Abstract
Replacing regular urea (RU) by slow-release urea (SRU) at two levels of non-protein nitrogen (NPN) in concentrate, offered with low-quality roughage, was evaluated in beef steers on dry matter intake (DMI), ruminal fermentation parameters, plasma urea nitrogen (PUN), total tract apparent digestibility of diets and in situ degradability of nitrogen sources. Eight ruminally cannulated steers were allocated into two 4x4 Latin squares, totalizing four treatments: 40 NPN/0 SRU: 40% of concentrate crude protein (CP) as NPN, resulting from 0% of SRU and 100% of RU; 40 NPN/50 SRU: 40% of concentrate CP as NPN, resulting from 50% of SRU and 50% of RU; 40 NPN/100 SRU: 40% of concentrate CP as NPN, resulting from 100% of SRU and 0% of RU; 80 NPN/100 SRU: 80% of concentrate CP as NPN, resulting from 100% of SRU and 0% of RU. Results showed that partial substitution of regular urea by slow-release urea did not alter dry matter intake, pattern of ruminal fermentation or plasma urea nitrogen concentrations and increased the total tract apparent digestibility of crude protein in steers diets. The increase in non-protein nitrogen content in crude protein of the concentrate could compromise feed intake and the efficiency of nutrient utilization in the steers fed complete diets based on low quality forage.
Controlled release; feed intake; non-protein nitrogen; short chain fatty acids
INTRODUCTION
Urea is the non-protein nitrogen source most often used in replacement of true protein feeds, as its use enables a reduction in the costs of cattle feeding (Silva et al. 2001Silva RMN, Valadares RFD, Valadares Filho SC, Cecon PR, Campos JMS, Oliveira GA, Oliveira AS. Uréia para vacas em lactação. 1. Consumo, digestibilidade, produção e composição do leite. Rev Bras Zootec. 2001; 30:1639-1649.). Urea is quickly degraded by urease from the rumen to ammonia (Cunningham 2004Cunningham JG. Tratado de fisiologia veterinária. 3rd.ed. Rio de Janeiro: Guanabara Koogan; 2004.), and, in the presence of carbohydrates, is used by the microorganisms for microbial protein synthesis (Butler 1998Butler WR. Review: Effect of protein nutrition on ovarian and uterine physiology in dairy cattle. J Anim Sci. 1998; 81: 2533-2539.). However, a detrimental aspect of urea utilization is its rapid solubilization and losses of almost 40% of nitrogen can occur, if there is no synchronization between ammonia release and carbohydrate availability in the rumen (Rodrigues 2003Rodrigues AA. Utilização de nitrogênio não-protéico em dietas de ruminantes. In: Simpósio Goiano sobre manejo e nutrição de bovinos de corte e leite: Proceedings: Goiânia: CBNA, 2003.). Besides, risks of intoxication for the animal are critical in using urea-containing supplements, especially to grazing cattle (Bartley et al. 1976Bartley EE, Davidovich AD, Barr GW, Griffel GW, Dayton AD, Deyoe CW, et al. Ammonia toxicity in cattle. I. Rumen and blood changes associated with toxicity and treatment methods. J Anim Sci. 1976; 43: 835-841.).
The development of products that slowly release urea, generically called slow-release urea (SRU), have been studied as an alternative for the disadvantages of high ruminal solubilization, high renal excretion and ammonia toxicity of urea. Moreover, the utilization of SRU can improve the acceptability of regular urea (RU) that has low palatability. Oliveira Júnior et al. (2004)Oliveira Júnior RC, Pires AV, Fernandes JJR, Susin I, Santos FAP, Araújo RC. Substituição total do farelo de soja por uréia ou amiréia, em dietas com alto teor de concentrado, sobre o nitrogênio amoniacal ruminal, os parâmetros sanguíneos e o metabolismo do nitrogênio em bovinos de corte. Rev Bras Zootec. 2004; 33: 738-748.observed increased dry matter intake (DMI) in the steers fed diets containing SRU compared with control group and small alteration in rumen fermentation pattern. The inclusion of SRU in beef steers diets did not affect growth performance, carcass dressing and rumen fermentation pattern compared to control diet where the crude protein (CP) source was soybean meal (Pinos-Rodriguez et al. 2010Pinos-Rodríguez JM, González-Muñoz SS, Bárcena R, Salem A. Effects of a slow-release coated urea product on growth performance and ruminal fermentation in beef steers. Ital. J Anim Sci. 2010; 9:16-19.).
The objective of the present study was to evaluate different levels of substitution of RU by SRU in a fixed level of concentrate on dry matter intake, rumen fermentation pattern, in situ degradability of nitrogen feed sources and total tract dry matter apparent digestibility in beef steers fed grass hay and concentrates.
MATERIAL AND METHODS
The trial was conducted at the Agência Paulista de Tecnologia dos Agronegócios (APTA), Andradina-SP, Brazil, according to the guidelines established by São Paulo State University (Brazil) Ethical Committee for Animal Research (CEEA), protocol number 88/2006.
Eight ruminally cannulated Nellore steers (374.4 ± 42.0 kg of BW) were housed in metabolic cages equipped with individual feed bunks and water fountains. The experimental design was a replicated 4x4 Latin square, totalizing 32 experimental units (eight per treatment). Animals were fed daily chopped Brachiaria brizantha hay ad libitum and 0.6% of BW of concentrate (approximately 2.0 kg), divided in two meals delivered at 800 and 1600 h. Grass hay and concentrate were roughly mixed by hand in the bunk before cattle had access to the meal.
The CP in the diets was balanced to 40% of concentrate non-protein nitrogen (NNP). The three different levels of SRU product (0.50 and 100%) were used in order to test the effect of urea solubility in diets usually adopted in field conditions.
The treatments were 40 NPN/0 SRU: 40% of concentrate crude protein (CP) as non-protein nitrogen (NPN), resulting from 0% of slow-release urea (SRU) (Optigen Alltech Inc., Nicholasville, KY, EUA) and 100% of regular urea (RU); 40 NPN/50 SRU: 40% of concentrate CP as NPN, resulting from 50% of SRU and 50% of RU; 40 NPN/100 SRU: 40% of concentrate CP as NPN, resulting from 100% of SRU and 0% of RU; 80 NPN/100 SRU: 80% of concentrate CP as NPN, resulting from 100% of SRU and 0% of RU.
The fourth treatment (80/100) tested the increase in NPN level in relation to total CP in the diet. The feeding regime adopted aimed to reproduce grazing conditions in the tropical areas, especially in dry season, where forage had been the main feed source and diet lacked adequate protein supply for microbial growth.
Urea {CO (NH2)2, 46.5% N} was used as regular urea. For slow-release urea production, the granules of regular urea were covered by an inert biodegradable polymer, which allowed slower rate of urea release in the rumen compared with regular urea (Tedeschi et al. 2002Tedeschi LO, Baker MJ, Ketchen DJ, Fox DG. Performance of growing and finishing cattle supplemented with a slow-release urea product and urea. Can J Anim Sci. 2002; 82:567-573.). Proximate analysis of feedstuffs is shown in Table 1.
The trial had four periods of 11 d, totalizing 44 experimental days. For dry matter intake measurement, all feeders were examined every morning at 0630 h. If there were no refusals, the amount of offered hay was raised by 10%. If there was a ~ 10% surplus, hay was kept at the same level, and if the surplus was 10% higher, the hay offered was reduced by 10%. At the last six days of each period, feed surplus from each steer was collected and weighed to calculate the feed intake.
Ruminal fluid samples were collected at the last day of each period, through the ruminal cannula with a vacuum pump at 0, 2, 4, 6, 8 and 10 h after the morning meal. At this day, the evening meal was offered after the collection of the 10 h sample. Approximately 500 mL of rumen fluid was collected at each sampling time from three different parts of the rumen. After the collection of samples, the remaining ruminal fluid was returned to the pre-ventricule. Immediately after the collection, 100 mL of rumen fluid was used for pH determination with a portable digital pH meter (HANNA instruments Limited HI8424, Bedfordshire, UK) previously calibrated with solutions of pH 4.0 and 7.0.
For the short-chain fatty acids (SCFA) analyses, which included acetate, propionate and butyrate, approximately 100 mL of ruminal fluid was centrifuged at 2000 x g for 20 min and 2.0 mL of the supernatant was added to 0.4 mL of formic acid and frozen at - 20ºC for further analyses, according to Erwin et al. (1961). Short-chain fatty acids were measured by gas chromatography (Finnigan 9001, Thermo Scientific, West Palm Beach, USA) using a glass column of 1.22 m of length and 0.63 cm of diameter packed with 80/120 Carbopack B-DA/4% (Supelco, Sigma-Aldrich, St. Louis, MO).
For ammonia nitrogen (NH3-N) concentration determination, 2.0 mL of the supernatant was added to 1.0 mL of 1 N H2SO4 solution and the centrifuge tubes were immediately frozen until the colorimetric analyses, according to the method described by Kulasek (1972)Kulasek GA. A micromethod for determination of urea in plasma, whole blood and blood cells using urease and phenol reagent. Pol Arch Weter. 1972; 15: 801-810. and adapted by Foldager (1977)Foldager J. Protein requirement and non protein nitrogen for high producing cow in early lactation [PhD Thesis]. Michigan, United States of America: Michigan State University; 1977..
For the measurement of plasma urea nitrogen (PUN) concentration, blood samples were collected at the 10th d at 0, 2, 4, 6, 8 and 10 h after morning feeding by jugular vein puncture in heparinized vacuum tubes. Blood was centrifuged at 1500 x g during 15 min and stored at -20°C for later analysis by enzymatic colorimetric methodology (Chaney and Marbach 1962Chaney AL, Marbach EP. Modified reagents for determination of urea and ammonia. Clin Chem. 1962; 8: 130-146.) using a commercial kit (LaborLab(r), Guarulhos, Brazil). Readings were made by spectrophotometry and the conversion to PUN was calculated by the multiplication of urea concentration in plasma (mg/dL) by the factor 0.47.
At the end of the fourth experimental period, an assay for in situruminal degradability characterization of nitrogen sources (regular urea, slow-release urea, soybean meal and corn gluten meal) was done. For this, following Mehrez and Ørskov (1977)Mehrez AZ, Ørskov ER. A study of the artificial fiber bag technique for determining the digestibility of feeds in the rumen. J Agr Sci.1977; 88: 645-665., two animals from treatment 40/0 were used. Nylon bags with a porosity of 50 μm (10.0 x 19.0 cm) were filled with approximately 8.0 g of feed previously dried at 55ºC for 72 h. Bags were weighed, tied and stored in a refrigerator (5ºC) before use. They were attached to the rumen cannula by a nylon thread with a minimum of 50 cm length and incubated in triplicate for 1, 2, 4, 8, 16, 24 and 32 h after morning feeding. After incubation, all the bags were washed thoroughly by hand and dried at 55ºC for 72 h for later weighing and chemical analyses. Degradability at time zero was measured by washing the bags in water (39ºC) for 15 min (Cummings et al. 1983Cummings KA, Nocek JE, Polan CE, Herbein JH. Nitrogen degradability and microbial protein synthesis in calves fed diets of varying degradability by the bag technique. J Dairy Sci. 1983; 66: 2356-2364.). In situ crude protein degradability estimate was calculated by the following formula: CPDg% = [(CP in original sample - CP in post rumen sample*100/CP in original sample], where CPDg% is the crude protein degradability in percentage.
Digestibility trial consisted of five days from the seven to eleven days of each experimental period. Total feces collection was done in collector boxes behind the digestibility cages. After the weighing of the collected material, 10% of feces samples were stored in plastic bags and maintained at -20°C until the analysis. During the same period, samples of feed (hay and concentrate) were daily collected and stored under refrigeration for further analysis. Feed and fecal samples were dried at 55ºC for 72 h and ground to pass a 1-mm screen. Composite samples per cow were used to determine the DM (method 934.01; AOAC 1990Association of Official Analytical Chemists - AOAC. Official Methods of Analysis. 15.ed., v.2. Washington, D.C. 1990, 1278p.); OM (method 924.05; AOAC 1990Association of Official Analytical Chemists - AOAC. Official Methods of Analysis. 15.ed., v.2. Washington, D.C. 1990, 1278p.); CP by total N determination using the micro-Kjeldahl technique (method 920.87; AOAC 1990Association of Official Analytical Chemists - AOAC. Official Methods of Analysis. 15.ed., v.2. Washington, D.C. 1990, 1278p.); ether extract (EE) determined gravimetrically after extraction using petroleum ether in a Soxhlet extractor (method 920.85; AOAC 1990Association of Official Analytical Chemists - AOAC. Official Methods of Analysis. 15.ed., v.2. Washington, D.C. 1990, 1278p.); NDF (with heat-stable α-amylase) and ADF according to Van Soest et al. (1991)Van Soest PJ, Robertson JB, Lewis BA. Methods of dietary fiber, neutral detergent fiber, and non starch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991; 74:3583-3597.. The value of non-fiber carbohydrates (NFC) was estimated by the formula: NFC (% DM) = 100 - (CP + NDF + EE + Ash) according to Hall (2001)Hall MB. Recent advanced in non-ndf carbohydrates for the nutrition of lactating cows. In: International Symposium in Dairy Cattle: Proceedings: Lavras: UFLA-FAEPE; 2001. p. 139-148.. Total digestible nutrient (TDN) was estimated by the formula: TDN = DCP + 2.25 x DEE + DNDF + DNFC according to NRC (2001)National Research Council - NRC. Nutrient requeriments of dairy cattle. 7.ed. Washington, D.C.: 2001. 381p..
Results were analyzed by Statistical Analysis System software (SAS Inst., Inc., Cary, NC, 2008Statistical Analysis System - SAS. SAS User's guide: Statistics. Cary: SAS Institute, 2008.), after verifying residue normality by Shapiro-Wilk test (PROC UNIVARIATE). The model of variance analysis accounted for the fixed effect of treatment and the random effects of square, animal nested in square and period. The degrees of freedom of the treatment were separated by three orthogonal contrasts:
-
• Contrast 1)
Linear effect of regular urea replacement by slow-release urea in the concentrate with 40% of CP as NPN:
-
• Contrast 2)
Non-linear association of regular urea replacement by slow-release urea in the concentrate with 40% of CP as NPN:
-
• Contrast 3)
40% (0% + 50% + 100% of regular urea replacement by slow-release urea) vs. 80% of NPN in the CP fraction of the concentrate with 100% of NPN offered as slow-release urea.
The variables ruminal pH, total concentration and molar proportion of SCFA, NH3-N concentration and PUN were analyzed by MIXED procedure of SAS with repeated measures (Wang and Goonewardene 2004Wang, Z, Goonewardene L A. The use of MIXED models in the analysis of animal experiments with repeated measures data. Can J Anim Sci. 2004; 84:1-11.). Effects were considered significant at p≤ 0.05.
RESULTS AND DISCUSSION
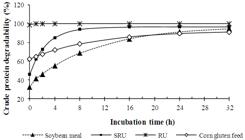
The adjusted curves of CP degradability for N sources are presented in Figure 1. It showed higher disappearance of SRU in relation to true protein sources (soybean meal and corn gluten feed) at the first 4 h of incubation, although lower than RU. Therefore, the degradability curve of SRU was in agreement of the proposal of the controlled release urea by means of an involucre of inert biodegradable polymer.
Degradation curve of crude protein (CP) for evaluated feeds (soybean meal; SRU: slow-release urea; RU: regular urea; corn gluten feed).
Data on feed intake are shown in Table 2. There was no effect (P>0.05) of RU substitution by SRU on DM, CP, NDF and NFC intake. In accordance with these results, Tedeschi et al. (2002)Tedeschi LO, Baker MJ, Ketchen DJ, Fox DG. Performance of growing and finishing cattle supplemented with a slow-release urea product and urea. Can J Anim Sci. 2002; 82:567-573., for crossbred growing and finishing steers, reported that DMI did not differ between the animals fed RU or SRU in total mixed ration. Probably, the high fermentability of the carbohydrate sources in the diet and the continuous supply of nutrients on ad libitum intake resulted continuous fermentation and sufficient N requirements to support the fermentation. Conversely, Akay et al. (2004)Akay V, Tikofsky J, Holtz C, Dawson KA. Optigen(r) 1200: controlled release of non-protein nitrogen in the rumen. In: Nutritional Biotechnology in the feed and food industries, Alltech's twenty first annual symposium: Proceedings: Nottingham: Nottingham University Press; 2004. p.179-185. observed reduction of 0.89 kg/d in DMI of lactating dairy cows fed SRU in relation to the animals that received RU without effect on milk yield. The present results suggested that using slow-release could maintain the feed intake as well as the production levels achieved with the common diets containing regular urea for beef cattle.
The increment in NPN content in concentrate from 40 to 80% did not affect DM and NDF intake. Oliveira et al. (2004)Oliveira MMNF, Torres, CAA, Valadares Filho SC, Santos ADF, Properi CP. Ureia para vacas leiteiras no pós-parto: Desempenhos produtivo e reprodutivo. Rev Bras Zootec. 2004; 33: 2266-2273. reported drop in DMI as the RU contents in dairy cows diets increased, which was considered a reflex of the low palatability of RU (Chalupa et al. 1979Chalupa WCA, Baile CA, McLaughlin CL. Effect of introduction of urea on feeding behaviour of Holstein heifers. J Dairy Sci. 1979; 62: 1278-1284.).
In the present study, the inclusion of a slow-release NPN did not negatively impacted feed intake. This effect was expected as SRU was hydrolyzed slowly than RU and in a similar rate than fiber digestion (Tedeschi et al. 2002Tedeschi LO, Baker MJ, Ketchen DJ, Fox DG. Performance of growing and finishing cattle supplemented with a slow-release urea product and urea. Can J Anim Sci. 2002; 82:567-573.), which could result in greater fiber degradation and ruminal turnover rates. Consequently, the rumen fill effects on feed intake would be lessened (Van Soest 1994Van Soest PJ. Nutritional ecology of ruminant. 2nd.ed. Ithaca: Cornell University Press; 1994.). However, in the present study, cattle was fed grass hay, which had higher NDF content, which could explain the results, since the lower responsiveness of the animal to NPN occurred in the diets with high NDF and small fraction of degradable carbohydrates in the rumen (Van Soest 1994Van Soest PJ. Nutritional ecology of ruminant. 2nd.ed. Ithaca: Cornell University Press; 1994.).
Conversely, the crude protein intake was decreased and non-fiber carbohydrate intake increased when cattle were fed diets with higher NPN contents. This was unexpected because no changes in DMI occurred across the different diets, suggesting that cattle might have selected for a lower protein diet. However, because the high CP and NFC of concentrate, it would be impossible to select for a low protein and high NFC diet concomitantly. The reason for these results was unclear but it could demonstrate that cattle were able to adjust their feed consumption in order to optimize the nitrogen requirements for rumen fermentation.
For ruminal pH, there was no interaction between the treatment and sampling time (P>0.05). There was neither effect of RU substitution by SRU (P>0.05), nor effect of NNP content in CP of concentrate (P>0.05) (Table 3). Oliveira Júnior et al. (2004)Oliveira Júnior RC, Pires AV, Fernandes JJR, Susin I, Santos FAP, Araújo RC. Substituição total do farelo de soja por uréia ou amiréia, em dietas com alto teor de concentrado, sobre o nitrogênio amoniacal ruminal, os parâmetros sanguíneos e o metabolismo do nitrogênio em bovinos de corte. Rev Bras Zootec. 2004; 33: 738-748. did not observe differences in pH of the steers fed true protein feed (soybean meal) or NPN sources (RU or extruded starch-urea). It was noteworthy that cattle were fed roughage-based diets in the present study, therefore the high pH values observed were expected despite the feeding of concentrate. Also, SRU effects on rumen pH were not expected, unless it markedly altered short chain fatty acids profile in the rumen.
For total SCFA concentration, there was no interaction between the treatment and sampling time (P>0.05) (Table 3). Total SCFA concentration showed negative linear association (P>0.05) in function of the increase in SRU content in NPN of concentrate, association verified by contrast 1 (linear effect of RU substitution by SRU with 40% of NPN in the concentrate). Nevertheless, replacing RU for SRU did not affect the molar concentrations of acetate, propionate, butyrate and the acetate to propionate ratio (P>0.05).
No interaction between the treatment and sampling time was observed for acetate molar proportion (P>0.05). However, there was an effect of NPN content in CP of the concentrate. Molar proportion of acetate in the group submitted to 80% NPN supplements was higher than in the group receiving concentrates with 40% of NPN in CP (Table 3). For propionate molar proportion, an interaction was observed between the treatment and sampling time (P>0.05). The same was observed for Ac:Pr ratio (P>0.05) (Table 3). However, studies on the effects of treatment in each time separately did not show the effect of substitution of RU by SRU (P>0.05), nor the effect of NNP content increase in CP of the concentrate (P>0.05). For molar proportion of butyrate, an interaction between the treatment and sampling time (P>0.05) was observed, although it was not possible to show the effect of RU substitution by SRU when the analysis was performed within sampling time. However, the animals that received 80/100 concentrate showed lower molar proportion of butyrate when compared with those fed 40% of NNP concentrates (Table 3). Some other studies supported the present findings. Devant et al. (2001)Devant M, Ferret A, Calsamiglia, S, Casals R, Gasa, J. Effect of nitrogen source in high-concentrate, low protein beef cattle diets on microbial fermentation studied in vivo and in vitro. J Anim Sci. 2001; 79: 1944-1953. did not observe difference in the total SCFA concentration in dairy cows fed soybean meal or RU and Oliveira-Júnior et al. (2004)Oliveira Júnior RC, Pires AV, Fernandes JJR, Susin I, Santos FAP, Araújo RC. Substituição total do farelo de soja por uréia ou amiréia, em dietas com alto teor de concentrado, sobre o nitrogênio amoniacal ruminal, os parâmetros sanguíneos e o metabolismo do nitrogênio em bovinos de corte. Rev Bras Zootec. 2004; 33: 738-748. did not observe effect of RU substitution by extruded starch-urea on total SCFA concentration in beef steers.
Mean ruminal NH3-N concentrations of all the treatments in this study were above the optimum range for good growth of rumen microorganisms according to Satter and Slyter (1974)Satter LD, Slyter LL. Effect of ammonia concentration on ruminal microbial protein production in vitro. Brit J Nutr. 1974; 32:199-208. (5.0 to 8.0 mg of NH3-N by dL of ruminal fluid), and above the maximum threshold of 10 mg/dL for optimization of digestibility of low quality forage (Krebs and Leng 1984Krebs G, Leng RA. The effect of supplementation with molasses/urea blocks on ruminal digestion. Proceedings of the Australian Society of Animal Production. 1984; 15:704.). Mean concentrations found in this study were also close to the recommended by Leng (1990)Leng RA. Factors affecting the utilization of 'poor-quality' forages by ruminants particularly under tropical conditions. Nutr Res Rev. 1990; 3: 277-303. (16.4 mg/dL) for enhancement of voluntary intake of low quality forage.
No effect of interaction between the treatments and sampling times (P>0.05), RU substitution by SRU (P>0.05), and no effects of concentrate NNP content were observed for rumen NH3-N (P>0.05). In the current study, it was expected that feeding slow-release as well as increasing diet NNP would alter the rumen concentration of ammonia throughout the day. The degradation of RU into NH3-N occurred almost instantly in the rumen due to its high solubility, increasing the ammonia pool very rapidly. Differently, the SRU could promote gradual delivery of NPN in the rumen. However, the treatments failed to alter the rumen ammonia concentrations, which were also not affected postprandially. What could contribute for this was the fact that diets were tested as total mixed rations. This could have impacted the effect of SRU and NNP level. Animals that ingest forage along with concentrate in small meals are submitted to a different pattern from what is observed when they are supplemented in pasture when they ingest high quantities of supplement in one single meal once a day that is more compatible with the product propose. Regular urea, fed to steers probably due to its rapid hydrolysis to ammonia in the rumen, increased in 58% mean ammonia concentration compared with SRU. Treatments were mixed into the basal diets at 1.8% of diet DM (Taylor-Edwards et al. 2009Taylor-Edwards CC, Hibbard G, Kitts SE, McLeod KR, Axe DE, Vanzant ES, et al. Effects of slow-release urea on ruminal digesta characteristics and growth performance in beef steers. J Anim Sci. 2009; 87:200-208.).
Regarding the PUN, no effect of interaction between the treatments and sampling times (P>0.05) as well as effect of concentrate NNP content (P>0.05) were observed. Replacing RU for SRU affected the PUN levels in a non-linear trend (Table 3). It was expected (as SRU was added into the diet) that the rumen ammonia pool would decrease in the early hours post-feeding, thereby affecting the PUN in the same manner. However, there was no effect of sampling time of PUN and rumen NH3-N as well. Butler (1998)Butler WR. Review: Effect of protein nutrition on ovarian and uterine physiology in dairy cattle. J Anim Sci. 1998; 81: 2533-2539. reported low fluctuation of PUN during the day (between 0.02 and 0.03 mg/dL). However, this fluctuation occurred in lower intensity in the animals fed TMR diets, likewise animals in the present experiment, when compared with animals fed forage and concentrate separately, according to Gustafsson and Palmquist (1993)Gustafsson AH, Palmquist DL. Diurnal variation of rumen ammonia, serum urea, and milk urea in dairy cows at high and low yields. J Dairy Sci. 1993; 76: 475-484.. Also, despite both the feeds (hay and concentrate) were offered together, manual mixture could allow selection of the feeds by the animals (Forbes 1995Forbes JM. The voluntary food intake and diet selection in farm animals. Wallingford: CAB International; 1995.) and, by consequence, different dynamics of intake, which could affect the PUN levels. Blood urea nitrogen concentration was lower for lactating dairy cows fed SRU and soybean meal than RU. Probably, more urea was produced in the liver and then excreted in urine for the cows consuming RU (Xin et al. 2010Xin HS, Schaefer DM, Liu QP, Axe DE, Meng QX. Effects of polyurethane coated urea supplement on in vitro ruminal fermentation, ammonia release dynamics and lactating performance of Holstein dairy cows fed a steam-flaked corn-based diet. Asian-Aust. J Anim Sci. 2010; 4:491-500.).
Digestibility coefficients for DM and its fraction along with TDN of diets are presented in Table 4. There was no effect of RU substitution by SRU on digestibility of DM, NDF, ADF, EE, NFC and OM, as well as TDN percentage of diets. Akay et al. (2004)Akay V, Tikofsky J, Holtz C, Dawson KA. Optigen(r) 1200: controlled release of non-protein nitrogen in the rumen. In: Nutritional Biotechnology in the feed and food industries, Alltech's twenty first annual symposium: Proceedings: Nottingham: Nottingham University Press; 2004. p.179-185. found that the use of encapsulated urea allowed higher microbial protein synthesis in vitro and faster nutrient utilization in relation to control diet, increasing the digestibility of NDF, ADF, total carbohydrates and OM in values of 16.6, 6.8, 4.0 and 8.0%, respectively, compared with the control group. Owens and Zinn (1988)Owens FN, Zinn RA. Protein metabolism of ruminant animals. In: Church DC, editor. The Ruminant Animal, Digestive Physiology and Nutrition. New Jersey: Prentice Hall; 1988. p. 227-249. reported that the compounds with controlled release nitrogen, such as extruded-urea starch, biuret and most of the complexes of urea and formaldehyde or molasses avoided intoxication by NH3-N, without however, affecting the nutrients utilization, which was in agreement with the results presented in this study.
On the other hand, in the present study replacing RU by SRU linearly increased (P>0.05) CP digestibility of the diet. There was an increase of 3.58% (2.85 percentage units) when 100% of RU was substituted by SRU in the CP of the concentrate. The results suggested that SRU allowed a better use of dietary non-protein nitrogen by the ruminal microorganisms, corroborating with a numerical decrease in rumen NH3-N in the 40/100 treatment and a drop in PUN levels as SRU as included in the diet. For Holstein x Nellore crossbred steers fed 0, 15.5, 31 and 46.5% of dietary N as NPN, ruminal digestibility of CP linearly increased with crescent dietary levels of NPN, likely because of absorption of NPN as ammonia before the abomasum (Chizzotti et al. 2008Chizzotti FHM, Pereira OG, Tedeschi LO, Valadares Filho SC, Chizzotti ML, Leão MI, et al. Effects of dietary nonprotein nitrogen on performance, digestibility, ruminal characteristics, and microbial efficiency in crossbred steers. J Anim Sci. 2008; 86:1173-1181.).
The content of NPN in CP of the concentrate did not influence the EE or NFC digestibilities (P>0.05). However, the treatment with 80% NNP had lower CP, NDF and ADF digestibilities (P>0.05). The percentage of TDN decreased with an increase in NNP percentage. When Silva et al. (2001)Silva RMN, Valadares RFD, Valadares Filho SC, Cecon PR, Campos JMS, Oliveira GA, Oliveira AS. Uréia para vacas em lactação. 1. Consumo, digestibilidade, produção e composição do leite. Rev Bras Zootec. 2001; 30:1639-1649. studied lactating cows and evaluated the inclusion of increasing levels of RU in diet in substitution of soybean meal, no effects on total tract apparent digestibility of DM, OM, CP, total carbohydrates and NDF were also observed. The explanation for the negative effects of increasing NPN content of the concentrate on fiber digestibility in the current study is not clear. It could be speculated that the 80/100 treatment might have enhanced fibrolytic enzyme activity (Pan et al. 2003Pan J, Suzuki T, Koike S, Ueda K, Kobaiashi Y, Tanaka K, et al. Effects of urea infused into the rumen on liquid- and particle-associated fibrolytic enzyme activities in steers fed low quality grass hay. Anim Feed Sci. Tech. 2003; 104:13-27.) so that fiber digestion was increased. However, rumen fermentation was not greatly modified by that treatment as seen by results on short chain fatty acids and rumen ammonia. On the other hand, the resulted lower crude protein digestibility was likely a consequence of unbalanced nitrogen to digestible organic matter content ratio in the rumen, whereby non-protein nitrogen was not completely utilized for the microbial growth. In addition, the decrease in diet TDN could be a consequence of the lower digestibility of cell wall fraction in this treatment. Despite the reduction in fiber digestibility, it should be born in mind that feed intake was not compromised even though cattle were fed high-roughage diets.
CONCLUSIONS
Partial substitution of regular urea by urea coated with an inert polymer, when offered at a maximum of 40% of non-protein nitrogen of the concentrate in total mixed ration with low quality forage did not alter dry matter intake of steers.
Partial substitution of regular urea by slow-release urea did not alter the pattern of ruminal fermentation or plasma urea nitrogen concentrations of steers in the conditions of the present experiment.
Regular urea substitution by slow-release urea in the concentrate offered with low quality forage in total mixed ration increased total tract apparent digestibility of crude protein.
The increase in non-protein nitrogen content in crude protein of the concentrate could compromise feed intake and the efficiency of nutrient utilization in the steers fed complete diets based on low quality forage.
ACKNOWLEGMENTS
The authors thank the employees of APTA (Pólo Regional Extremo Oeste, Andradina, SP, Brazil) for the good care of the animals. Also, Ari de Castro, Gilson de Godoy and Simi Aflalo (Laboratory of Bromatology, Department of Nutrition and Animal Production, FMVZ-USP, Pirassununga, Brazil) for all the help with laboratory analysis are gratefully acknowledge.
REFERENCES
- Association of Official Analytical Chemists - AOAC. Official Methods of Analysis. 15.ed., v.2. Washington, D.C. 1990, 1278p.
- Akay V, Tikofsky J, Holtz C, Dawson KA. Optigen(r) 1200: controlled release of non-protein nitrogen in the rumen. In: Nutritional Biotechnology in the feed and food industries, Alltech's twenty first annual symposium: Proceedings: Nottingham: Nottingham University Press; 2004. p.179-185.
- Bartley EE, Davidovich AD, Barr GW, Griffel GW, Dayton AD, Deyoe CW, et al. Ammonia toxicity in cattle. I. Rumen and blood changes associated with toxicity and treatment methods. J Anim Sci. 1976; 43: 835-841.
- Butler WR. Review: Effect of protein nutrition on ovarian and uterine physiology in dairy cattle. J Anim Sci. 1998; 81: 2533-2539.
- Chalupa WCA, Baile CA, McLaughlin CL. Effect of introduction of urea on feeding behaviour of Holstein heifers. J Dairy Sci. 1979; 62: 1278-1284.
- Chaney AL, Marbach EP. Modified reagents for determination of urea and ammonia. Clin Chem. 1962; 8: 130-146.
- Chizzotti FHM, Pereira OG, Tedeschi LO, Valadares Filho SC, Chizzotti ML, Leão MI, et al. Effects of dietary nonprotein nitrogen on performance, digestibility, ruminal characteristics, and microbial efficiency in crossbred steers. J Anim Sci. 2008; 86:1173-1181.
- Cummings KA, Nocek JE, Polan CE, Herbein JH. Nitrogen degradability and microbial protein synthesis in calves fed diets of varying degradability by the bag technique. J Dairy Sci. 1983; 66: 2356-2364.
- Cunningham JG. Tratado de fisiologia veterinária. 3rd.ed. Rio de Janeiro: Guanabara Koogan; 2004.
- Devant M, Ferret A, Calsamiglia, S, Casals R, Gasa, J. Effect of nitrogen source in high-concentrate, low protein beef cattle diets on microbial fermentation studied in vivo and in vitro. J Anim Sci. 2001; 79: 1944-1953.
- Erwin ES, Marco GJ, Emery, EM. Volatile fatty acids analyses of blood and rumen fluid by gas chromatography. J Dairy Sci. 1961; 44: 1768-1771.
- Foldager J. Protein requirement and non protein nitrogen for high producing cow in early lactation [PhD Thesis]. Michigan, United States of America: Michigan State University; 1977.
- Forbes JM. The voluntary food intake and diet selection in farm animals. Wallingford: CAB International; 1995.
- Fox DG,Tedeschi LO, Tylutki TP, Russell JB, Van Amburgh ME, Chase LE, et al. The Cornell Net Carbohydrate and Protein System model for evaluating herd nutrition and nutrient excretion. Anim Feed Sci Tech. 2004; 112: 29-78.
- Gustafsson AH, Palmquist DL. Diurnal variation of rumen ammonia, serum urea, and milk urea in dairy cows at high and low yields. J Dairy Sci. 1993; 76: 475-484.
- Hall MB. Recent advanced in non-ndf carbohydrates for the nutrition of lactating cows. In: International Symposium in Dairy Cattle: Proceedings: Lavras: UFLA-FAEPE; 2001. p. 139-148.
- Krebs G, Leng RA. The effect of supplementation with molasses/urea blocks on ruminal digestion. Proceedings of the Australian Society of Animal Production. 1984; 15:704.
- Kulasek GA. A micromethod for determination of urea in plasma, whole blood and blood cells using urease and phenol reagent. Pol Arch Weter. 1972; 15: 801-810.
- Leng RA. Factors affecting the utilization of 'poor-quality' forages by ruminants particularly under tropical conditions. Nutr Res Rev. 1990; 3: 277-303.
- Mehrez AZ, Ørskov ER. A study of the artificial fiber bag technique for determining the digestibility of feeds in the rumen. J Agr Sci.1977; 88: 645-665.
- National Research Council - NRC. Nutrient requeriments of dairy cattle. 7.ed. Washington, D.C.: 2001. 381p.
- Oliveira MMNF, Torres, CAA, Valadares Filho SC, Santos ADF, Properi CP. Ureia para vacas leiteiras no pós-parto: Desempenhos produtivo e reprodutivo. Rev Bras Zootec. 2004; 33: 2266-2273.
- Oliveira Júnior RC, Pires AV, Fernandes JJR, Susin I, Santos FAP, Araújo RC. Substituição total do farelo de soja por uréia ou amiréia, em dietas com alto teor de concentrado, sobre o nitrogênio amoniacal ruminal, os parâmetros sanguíneos e o metabolismo do nitrogênio em bovinos de corte. Rev Bras Zootec. 2004; 33: 738-748.
- Owens FN, Zinn RA. Protein metabolism of ruminant animals. In: Church DC, editor. The Ruminant Animal, Digestive Physiology and Nutrition. New Jersey: Prentice Hall; 1988. p. 227-249.
- Pan J, Suzuki T, Koike S, Ueda K, Kobaiashi Y, Tanaka K, et al. Effects of urea infused into the rumen on liquid- and particle-associated fibrolytic enzyme activities in steers fed low quality grass hay. Anim Feed Sci. Tech. 2003; 104:13-27.
- Pinos-Rodríguez JM, González-Muñoz SS, Bárcena R, Salem A. Effects of a slow-release coated urea product on growth performance and ruminal fermentation in beef steers. Ital. J Anim Sci. 2010; 9:16-19.
- Rodrigues AA. Utilização de nitrogênio não-protéico em dietas de ruminantes. In: Simpósio Goiano sobre manejo e nutrição de bovinos de corte e leite: Proceedings: Goiânia: CBNA, 2003.
- Satter LD, Slyter LL. Effect of ammonia concentration on ruminal microbial protein production in vitro. Brit J Nutr. 1974; 32:199-208.
- Statistical Analysis System - SAS. SAS User's guide: Statistics. Cary: SAS Institute, 2008.
- Silva RMN, Valadares RFD, Valadares Filho SC, Cecon PR, Campos JMS, Oliveira GA, Oliveira AS. Uréia para vacas em lactação. 1. Consumo, digestibilidade, produção e composição do leite. Rev Bras Zootec. 2001; 30:1639-1649.
- Taylor-Edwards CC, Hibbard G, Kitts SE, McLeod KR, Axe DE, Vanzant ES, et al. Effects of slow-release urea on ruminal digesta characteristics and growth performance in beef steers. J Anim Sci. 2009; 87:200-208.
- Tedeschi LO, Baker MJ, Ketchen DJ, Fox DG. Performance of growing and finishing cattle supplemented with a slow-release urea product and urea. Can J Anim Sci. 2002; 82:567-573.
- Tylutki TP, Fox DG, Durbal VM, Tedeschi LO, Russell JB, Van Amburgh ME, et al. Cornell Net Carbohydrate and Protein System: A model for precision feeding of dairy cattle. Ani Feed Sci Tech. 2008; 143:174-202.
- Van Amburgh ME, Chase LE, Overton TR, Ross DA, Recktenwald EB, Higgs RJ, et al. Updates to the Cornell Net Carbohydrate and Protein System v6.1 and implications for ration formulation. Proc Cornell Nutr Conf. 2010; 1-16.
- Van Soest PJ, Robertson JB, Lewis BA. Methods of dietary fiber, neutral detergent fiber, and non starch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991; 74:3583-3597.
- Van Soest PJ. Nutritional ecology of ruminant. 2nd.ed. Ithaca: Cornell University Press; 1994.
- Xin HS, Schaefer DM, Liu QP, Axe DE, Meng QX. Effects of polyurethane coated urea supplement on in vitro ruminal fermentation, ammonia release dynamics and lactating performance of Holstein dairy cows fed a steam-flaked corn-based diet. Asian-Aust. J Anim Sci. 2010; 4:491-500.
- Wang, Z, Goonewardene L A. The use of MIXED models in the analysis of animal experiments with repeated measures data. Can J Anim Sci. 2004; 84:1-11.
Publication Dates
-
Publication in this collection
Jan-Feb 2015
History
-
Received
04 July 2013 -
Accepted
18 Sept 2014