Abstract
This study aimed to evaluate the in vitro anthelmintic activity and antibacterial activity of the extracts from the leaves and fruits of Garcinia indica (Dupetit-Thouars) Choisy and Garcinia cambogia(Gaertn.) Desr. using the Indian earthworm Pheretima posthuma. Two concentrations (25 and 50 mg/mL) of various extracts such as petroleum ether, chloroform, ethyl acetate, methanol and water were tested. Albendazole at the concentrations of 25 and 50 mg/mL was used as the standard reference. Significant anthelmintic effects of the fruits and leaves of G. cambogia and G. indica (P<0.05) were observed and the results were expressed in terms of paralysis and death time. All the extracts showed the dose dependent paralysis and death of earthworms. Among all the extracts used, methanol extract exhibited the highest activity. G. cambogia leaf extract (50 mg/mL) had 30% faster paralysis effect on earthworms than the standard reference. Furthermore, the antimicrobial activity of the methanol extracts of the fruits and leaves showed significant (P<0.05) activity against Gram-positive and Gram-negative bacteria. At a concentration of 500 µg/mL, G. indica fruit extract presented higher zones of inhibition against Pseudomonas aeruginosa and Staphylococcus aureus. Hence, it could be concluded that the leaves and fruits of G. indica and G. cambogia contained active anthelmintic and antibacterial phytochemicals, which could find their applications in pharmaceuticals.
anthelmintic; antibacterial; Garcinia cambogia ; Garcinia indica ; Minimal Inhibitory Concentration
INTRODUCTION
Nematode infections cause severe health problems worldwide in humans and animals (Tariq et al. 2009Tariq KA, Chishti MZ, Ahmad F, Shawl AS. Anthelmintic activity of extracts of Artemisia absinthium against ovine nematodes. Vet Parasitol. 2009; 160: 83-88.; Das et al. 2011Das SS, Dey M, Ghosh AK. Determination of the anthelmintic activity of the leaf and bark extract of Tamarindus Inidca Linn. Indian J Pharm Sci. 2011; 73(1): 104-107.). In animals, these parasites cause frequent economic losses due to mortality (Agrawal and Banerjee 2007Agrawal MC, Banerjee PS. Problems confronting helminthic diseases of domestic animals in India. J Parasit Dis. 2007; 31(1): 3-13.). In human beings, they cause mal-absorption, diarrhea, anaemia and other intestinal problems (Kumar et al. 2012Kumar HKS, Raju MBV, Dinda SC, Sahu S. Evaluation of anthelmintic activity of Bambusa arundinacea. Asian J Pharm Tech. 2012; 2(2): 62-63.). More than two billion people are infected with different intestinal worms worldwide (Somvanshi et al. 2014Somvanshi VS, Ellis BL, Hu Y, Aroian RV. Nitazoxanide: Nematicidal mode of action and drug combination studies. Mol Biochem Parasit. 2014; 193: 1-8.). The nematode infections are more prevalent in developing countries due to poor sanitation and hygiene (Dhar et al. 1982Dhar DN, Sharma RL, Bansal GC. Gastro-intestinal Nematodes in sheep in Kashmir. Vet Parasitol. 1982; 11: 271-277.). Nematode species such as Haemonchus contortus and Bunostomum trigonocephalum that feed on the blood were recognized for clinical and sub-clinical indications causing excessive commercial loss in ruminants (Agrawal and Banerjee 2007Agrawal MC, Banerjee PS. Problems confronting helminthic diseases of domestic animals in India. J Parasit Dis. 2007; 31(1): 3-13.). The gastrointestinal nematodes are normally treated with chemically synthesized drugs. Control of nematodes with synthetic anthelmintics for longer periods has made them drug resistant (Lakshmanan et al. 2011Lakshmanan B, Mazumder PM, Sasmal D, Ganguly S, Jena SS. In Vitro anthelmintic activity of some 1-substituted imidazole derivatives. Acta Parasitol Globalis. 2011; 2(1): 1-5.). The other drawbacks of the existing anthelmintic drugs are side effects such as headache, loss of appetite, diarrhoea and vomiting (Goodman and Gilman 2001Goodman LS, Gilman A. The Pharmacological basis of Therapeutics. 10. Ed. New York: Mc graw Hill Medical Publishing Division, 2001: 1121p.). In order to overcome these drawbacks, phyto-medicines, which offer greater advantages over synthetic drugs have been developed (Hammond et al. 1997Hammond JA, Fielding D, Bishop SC. Prospects for plant anthelmintics in tropical veterinary medicine. Vet Res Commun. 1997; 21: 213-228.). Since time immemorial, humans have depended on traditional medicines derived from the plants for curing their ailments (Silva et al. 2008Silva RP, Holanda CMCX, Barbosa VSA, Oliveira DP, Lima NA, Camara ACJ, et al. Effect of Medicinal Plants on the Parasitemia of Trypanosoma cruzi and on the Biodistribution of Sodium Pertechnetate (Na99mTcO4). Braz Arch Biol Technol. 2008; 51: 209-214.). Reports from several parts of the world have shown that plant species could efficiently decrease parasite infections and could be promising alternatives to the conventional anthelmintics (Tariq and Tantry 2012Tariq KA, Tantry MA. Preliminary studies on plants with anthelmintic properties in Kashmir - the North-West temperate Himalayan region of India. Chinese Med. 2012; 3: 106-112.).
The genus Garcinia (Clusiaceae family) includes about 200 species throughout the world. Of these, 36 species have been reported from India. G. indica and G. cambogia species endemic to Western Ghats have been reported to possess anthelmintic activity (Abraham et al. 2006Abraham Z, Malik SK, Rao GE, Narayanan SL, Biju S. Collection and characterisation of Malabar tamarind [Garcinia cambogia (Gaertn) Desr.] .Genet Resour Crop Ev. 2006; 53: 401-406.). The compounds derived from the parts of G.indica (kokum) have been studied for anti-obesity, anticancer, anti-diabetic, antioxidant and antimicrobial properties (Hemshekhar et al. 2011). Kokum fruits have been used in the preparation of 'amrutkokum', a drink commonly used to relieve the sunstroke. Kokum has been used for its therapeutic properties as a good appetizer, remedy for flatulence (Dushyantha et al. 2010Dushyantha DK, Girish DN, Suvarna VC, Dushyantha DK. Native lactic acid bacterial isolates of kokum for preparation of fermented beverage. European J Biol Sci. 2010; 2(1): 21-24.). Anthelmintic activity of the fruit rind of Kokum was reported by Swapna et al. (2012)Swapna P, Elumalai A, Jayasri P. Evaluation of anthelmintic activity of Garcinia indica choisy fruits. Int J Adv Life Sci. 2012; 1: 85-88.. There were no reports of the anthelmintic activity of the extracts from the leaves of Kokum tree.
Garcinia cambogia, also called as Malabar tamarind or Kodampuli is famous for its anti-obesity property of the fruit rind (Jena et al. 2002Jena BS, Jayaprakasha GK, Singh RP, Sakariah KK. Chemistry and Biochemistry of (-)-Hydroxycitric Acid from Garcinia. Journal Agr Food Chem. 2002; 50: 10-22.). Malabar tamarind has been known to have different secondary metabolites such as benzophenones, flavonoids and xanthones (Iinuma et al. 1998Iinuma M, Ito T, Miyake R, Tosa H, Tanaka T, Chelladurai V. A xanthone from Garcinia cambogia. Phytochemistry 1998; 4; 7(6): 1169-1170.; Koshy et al. 2001Koshy AS, Anila L, Vijayalakshmi NR. Flavonoids from Garcinia cambogia lower lipid levels in hypercholesterolemic rats. Food Chem. 2001; 79: 289-294.; Masullo et al. 2008Masullo M, Bassarello C, Suzuki H, Pizza C, Piacente S. Polyisoprenylated benzophenones and an unusual polyisoprenylated tetracyclic xanthone from the fruits of Garcinia cambogia. J Agr Food Chem. 2008; 56: 5205-5210.). The biological activities of these secondary metabolites include antimalarial, antiviral, cytotoxic, antioxidant and anticancer properties (Ito et al. 1998Ito C, Itoigawa M, Furukawa H, Rao KS, Enjo F, Bu P, Takayasu J, Tokuda H, Nishino H. Xanthones as inhibitors of Epstein-Barr virus activation. Cancer Lett. 1998; 132: 113-117.; Hay et al. 2004Hay A, Helesbeux J, Duval O, Labaied M, Grellier P, Richomme P. Antimalarial xanthones from Calophyllum caledonicum and Garcinia vieillardii. Life Sci. 2004; 75: 3077-3085.; Matsumoto et al. 2005Matsumoto K, Akao Y, Ohguchi K, Ito T, Tanaka T, Iinuma M, Nozawa Y. Xanthones induce cell-cycle arrest and apoptosis in human colon cancer DLD-1 cells. Bioorgan Med Chem. 2005; 13: 6064-6069.; Wang et al. 2011Wang JJ, Sanderson BJS, Zhang W. Cytotoxic effect of xanthones from pericarp of the tropical fruit mangosteen (Garcinia mangostana Linn) on human melanoma cells. Food Chem Toxicol. 2011; 49: 2385-2391.). The fruits, leaves and roots of this plant have been explored (Tharachand et al. 2013Tharachand, Selvaraj I, Avadhani M. Medicinal properties of Malabar tamarind [Garcinia Cambogia (Gaertn) DESR.] . Int J Pharm Sci Rev Res. 2013; 19(2): 101-107.). The preliminary in vitro anthelmintic activity of G. cambogia leaves and fruits have been reported by Mathew et al. (2011)Mathew GE, Mathew B, Sheneeb MM, Nyanthara, Haribabu Y. Anthelmintic activity of leaves of Garcinia cambogia. Int J Res Pharm Sci. 2011; 2(1): 63-65. and Rajendran et al. (2011)Rajendran V, Rathinambal V, Gopal V. In vitro anthelmintic activity of fresh juice and ethanolic extract of Garcinia cambogia (Clusiaceae). Ann Biol Res. 2011; 2(2): 50-53.. Rajendran et al. (2011)Rajendran V, Rathinambal V, Gopal V. In vitro anthelmintic activity of fresh juice and ethanolic extract of Garcinia cambogia (Clusiaceae). Ann Biol Res. 2011; 2(2): 50-53. reported the anthelmintic activity of the fresh juice and the ethanol extract of the fruit of G.cambogia. Mathew et al. (2011)Mathew GE, Mathew B, Sheneeb MM, Nyanthara, Haribabu Y. Anthelmintic activity of leaves of Garcinia cambogia. Int J Res Pharm Sci. 2011; 2(1): 63-65. reported the anthelmintic activity exhibited by three different extracts, namely petroleum ether, chloroform and alcohol from the leaves of G.cambogia.
Antimicrobial activity of the preserved (salted and sun dried) fruit rind of Kokum against the bacterial strains has been reported. It has reported that the presence of furfural in kokum extract is responsible for its antimicrobial activity (Sutar et al. 2012Sutar RL, Mane SP, Ghosh JS. Antimicrobial activity of extracts of dried kokum (Garcinia indica C). Int Food Res J. 2012; 19(3): 1207-1210.). Shivakumar et al. (2011)Shivakumar S, Sandhiya S, Subhasree N, Agrawal A, Dubey GP. In vitro assessment of antibacterial and antioxidant activities of fruit rind extracts of Garcinia cambogia. L. Int J Pharm Pharm Sci. 2013; 5(2): 254-257. reported the in vitro antibacterial activity of the fruit rind extracts of G.cambogia using hexane, ethyl acetate and ethanol as the solvents for extraction. Evidently, To the best of knowledge, there have been only a few studies reports on the anthelmintic and antimicrobial activity of the fruit and leaf extracts of G.cambogia andG.indica. Therefore, the aim of this study was to evaluate the anthelmintic activity of various extracts from the fruits and leaves of G.cambogia and G.indica. The antibacterial activity of the methanol extract of the leaves and fruits of these plants was also evaluated.
MATERIAL AND METHODS
Plant material
The authentic samples of the fruits and leaves of G.cambogia and G.indica were collected from the National Bureau of Plant Genetic Resources (NBPGR) regional station, Thrissur, India. The collected leaves and fruits were shade dried at room temperature, homogenized to powder and stored in air-tight containers for further extraction.
Chemicals and reagents
All the chemicals such as solvents used in this study were of analytical grade purchased from Merck India, Mumbai, India. The media for antibacterial activity was purchased from Hi-media Laboratories Ltd, Mumbai, India.
Preparation of the extract
Different extracts of the leaves and fruits of G. cambogia and G. indica were prepared using Soxhlet apparatus (Borosil Glass works Ltd.), where petroleum ether, chloroform, ethyl acetate, methanol and water (successive extraction) were used as solvents. The extraction time was 6 h with the solvent maintained to continuously reflux over the sample. All the extracts were cooled to the room temperature and filtered. The solvents from the filtrates (except aqueous extract) were evaporated using a rotary evaporator (Rotavapor R210, Buchi, Switzerland). The dried extracts were stored for further studies (Raaman 2006Raaman N. Phytochemical techniques. New Delhi: New India Publishing Agency, 2006: 12p.).
Organisms for the study
Anthelmintic activity was carried out on the Indian adult earthworm (Pheretima posthuma), collected from the moist soil of the garden fields at VIT University. Adult earthworms (4 - 8 cm in length) were selected for the experiment. Antibacterial activity of the crude extracts of leaves and fruits of G.cambogia and G.indica were tested against human pathogenic bacterial strains, Bacillus cereus, Pseudomonas aeruginosa, Escherichia coli and Staphylococcus aureus. Pure bacterial strains used in this study were obtained from the Microbiology Laboratory, VIT University, Vellore.
Evaluation of anthelmintic activity
In vitro anthelmintic activity of all the extracts was evaluated as per Patil et al. (2015)Patil SA, Prabhakara CT, Halasangi BM, Toragalmath SS, Badami PS. DNA cleavage, antibacterial, antifungal and anthelmintic studies of Co(II), Ni(II) and Cu(II) complexes of coumarin Schiff bases: Synthesis and spectral approach. Spectrochim Acta A. 2015; 137: 641-651.. The earthworms were divided into individual groups (each group containing five organisms) for each treatment at two different concentrations. The standard drug albendazole at two different concentrations of 25 and 50 mg/mL was used for comparing the activity of the extracts with saline (0.89% NaCl w/v) as a control. Similarly, all the extracts were prepared at concentrations of 25 and 50 mg/mL as test. A volume of 10mL of each extract of both the concentrations were taken in Petri dishes. Five earthworms were added to each Petri dish. Earthworms were observed for their movements. The time taken for the worm to lose its movement (except when external stimulus was given) was considered for paralysis time and the time taken to lose its motility even in the presence of external stimulus (when dipped in warm water at 550C) and faded body color was considered for death time. Paralysis time and death time of each earthworm in the group was recorded.
Evaluation of antibacterial activity
The methanol extracts of the fruits and leaves of both the plants showed good anthelmintic activity compared to the standard drug albendazole. In order to study the antibacterial activity of these extracts, four food borne pathogenic bacteria (two Gram-positive and two Gram-negative) were selected. The antibacterial activity was carried out by agar-well diffusion method (Das et al. 2014Das A, Bhattacharya S, Mohammed AYH, Rajan SS. In vitro Antimicrobial Activity and Characterization of Mangrove Isolates of Streptomycetes Effective against Bacteria and Fungi of Nosocomial Origin. Braz Arch Biol Technol. 2014; 3: 349-356.; Naine et al. 2015Naine SJ, Devi CS, Mohanasrinivasan V, Vaishnavi B. Antimicrobial, Antioxidant and Cytotoxic Activity of Marine Streptomyces parvulus VITJS11 Crude Extract. Braz Arch Biol Technol. Epub Oct 14; doi: 10.1590/S1516-8913201400173.
https://doi.org/10.1590/S1516-8913201400...
). Gram-positive bacteria used in the study were Staphylococcus aureusand Bacillus cereus and Gram-negative bacteria were Pseudomonas aeruginosa and Escherichia coli (food borne pathogens). Standard antibiotic used was ampicillin at a concentration of 100 µg/mL. All the four methanol extracts were prepared at two different concentrations of 500 and 1000 µg/mL. The zones of growth inhibition were measured (in mm) after 24 h of incubation at 370C. The test was assayed in triplicates. Further, the Minimal Inhibitory Concentration (MIC) values of the methanol extracts were determined by micro dilution technique in Luria-Bertani (LB) broth (Hughes et al. 2013Hughes AFS, Lima FG, Lucchese AM, Neto AG, Uetanabaro APT. Antimicrobial Activity of Syagrus coronata (Martius) Beccari. Braz Arch Biol Technol. 2013; 2: 269-274.). The stock solutions of the extracts were prepared in their respective solvents. Serial dilutions of the methanol extracts were prepared in LB medium ranging from 10 to 500 µg/mL to the final volumes of 100 µL in 96-well microtiter plate. To the wells of the microtiterplate containing diluted methanol extracts, 100 µL of the bacterial suspension was added and incubated overnight at 370C. Bacteria cultured in LB broth without the extracts were used as control.
Statistical analysis
The data for anthelmintic activity was represented as mean ± SD of five earthworms in each group (n=5). The data for antibacterial activity was represented as mean ± SD of three replicates (n=3). Statistical analysis was performed using Statistical Package for Social Sciences version 19.0 (SPSS Inc., Chicago, IL, USA). All the data was submitted to the Analysis of Variance (ANOVA), followed by the post-hoc analysis of Duncan's Multiple Range Test (DMRT) in order to find the significant differences between the means of the groups. Values of P<0.05 were considered for significant differences.
RESULTS
Anthelmintic activity
The anthelmintic activity of the extracts was confirmed by observing the paralysis and death of earthworms. Among the fruit extracts of G.cambogia(Fig. 1), the methanol extract (50 mg/mL) paralyzed the earthworms earlier (15.6 min) than other extracts and albendazole (18.2 min) at the given concentration. The paralyzed earthworms in the methanol extract of fruit survived for less time (25.4 min) when compared to albendazole (27.8 min). The duration for the paralysis of the earthworms was longer in case of petroleum ether extract (25mg/mL), whereas the duration for the death of the earthworms was longer in case of ethyl acetate extract (25mg/mL).
Anthelmintic effect of Garcinia cambogia fruit extracts. Each value represents means ± SD. Means followed by different superscripts within a group are significantly different at P<0.05 according to Duncan’s Multiple Range Test (DMRT).
Among all the extracts of G. cambogia leaves (Fig. 2), the methanol extract at 50 mg/mL concentration took the least time (12 min) to paralyze the earthworms and those worms survive up to 16.6 min. The leaf extracts of G.cambogia showed better anthelmintic activity when compared to the corresponding extracts of the G.cambogia fruit. Furthermore, it was observed that the lower concentration of leaf extract (25 mg/mL) paralyzed the earthworms in virtually the same time taken by the fruit extract at higher concentration (50 mg/mL).
Anthelmintic effect of Garcinia cambogia leaf extracts. Each value represents means ± SD. Means followed by different superscripts within a group are significantly different at P<0.05 according to Duncan’s Multiple Range Test (DMRT).
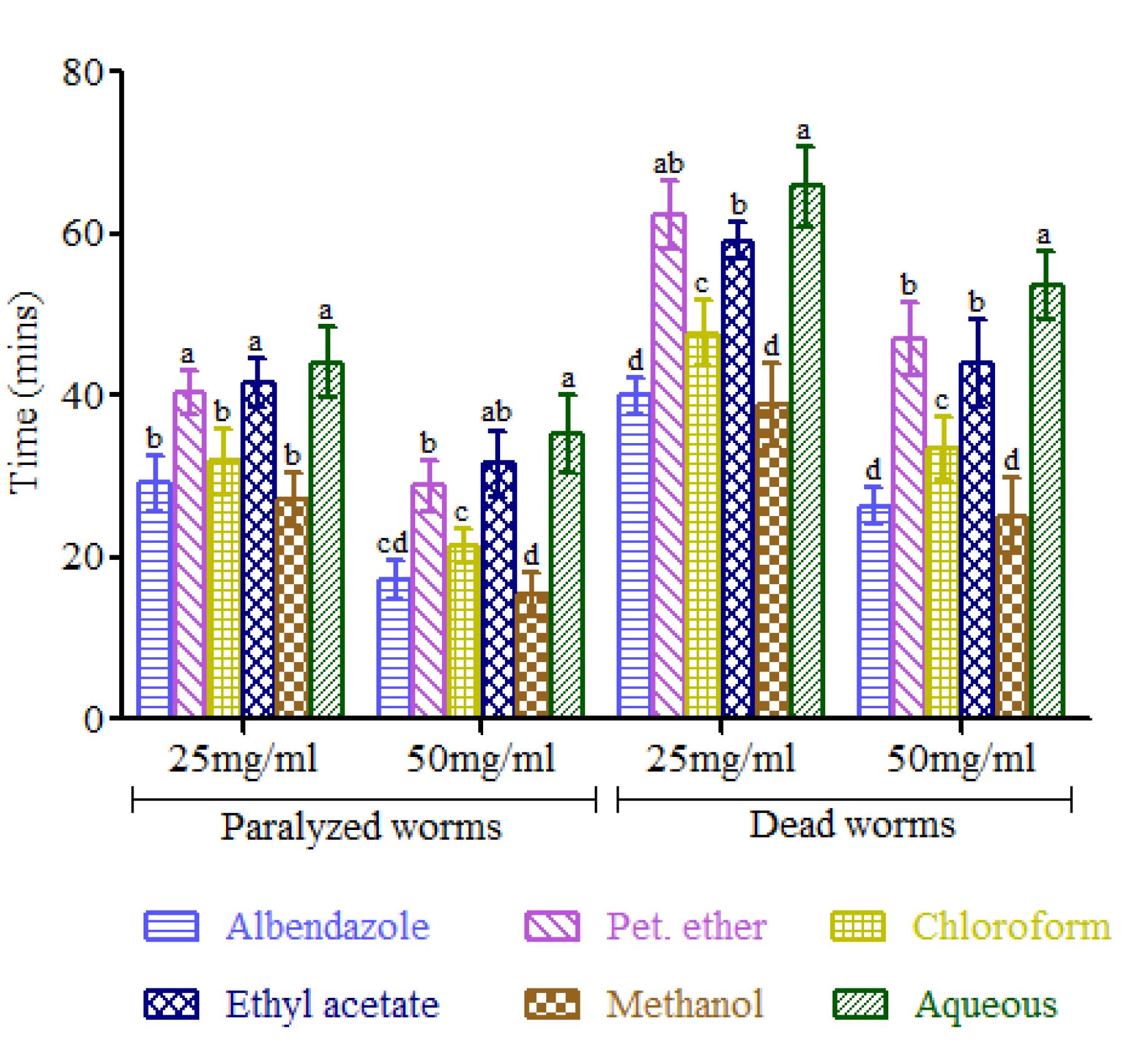
In G. indica, the methanol extract of the fruit (Fig. 3) at a concentration of 50 mg/mL paralyzed the earthworms in 15.6 min (10% faster effect than albendazole), whereas albendazole took 17.2 min. Even at the lower concentration of 25 mg/mL, the methanol extract showed 10% faster paralysis effect than albendazole. Similarly, the death time of the methanol extracts at both the concentrations corresponded with the standard reference. No significant difference was observed in paralysis time and death time between the two groups. Aqueous extract showed slower paralysis effect than other extracts at both the concentrations (25 and 50 mg/mL). At higher concentration (50 mg/mL), the pet ether and ethyl acetate extracts exhibited same effect on the death of earthworms.
Anthelmintic effect of Garcinia indica fruit extracts. Each value represents means ± SD. Means followed by different superscripts within a group are significantly different at P<0.05 according to Duncan’s Multiple Range Test (DMRT).
Anthelmintic effect of the leaves of G.indica with different extracts is shown in Figure 4. The leaf extracts of G.indica showed faster paralysis effect than their corresponding fruit extracts. In this case, except aqueous extract, all other extracts at a concentration of 50 mg/mL showed equivalent paralysis effect. The time taken by the paralyzed earthworms to die was almost the same in case of methanol extract and albendazole at both the concentrations. All the extracts showed dose dependent paralysis and death of the earthworms. It was found that the higher concentration (50 mg/mL) of all the extracts had a faster effect than the lower concentration (25 mg/mL).
Anthelmintic effect of Garcinia indica leaf extracts. Each value represents means ± SD. Means followed by different superscripts within a group differ significantly with each other at P<0.05 according to Duncan’s Multiple Range Test (DMRT).
Antibacterial activity
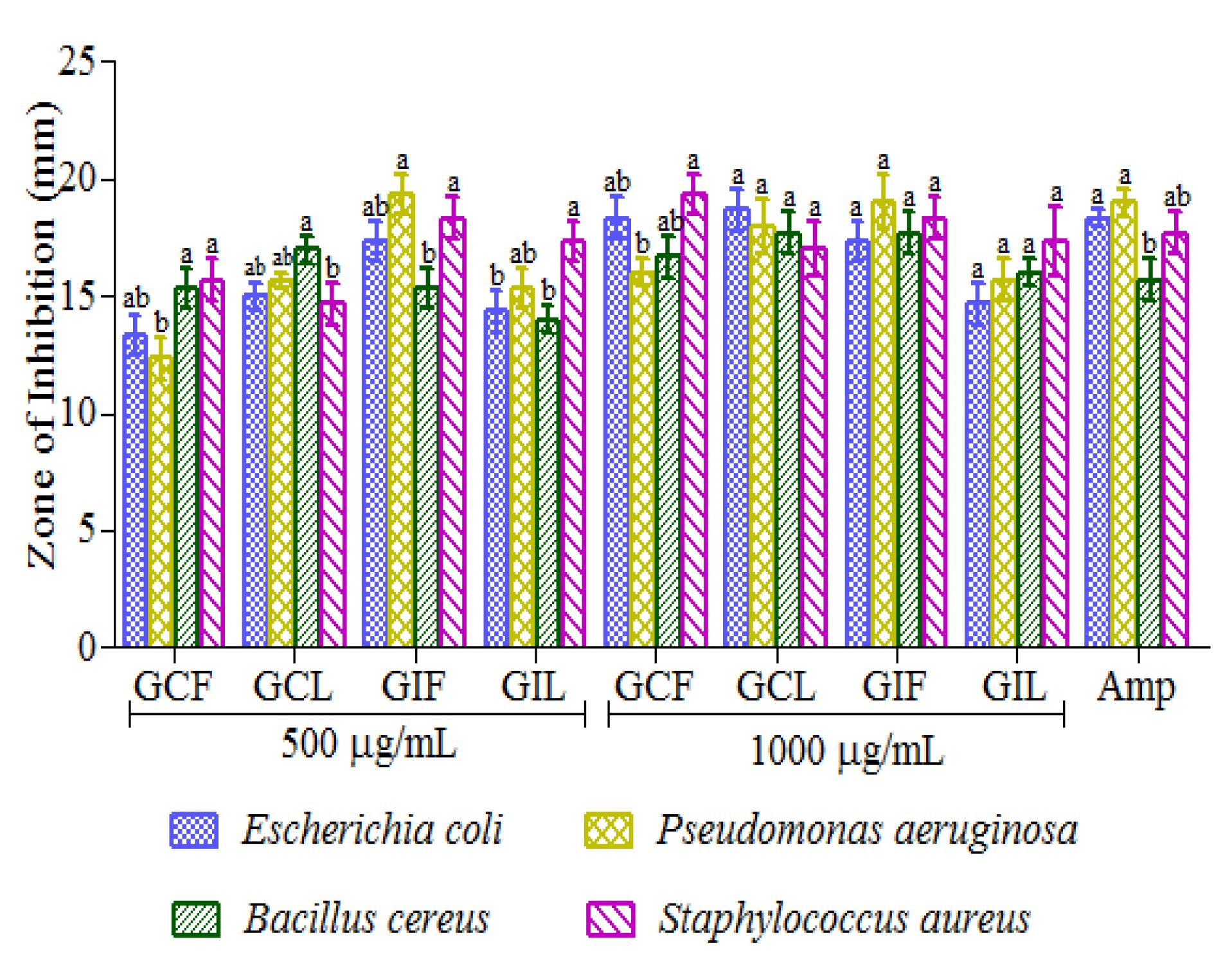
Antibacterial activity of methanol extracts of the fruits and leaves of G.cambogia and G.indica are depicted in Figure 5. The antibacterial activity was carried out through agar-well diffusion method. The zones of inhibition were considered for the effectiveness of the extract against the tested bacterial pathogens. All the extracts showed inhibitory effect against the test organisms. G.cambogia fruit extract (500 µg/mL) was more effective on Gram-positive bacteria, S. aureus (zone of inhibition being 15.6 mm) and B. cereus (15.3 mm). B. cereus (17 mm) was more susceptible to G. cambogia leaf extract than other tested bacterial strains. Fruit extract of G.indica showed highest antibacterial activity on both P. aeruginosa and S. aureus (19 mm). S. aureus (17.3 mm) was also highly susceptible to G. indica leaf extract. Among all the methanol extracts of G.cambogia and G.indica at a concentration of 500 µg/mL, P. aeruginosa and S. aureus were the most susceptible bacterial strains among the tested pathogens. At 1000 µg/mL concentration, all the extracts showed similar activity against all the bacterial strains (nearly equal range of inhibition zones). At this concentration, the effect shown by different extracts towards the tested bacterial strains was very low (ranged between 17 mm and 19 mm).
Antibacterial activity of Garcinia cambogia and G. indica fruit and leaf extracts. Each value represents means ± SEM in each group. Means followed by different superscript within a group differ significantly with each other at P<0.05 according to Duncan’s Multiple Range Test (DMRT). GCF – G. cambogia Fruit extract, GCL - G. cambogia Leaf extract, GIF - G. indica Fruit extract and GIL - G. indica Leaf extract.
Minimal inhibitory concentration (MIC) of the methanol extracts towards Gram-positive and Gram-negative bacteria are represented in Table 1. Among all the methanol extracts used in this study, G. indica fruit extract inhibited P. aeruginosa at a lower concentration (25 µg/mL). G. indica fruit extract showed significant difference in the inhibition between the Gram-positive and Gram-negative bacteria. B. cereus was the most resistant among all the tested strains (MIC value of 79 ± 7 towards G. cambogia fruit extract). The other extracts did not show any considerable differences between the Gram-positive and Gram-negative bacteria. All the extracts inhibited the tested bacterial strains within a concentration of 80 µg/mL.
DISCUSSION
Plants produce many phytochemicals to protect themselves from the microbial infections and other biological toxicities. Hence, plant materials can serve as the good sources of herbal medicines (Kim et al. 2013Kim S, Cho AR, Han J. Antioxidant and antimicrobial activities of leafy green vegetable extracts and their applications to meat product preservation. Food Control. 2013; 29: 112-120.). The antimicrobial and anthelmintic properties of plants have been explored throughout the world. The inhibitory effect of the plant extracts are attributed to the phytochemicals present in the plant parts.
The objectives of this study were to evaluate the in vitro anthelmintic activity of different extracts of G. cambogia andG.indica leaves and fruits in comparison to the reference drug albendazole. The extracts showing better anthelmintic activity were tested for their antibacterial activity. The in vitro anthelmintic effect of different extracts was carried out on Indian adult earthworm (Pheretima posthuma) due to its physiological and structural similarity with intestinal parasites such as Ascaris lumbricoides (Gogoi et al. 2014Gogoi B, Kakoti BB, Bora NS, Yadav P. In vitro antihelmintic activity of bark extract of Cinnamomum bejolghota (Buch-Ham.) in Indian adult earthworm (Pheretima posthuma) . Asian Pac J Trop Dis. 2014; 4(Suppl 2): S924-S927.). The results of this study revealed the anthelmintic activity of all the extracts of G.indica and G.cambogia leaves and fruits using solvents such as petroleum ether, chloroform, ethyl acetate, methanol and water. The methanol extract of the leaves of G.cambogia took the least time to paralyze the earthworms. The efficiency of the plant material was determined based on the loss of movement of the worm and death of the worm in in vitrostudies. Of all the extracts used in this study, methanol extract of both the fruits and leaves of G.cambogia and G.indica exhibited maximum effect. The anthelmintic effect (paralysis and death of earthworms) shown by the methanol extract was almost equal to the effect shown by the reference drug, albendazole in all the studied cases, with a maximum effect compared to other extracts, which could be ascribed to its polarity Methanol being a mid-polar solvent was capable of extracting both polar as well as non-polar components into it due to which broad range of phyto-constituents were available for the activity (Bae et al. 2012Bae H, Jayaprakasha GK, Crosby K, Jifon JL, Patil BS. Influence of Extraction Solvents on Antioxidant Activity and the Content of Bioactive Compounds in Non-pungent Peppers. Plant Food Hum Nutr. 2012; 67: 120-128.).
The in vitro anthelmintic activity of the methanol extracts could be either due to the damage of cellular integrity or neuromuscular coordination. The damage of cellular integrity could be achieved by the inhibition of tubulin polymerization and inhibition of enzymes in the glycolytic pathway. The damage of neuromuscular coordination could be caused in the parasite by hyperpolarizing the nerve membrane and inhibiting the enzyme acetylcholinesterase (Martin, 1997Martin RJ. Modes of action of anthelmintic drugs. Vet J. 1997; 154: 11-34.; Guest 2008Guest M. An investigation into the mode of action of the anthelmintic emodepside; a molecular, pharmacological and electrophysiological characterisation. University of Southampton: Faculty of Medicine, Health and Life sciences. PhD Thesis; 2008. P. 16-18.).
The preliminary antibacterial activity of the crude methanol extract was carried out by agar-well diffusion method at two different concentrations (500 and 1000 µg/mL). At 500 µg/mL concentration, G.indica fruit extract showed highest zone of inhibition against P. aeruginosa and S. aureus. At a concentration of 1000 µg/mL, the fruit extracts of both G.cambogia and G.indica showed maximum inhibitory effect against S. aureus. In addition, G.indica fruit extract showed maximum activity against P. aeruginosa. The inhibitory effect of G.indica fruit methanol extract towards P. aeruginosa was supported by previous reports. Sutar et al. (2012)Sutar RL, Mane SP, Ghosh JS. Antimicrobial activity of extracts of dried kokum (Garcinia indica C). Int Food Res J. 2012; 19(3): 1207-1210. reported that E.coli did not show any zone of inhibition when tested with methanol extract ofG.indica fruit. Contrary to this, in the present study E. coli was susceptible to the methanol extract of G.indicafruit. In another study, the antibacterial activity of ethanol extract of G.cambogia fruit was studied. It is reported that E. coli, P. aeruginosa and S. aureus showed zones of inhibition with ethanol extract (Shivakumar et al. 2013Shivakumar S, Sandhiya S, Subhasree N, Agrawal A, Dubey GP. In vitro assessment of antibacterial and antioxidant activities of fruit rind extracts of Garcinia cambogia. L. Int J Pharm Pharm Sci. 2013; 5(2): 254-257.).
In this study, methanol extract showed similar zones of inhibition with the mentioned strains of bacteria. The methanol extracts of G.cambogia andG.indica fruits and leaves showed significant bactericidal activity against the tested Gram-positive and Gram-negative bacteria. The antibacterial effect shown by all the extracts was similar on both Gram-negative and Gram- positive bacteria. This similarity could be related to the common solvent used for the extraction of compounds from the plant materials. The inhibitory effect shown by the extracts was due to the phyto-constituents present in the leaves and fruits of G.cambogia and G.indica. One of the reasons for the bactericidal activity of these phytochemicals could be due to membrane permeability resulting in the leakage of intracellular materials causing cell death (Karsha and Lakshmi 2010Karsha PV, Lakshmi OB. Antibacterial activity of black pepper (Piper nigrum Linn) with special reference to its mode of action on bacteria. Indian J Nat Prod Resour. 2010; 1(2): 213-215.).
CONCLUSION
Based on the results of this study, it could be concluded that the fruits and leaves of G.cambogia and G.indica tested in the form of crude extracts showed significant (P<0.05) in vitro anthelmintic activity at two different concentrations tested against Indian earthworm as determined by worm motility inhibition. The findings of this study suggested that G.cambogia leaves could become a source of anthelmintics instead of chemically synthesized drugs. However, further in vivo studies against different parasites of human and other animals at different doses are needed to determine the potential of G.cambogia as an anthelmintic against gastrointestinal worms. The methanol extract of G.indica fruit showed significant (P<0.05) MIC value at very low concentration. The results showed that the selected methanol extracts possessed active components capable of inhibiting the bacterial growth. These results presented the basis for selecting the plant species for further exploration of biologically important compounds. Future studies aiming the isolation and structure interpretation of the biologically active compounds present in the methanol extract of the leaves and fruits from G.cambogia and G.indica should be done.
ACKNOWLEDGEMENTS
The authors thank the management of VIT University, Vellore for providing the necessary laboratory facilities and infrastructure to carry out this work. Authors also thank the Director, NBPGR, New Delhi and NBPGR Regional Station, Thrissur for providing G. indica and G. cambogia leaf and fruit samples.
REFERENCES
- Abraham Z, Malik SK, Rao GE, Narayanan SL, Biju S. Collection and characterisation of Malabar tamarind [Garcinia cambogia (Gaertn) Desr.] .Genet Resour Crop Ev. 2006; 53: 401-406.
- Agrawal MC, Banerjee PS. Problems confronting helminthic diseases of domestic animals in India. J Parasit Dis. 2007; 31(1): 3-13.
- Bae H, Jayaprakasha GK, Crosby K, Jifon JL, Patil BS. Influence of Extraction Solvents on Antioxidant Activity and the Content of Bioactive Compounds in Non-pungent Peppers. Plant Food Hum Nutr. 2012; 67: 120-128.
- Das A, Bhattacharya S, Mohammed AYH, Rajan SS. In vitro Antimicrobial Activity and Characterization of Mangrove Isolates of Streptomycetes Effective against Bacteria and Fungi of Nosocomial Origin. Braz Arch Biol Technol. 2014; 3: 349-356.
- Das SS, Dey M, Ghosh AK. Determination of the anthelmintic activity of the leaf and bark extract of Tamarindus Inidca Linn. Indian J Pharm Sci. 2011; 73(1): 104-107.
- Dhar DN, Sharma RL, Bansal GC. Gastro-intestinal Nematodes in sheep in Kashmir. Vet Parasitol. 1982; 11: 271-277.
- Dushyantha DK, Girish DN, Suvarna VC, Dushyantha DK. Native lactic acid bacterial isolates of kokum for preparation of fermented beverage. European J Biol Sci. 2010; 2(1): 21-24.
- Gogoi B, Kakoti BB, Bora NS, Yadav P. In vitro antihelmintic activity of bark extract of Cinnamomum bejolghota (Buch-Ham.) in Indian adult earthworm (Pheretima posthuma) . Asian Pac J Trop Dis. 2014; 4(Suppl 2): S924-S927.
- Goodman LS, Gilman A. The Pharmacological basis of Therapeutics. 10. Ed. New York: Mc graw Hill Medical Publishing Division, 2001: 1121p.
- Guest M. An investigation into the mode of action of the anthelmintic emodepside; a molecular, pharmacological and electrophysiological characterisation. University of Southampton: Faculty of Medicine, Health and Life sciences. PhD Thesis; 2008. P. 16-18.
- Hammond JA, Fielding D, Bishop SC. Prospects for plant anthelmintics in tropical veterinary medicine. Vet Res Commun. 1997; 21: 213-228.
- Hay A, Helesbeux J, Duval O, Labaied M, Grellier P, Richomme P. Antimalarial xanthones from Calophyllum caledonicum and Garcinia vieillardii. Life Sci. 2004; 75: 3077-3085.
- Hemshekhar M, Sunitha K, Santhosh MS, Devaraja S, Kemparaju K, Vishwanath BS, Niranjana SR, Girish KS. An overview on genus Garcinia: phytochemical and therapeutical aspects. Phytochem Rev. 2011; 10: 325-351.
- Hughes AFS, Lima FG, Lucchese AM, Neto AG, Uetanabaro APT. Antimicrobial Activity of Syagrus coronata (Martius) Beccari. Braz Arch Biol Technol. 2013; 2: 269-274.
- Iinuma M, Ito T, Miyake R, Tosa H, Tanaka T, Chelladurai V. A xanthone from Garcinia cambogia. Phytochemistry 1998; 4; 7(6): 1169-1170.
- Ito C, Itoigawa M, Furukawa H, Rao KS, Enjo F, Bu P, Takayasu J, Tokuda H, Nishino H. Xanthones as inhibitors of Epstein-Barr virus activation. Cancer Lett. 1998; 132: 113-117.
- Jena BS, Jayaprakasha GK, Singh RP, Sakariah KK. Chemistry and Biochemistry of (-)-Hydroxycitric Acid from Garcinia. Journal Agr Food Chem. 2002; 50: 10-22.
- Karsha PV, Lakshmi OB. Antibacterial activity of black pepper (Piper nigrum Linn) with special reference to its mode of action on bacteria. Indian J Nat Prod Resour. 2010; 1(2): 213-215.
- Kim S, Cho AR, Han J. Antioxidant and antimicrobial activities of leafy green vegetable extracts and their applications to meat product preservation. Food Control. 2013; 29: 112-120.
- Koshy AS, Anila L, Vijayalakshmi NR. Flavonoids from Garcinia cambogia lower lipid levels in hypercholesterolemic rats. Food Chem. 2001; 79: 289-294.
- Kumar HKS, Raju MBV, Dinda SC, Sahu S. Evaluation of anthelmintic activity of Bambusa arundinacea. Asian J Pharm Tech. 2012; 2(2): 62-63.
- Lakshmanan B, Mazumder PM, Sasmal D, Ganguly S, Jena SS. In Vitro anthelmintic activity of some 1-substituted imidazole derivatives. Acta Parasitol Globalis. 2011; 2(1): 1-5.
- Martin RJ. Modes of action of anthelmintic drugs. Vet J. 1997; 154: 11-34.
- Masullo M, Bassarello C, Suzuki H, Pizza C, Piacente S. Polyisoprenylated benzophenones and an unusual polyisoprenylated tetracyclic xanthone from the fruits of Garcinia cambogia. J Agr Food Chem. 2008; 56: 5205-5210.
- Mathew GE, Mathew B, Sheneeb MM, Nyanthara, Haribabu Y. Anthelmintic activity of leaves of Garcinia cambogia. Int J Res Pharm Sci. 2011; 2(1): 63-65.
- Matsumoto K, Akao Y, Ohguchi K, Ito T, Tanaka T, Iinuma M, Nozawa Y. Xanthones induce cell-cycle arrest and apoptosis in human colon cancer DLD-1 cells. Bioorgan Med Chem. 2005; 13: 6064-6069.
- Naine SJ, Devi CS, Mohanasrinivasan V, Vaishnavi B. Antimicrobial, Antioxidant and Cytotoxic Activity of Marine Streptomyces parvulus VITJS11 Crude Extract. Braz Arch Biol Technol. Epub Oct 14; doi: 10.1590/S1516-8913201400173.
» https://doi.org/10.1590/S1516-8913201400173 - Patil SA, Prabhakara CT, Halasangi BM, Toragalmath SS, Badami PS. DNA cleavage, antibacterial, antifungal and anthelmintic studies of Co(II), Ni(II) and Cu(II) complexes of coumarin Schiff bases: Synthesis and spectral approach. Spectrochim Acta A. 2015; 137: 641-651.
- Raaman N. Phytochemical techniques. New Delhi: New India Publishing Agency, 2006: 12p.
- Rajendran V, Rathinambal V, Gopal V. In vitro anthelmintic activity of fresh juice and ethanolic extract of Garcinia cambogia (Clusiaceae). Ann Biol Res. 2011; 2(2): 50-53.
- Shivakumar S, Sandhiya S, Subhasree N, Agrawal A, Dubey GP. In vitro assessment of antibacterial and antioxidant activities of fruit rind extracts of Garcinia cambogia. L. Int J Pharm Pharm Sci. 2013; 5(2): 254-257.
- Silva RP, Holanda CMCX, Barbosa VSA, Oliveira DP, Lima NA, Camara ACJ, et al. Effect of Medicinal Plants on the Parasitemia of Trypanosoma cruzi and on the Biodistribution of Sodium Pertechnetate (Na99mTcO4). Braz Arch Biol Technol. 2008; 51: 209-214.
- Somvanshi VS, Ellis BL, Hu Y, Aroian RV. Nitazoxanide: Nematicidal mode of action and drug combination studies. Mol Biochem Parasit. 2014; 193: 1-8.
- Sutar RL, Mane SP, Ghosh JS. Antimicrobial activity of extracts of dried kokum (Garcinia indica C). Int Food Res J. 2012; 19(3): 1207-1210.
- Swapna P, Elumalai A, Jayasri P. Evaluation of anthelmintic activity of Garcinia indica choisy fruits. Int J Adv Life Sci. 2012; 1: 85-88.
- Tariq KA, Tantry MA. Preliminary studies on plants with anthelmintic properties in Kashmir - the North-West temperate Himalayan region of India. Chinese Med. 2012; 3: 106-112.
- Tariq KA, Chishti MZ, Ahmad F, Shawl AS. Anthelmintic activity of extracts of Artemisia absinthium against ovine nematodes. Vet Parasitol. 2009; 160: 83-88.
- Tharachand, Selvaraj I, Avadhani M. Medicinal properties of Malabar tamarind [Garcinia Cambogia (Gaertn) DESR.] . Int J Pharm Sci Rev Res. 2013; 19(2): 101-107.
- Wang JJ, Sanderson BJS, Zhang W. Cytotoxic effect of xanthones from pericarp of the tropical fruit mangosteen (Garcinia mangostana Linn) on human melanoma cells. Food Chem Toxicol. 2011; 49: 2385-2391.
Publication Dates
-
Publication in this collection
May-Jun 2015
History
-
Received
05 Feb 2015 -
Accepted
30 Mar 2015