyll fluorescence to estimate the effect of photoinhibition in outdoor cultures of Spirulina platensis. 1994; J Appl Phycol., 6: 31-34.) (Table 2). The latter is caused by the photo-oxidation reaction inside the cell due to excess light that cannot be absorbed by the photosynthetic apparatus (Richmond 1986).
The photoperiod and temperature could increase or decrease the level of protein expression. Under the optimum condition, many proteins over-expressed and under the adverse condition some peptides expression was inhibited. In order to score and preserve the banding pattern, the gel was scanned under BIO-RAD GS -700 imaging densitometer. The density of bands show the quantity of particular peptide as suggested, which indicated that this peptide was expressed in large amount and light band showed low level expression. The expression of 14.0 and 25.2 kDa peptides was 5 mm in the all light dark conditions (Fig. 4A, 4B). A novel 47.9 kDa (4 mm) peptide was expressed in large amount at alternate light and dark period at 27-36 ºC condition (Fig. 4A). Due to high metabolic activity in the both 12:12 hour light dark condition most proteins (14.0, 24.2, 25.2 and 34.3 kDa) expressed in large amount (more than 3 mm) as compared to control and other culture conditions. The other two peptides (37.0 and 42.7 kDa) were expressed at more than 3 mm level only in the ALR and NDL i.e. provide alternate light with same temperature but differ in the intensity of light (Fig. 4A, E). Expression of some other peptides depended on the photoperiod and the intensity of light e.g. 53.1, 57.2, 110.1 and 117.3 kDa peptides expressed in 3 to 5 mm level in both alternate light conditions with same light intensity but different temperatures (Fig. 4A, 4B, and 4F). Two novel proteins (47.9 and 67.0 kDa) were uniquely and highly expressed only in the ALR condition. These results tended to agree with Rajendran et al. (2007)Rajendran UM, Elango K, Anand N. Effects of a Fungicide, an Insecticide and a Bio-pesticide on Tolypothrix scytonemoides Pest. Biochem. Physiol. 2007; 87: 64-171. who reported that the production of novel proteins or the increased production of already existing proteins resulted under stress conditions due to stress response.
Densitometric Analysis of SDS-PAGE banding pattern in Oscillatoria agardhii. (ALR- Alternate Light at 27-36ºC, ALC- Alternate Light at 20-25ºC, CLR- Continuous Light at 27-36ºC, CLC- Continuous Light at 20-25ºC, NDL- Natural Day Light at 27-36ºC, Control).
CONCLUSION
These studies were conducted with a view to determine the deleterious and differential effects of temperature and photoperiod on the growth and protein profiling of O. agardhii. The proteomic study of the differential protein profiles under different environmental conditions revealed that O. agardhii showed both the constancy of protein profiles and variability of the relative abundance of some abundant proteins under different culture conditions. The effect of both the temperatures (27-36ºC and 20-25ºC) was probably responsible for the increase or decrease in the level of protein expression whereas the effect of alternate light and continuous light induced or repressed the expression of some peptides. Hence some novel proteins could be expressed and expression of some existing peptide could be repressed. In continuous light, algal growth might be reduced due to photoinhibition and photo-oxidation.
ACKNOWLEDGEMENTS
We thank to the Council of Scientific and Industrial Research, New Delhi for financial assistance for this study.
REFERENCES
- Berges JA, Fisher AE, Harrison PJ. A comparison of Lowry, Bradford and Smith protein using different protein standards and protein isolated from the marine diatom Thalassiosira pseudonana assays. Mar Biol. 1993; 115, 187-193.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976; 72, 248-254.
- Chan LL, Hodgkiss IJ, Wan JMF, Lum JHK, Mak ASC, Sit WH, Lo SCL. Proteomic study of a model causative agent of harmful algal blooms, Prorocentrum triestinum II, The use of differentially expressed protein profiles under different growth phases and growth conditions for bloom prediction. Proteomics. 2004; 4,3214-3226.
- Fabregas J, Maseda A, Domınguez A, Ferreira M, Otero A. Changes in the cell composition of the marine microalga, Nannochloropsis gaditana, during a light dark cycle. Biotechnol Lett. 2002; 24:1699-1703.
- Falkowski PG, Dubinsky Z and Wyman K. Growth irradiance relationships in phytoplankton. Limnol Oceanogr. 1985; 30: 311-321.
- Foy RH. Interaction of temperature and light on the growth rates of two planktonic Oscillatoria species under a short photoperiod regime. Bri Phycol J. 1983; 18, 267-273.
- Healey FP. Interacting effects of light and nutrient limitation on the growth rate of Synechococcus linearis (Cynanophyceae). Phycol. 1985; 21: 34- 46.
- Jensen S and Knusten G. Influence of light and temperature on photoinhibition of photosynthesis in Spirulina plantensis. J App Phycol. 1993; 5: 495-504.
- Kumar M, Rawat S, Singh GP. Impact of different culture conditions on growth and pigment contents of spirulina platensis (jal mahal isolate). J Indian bot Soc. 2008; 87 (3 & 4): 267-271.
- Lorenzen H, Hesse M. Synchronous cultures. Bot. Monogr., Algl physiol & biochem. 1974; 10: 894-908.
- Luning K, Dring MJ. Continuous under water tight measurements near Helgoland (North Sea) and its significance for characteristic light limits in the sub littoral region. Heg Wis Meentrs. 1979; 32:403-424.
- Millie DF, lngram DA, Dionigi CP. Pigment and photosynthetic responses of Oscillatoria agardhii (Cyanophyta) to photon flux density and spectral quality. Phycol. 1990; 26: 660-666.
- Pandey R, Chauhan S, Singhal GS, Kale RK. Spectral Light Quality Affects Protein Profile of Synechococcus sp.PCC 7942: A Comparative 2-Dimensional Gel Electrophoresis (2-DGE) Analysis, Curr Microbiol. 2000; 40: 297-301.
- Post AF, De Wit R, Mur LR. Interactions between temperature and light intensity on growth and photosynthesis of the cyanobacterium Oscillatoria agardhii. J. Plank Res. 1985a; 7: 487-49.
- Rajendran UM, Elango K, Anand N. Effects of a Fungicide, an Insecticide and a Bio-pesticide on Tolypothrix scytonemoides Pest. Biochem. Physiol. 2007; 87: 64-171.
- Renaud SM, Parry DL, Luong VT, Kuo C, Padovan A, Sammy N. Effect of light intensity on the proximate biochemical and fatty acid composition of Isochrysis sp. and Nannochloropsis oculata for use in tropical aquaculture. J Appl Phycol. 1991; 3: 43-53.
- Reynolds CS. The ecology of freshwater phytoplankton. Cambridge University Press, London. 1984.
- Richmond A. Cell response to environmental factors. Richmond A (ed) Handbook of microalgal mass culture. 1986; 69-106.
- Richmond A. Spirulina, Cambridge University Press, Cambridge, Micro-algal Biotec. 1988; 85-121.
- Sambrook J, Fritsch EF, Maniatis T. (1989). Molecular Cloning: A Laboratory Manual. 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Sanchez-Saavedra MP, Voltolina D. Effect of photon fluence rates of white and blue-green light on growth efficiency and pigment content of three diatom species in batch cultures. Cienc Mar. 2002; 28: 273-279.
- Seyfabadi J, Ramezanpour Z, Khoeyi Z. Protein, fatty acid, and pigment content of Chlorella vulgaris under different light regimes. J Appl Phycol. 2011; 23: 721-726.
- Shuter B. A model of physiological adaptation in unicellular algae. Theor Biol. 1979; 78: 519-552.
- Singh GP, Srivastava P. Impact of varied culture conditions on growth and morphology of Ankistrodesmus Fusiformis. J lndian bot Soc. l99l; 71: 341-345.
- Srivastava P. Studies on the experimental cultures of certain Chlorococcales, Ph.D. Thesis Osmania University, Hyderabad, 1967.
- Tandeau De Marsac NT. Phycobiliproteins and phycobilisomes: the early observations. Photosynth Res. 2003; 6: 197-205.
- Telford WG, Moss MW, Morseman JP, Allnutt FCT. Cryptomonad algal phycobilliproteins as fluorochromes for extracellular and intracellular antigen detection by flow cytometry. Cytometry. 2001; 44: 16-23.
- Van Liere L and Mur LR. Occurrence of Oscillatoria agardhii and some related species, a survey. Hypertrophic Ecosystems, 1980; 67-77.
- Vijaya V, Anand N. Blue light enhance the pigment synthesis in cyanobacterium Anabaena ambigua rao (nostacales), J Agrl Bio Sci. 2009; 4: 3.
- Vonshak A, Torzillo G, Tomaseli L. Use of chlorophyll fluorescence to estimate the effect of photoinhibition in outdoor cultures of Spirulina platensis. 1994; J Appl Phycol., 6: 31-34.
- Weber A, Jung K. Profiling Early Osmostress dependent gene expression in Escherichia coli using DNA Macroarrays." J Bacteriol. 2002; 184, 5502-5507.
- Zarrouk C. Contribution a retude du cyanophyceae. Influence dedivers facteurs physiques et chimiques sur la croissance et la photosynthse de Spirulina maxima (Setch et Gardner) Geitl. PhD, Paris. 1966.
Publication Dates
-
Publication in this collection
Nov-Dec 2014
History
-
Received
15 May 2013 -
Accepted
04 June 2014