Abstract
The acute effects of Glycine max (GM) on post prandial glycemia (PPG) in male Wistar rats were investigated. All substances were orally administered by gavage in overnight fasted animals. The elevation of PPG promoted by starch (1g/kg) was prevented by GM (2.5 mg/kg, 5.0 mg/kg, 7.5 mg/kg, 10.0 mg/kg, and 100.0 mg/kg). In conclusion GM showed potential antidiabetic effect.
Glycine max; soy; diabetes; acarbose; phytotherapy; post prandial glycemia
INTRODUCTION
The well-established beneficial effects on metabolism of extracts from soy have been attributed to the isoflavones11. Mezei O, Banz WJ, Steger RW, Peluso MR, Winters TA, Shay N. Soy isoflavones exert antidiabetic and hypolipidemic effects through the PPAR pathways in obese Zucker rats and murine RAW 264.7 cells. J Nutr. 2003;133(5):1238-43.
2. Ricketts ML, Moore DD, Banz WJ, Mezei O, Shay NF. Molecular mechanisms of action of the soy isoflavones includes activation of promiscuous nuclear receptors. A review. J Nutr Biochem. 2005;16(6):321-30.
3. Babu PV, Liu D, Gilbert ER. Recent advances in understanding the anti-diabetic actions of dietary flavonoids. J Nutr Biochem. 2013;24(11):1777-89.
4. Tsugane S, Sawada N. The JPHC study: design and some findings on the typical Japanese diet. Jpn J Clin Oncol. 2014;44(9):777-82.-55. Wang Q, Ge X, Tian X, Zhang Y, Zhang J, Zhang P. Soy isoflavone: The multipurpose phytochemical (Review). Biomed Rep. 2013;1(5):697-701.. In agreement with this affirmation we previously demonstrated66. Guo TL, Wang Y, Xiong T, Ling X, Zheng J. Genistein modulation of streptozotocin diabetes in male B6C3F1 mice can be induced by diet. Toxicol Appl Pharmacol. 2014;280(3):455-66. activation of peroxisome proliferator-activated receptors α by using a methanolic fraction from soybean seeds rich in isoflavones (daidzin, glycitin, genistin, daidzein, malonylglycitin, malonylgenistin, genistein, glycitein, and malonyldaidzin).
In addition, several studies reported antidiabetic properties of isoflavones from soy, not only in pre-clinical models77. Malarde L, Groussard C, Lefeuvre-Orfila L, Vincent S, Efstathiou T, Gratas-Delamarche A. Fermented soy permeate reduces cytokine level and oxidative stress in streptozotocin-induced diabetic rats. J Med Food. 2015;18(1):67-75.,88. Mueller NT, Odegaard AO, Gross MD, Koh WP, Yu MC, Yuan JM, et al. Soy intake and risk of type 2 diabetes in Chinese Singaporeans [corrected]. Eur J Nutr. 2012;51(8):1033-40. but also in humans99. Talaei M, Pan A. Role of phytoestrogens in prevention and management of type 2 diabetes. World J Diabetes. 2015;6(2):271-83.,10 10. Zimmermann C, Cederroth CR, Bourgoin L, Foti M, Nef S. Prevention of diabetes in db/db mice by dietary soy is independent of isoflavone levels. Endocrinology. 2012;153(11):5200-11..
The mechanisms by which isoflavones from soy produce antidiabetic effects include: antioxidant and anti-inflammatory properties88. Mueller NT, Odegaard AO, Gross MD, Koh WP, Yu MC, Yuan JM, et al. Soy intake and risk of type 2 diabetes in Chinese Singaporeans [corrected]. Eur J Nutr. 2012;51(8):1033-40. protection of beta cells1111. Gilbert ER, Liu D. Anti-diabetic functions of soy isoflavone genistein: mechanisms underlying its effects on pancreatic beta-cell function. Food Funct. 2013;4(2):200-12., and stimulation of insulin release1212. Curtis PJ, Sampson M, Potter J, Dhatariya K, Kroon PA, Cassidy A. Chronic ingestion of flavan-3-ols and isoflavones improves insulin sensitivity and lipoprotein status and attenuates estimated 10-year CVD risk in medicated postmenopausal women with type 2 diabetes: a 1-year, double-blind, randomized, controlled trial. Diabetes Care. 2012;35(2):226-32..
However, there are few studies supporting that this herbal preparations could show acute beneficial effect in diabetes1313. Genovese MI, Davila J, Lajolo FM. Isoflavones in Processed Soybean Products from Ecuador. Braz Arch Bio Technol. 2006;.49 (5): 853-59.. Moreover, considering that genistein, the main isoflavone present in the soy1414. Lee DS, Lee SH. Genistein, a soy isoflavone, is a potent alpha-glucosidase inhibitor. FEBS Lett. 2001;501(1):84-6. inhibits alpha-glucosidade1515. Bergmeyer HU, Bernt E. 1974. Determination of glucose with glucose oxidase and peroxidase. In Methods of Enzymatic Analysis, Bergmeyer HU (ed.). Academic Press: Weinhein, London; 1205-1215., the possibility of an acute effect of extracts of GM on post prandial glycemia should be investigated. Furthermore, there is no dearth of reports evaluating if the inhibition of alpha-glucosidase has pharmacological significance in vivo conditions.
Thus, the present study was carried out to verify if the isoflavones from soy would show acute effects on postprandial glycemia.
MATERIALS AND METHODS
PLANT MATERIALS: Capsules (Soyfemme(r) from Aché Laboratories - Guarulhos, SP, Brazil) containing Isoflavones (40%) from dried extracts of Glycine max (L.) Merr (GM) were used. Immediately before the administration, the contents of the capsules were removed and dissolved in water.
EXPERIMENTAL PROCEDURES: One hundred and thirty male Wistar adult rats (Rattus norvegicus) weighing 250-300g were used. The rats were maintained under controlled temperature (23ºC) and photoperiod (12 h light/12 h dark). All animals received free access to standard commercial laboratory diet (Nuvilab(r), Curitiba, PR, Brazil). The manipulation of the animals followed the Brazilian animal protection law. All substances were orally administered through a gastric tube (gavage) in overnight (15-h) fasted rats and blood was collected by decapitation for glucose evaluation1616. Kim JH, Kang MJ, Choi HN, Jeong SM, Lee YM, Kim JI. Quercetin attenuates fasting and postprandial hyperglycemia in animal models of diabetes mellitus. Nutr Res Pract. 2011;5(2):107-11..
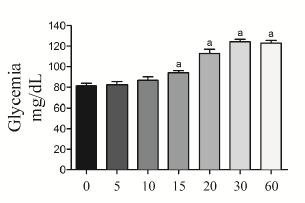
Acute effect of soluble starch (1000 mg/kg) on glycemia. Glycemia was measured at 0 (baseline values), 5, 10, 15, 20, 30 and 60 min after the administration of soluble starch. We chose the dose of soluble starch on the basis of a previous study1717. DiNicolantonio JJ, Bhutani J, O'Keefe JH. Acarbose: safe and effective for lowering postprandial hyperglycaemia and improving cardiovascular outcomes. Open Heart. 2015;2(1):e000327.. The results are presented in the Figure 1.
Effect of the acute administration (oral gavage) of soluble starch 1000 mg/kg (SS) on glycemia in 15-h fasted rats. The blood glucose concentrations were evaluated at 0, 5, 10, 15, 20, 30 and 60 min after SS administration. The results are presented as mean ± standard error of the mean. n=5 for each time. Newman-Keuls multiple comparison test. ap < 0.05 for comparisons between 0 min vs. 10 min, 15 min, 20 min, 30 min and 60 min.
Acute effect of acarbose (0.1 mg/kg, 1.0 mg/kg or 10.0 mg/kg) on the elevation of glycemia after the administration ofsoluble starch (1000 mg/kg). Glycemia was measured 30 min after the simultaneous administration of soluble starch plus acarbose (0.1 mg/kg, 1.0 mg/kg or 10.0 mg/kg). The control group received only vehicle. Furthermore, an additional control group which received simultaneous administration of vehicle plus soluble starch was included. The results are showed in the Figure 2.
Effect of the acute administration (oral gavage) of soluble starch 1000 mg/kg (SS) plus acarbose on glycemia in 15-h fasted rats. The blood glucose concentrations were evaluated at 30 min after the administration of SS plus vehicle or SS plus acarbose (0.1, 1.0 and 10.0 mg/kg). The control group received oral (gavage) vehicle. The results are presented as mean ± standard error of the mean. n=6 for each group. Newman-Keuls multiple comparison test. ap < 0.05 when compared with control group; bp < 0.05 when compared with SS + vehicle group.
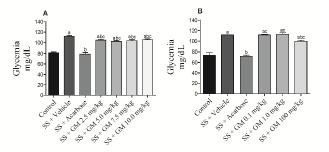
Acute effect of GM (2.5 mg/kg, 5.0 mg/kg, 7.5 mg/kg or 10.0 mg/kg) on the elevation of glycemia after the administration ofsoluble starch (1000 mg/kg). Glycemia was measured 30 min after the simultaneous administration of soluble starch plus GM (2.5 mg/kg, 5.0 mg/kg, 7.5 mg/kg or 10.0 mg/kg). The control group received only vehicle. The positive control group received simultaneous administration of soluble starch plus acarbose (10 mg/kg). Furthermore, an additional control group which received simultaneous administration of vehicle plus soluble starch was included. The results are showed in the Figure 3A.
Effect of the acute administration (gavage) of vehicle (Control), soluble starch 1000 mg/kg (SS) plus vehicle, SS plus acarbose 10.0 mg/kg, and SS plus Glycine max (GM) in the doses of 2.5, 5.0, 7.5 and 10.0 mg/kg (Fig. 3A) or GM 0.1, 1.0 and 100 mg/kg (Fig. 3B) in 15-h fasted rats. The glycemia was evaluated at 30 min after the administration of SS. The results are presented as mean ± standard error of the mean. n=5 for each group. Newman-Keuls multiple comparison test. ap < 0.05 when compared with control group; bp < 0.05 when compared with SS + vehicle group; cp < 0.05 when compared with SS + acarbose group.
Acute effect of GM (0.1 mg/kg, 1.0 mg/kg or 100.0 mg/kg) on the elevation of glycemia after the administration ofsoluble starch (1000 mg/kg). Glycemia was measured 30 min after the simultaneous administration of soluble starch plus GM (0.1 mg/kg, 1.0 mg/kg, or 100.0 mg/kg). The control group received only vehicle. The positive control group received simultaneous administration of soluble starch plus acarbose (10 mg/kg). Furthermore, an additional control group that received simultaneous administration of vehicle plus soluble starch was included. The results are presented in the Figure 3B.
STATISTICAL ANALYSIS: The results are reported as means ± standard error of the means (SEM). Significance of differences between the groups was evaluated by Newman-Keuls multiple comparison test. A 95% level of confidence (P<0.05) was accepted for all comparisons.
RESULTS
In the first set of experiments, the acute effect of oral administration of soluble starch on glycemia was investigated. As shown in Figure 1, elevation (P<0.05) of glycemia was observed from 15 min and this difference was maintained 60 min and 120 min (not showed) later. Because increased (P<0.05) blood glucose was well established 30 min after the administration of soluble starch this time was used in the following experiments.
In the second set of experiments, the acute effect of increasing doses of acarbose on the elevation of glycemia promoted by the administration of soluble starch was evaluated. As shown in Figure 2, the dose of 1.0 mg/kg and 10 mg/kg impair (P<0.05) the elevation of glycemia promoted by soluble starch.
Considering that acarbose (10 mg/kg) impaired the elevation of glycemia promoted by the administration of soluble starch this dose was used in the following experiments.
In the third set of experiments, the acute effect of increasing doses of GM on the elevation of glycemia promoted by the administration of soluble starch was evaluated. As shown in Figure 3A, the doses of 2.5 mg/kg, 5.0 mg/kg, 7.5 mg/kg, and 10.0 mg/kg decrease (P<0.05) the intensity of elevation of glycemia promoted by soluble starch.
Because acarbose prevent 100% the elevation of glycemia we also express the results as percent of effect in comparison with acarbose. Thus, the percent of reduction in the elevation of glycemia after the administration of soluble starch in the groups SS + GM 2.5 mg/kg, SS + GM 5.0 mg/kg, SS + GM 7.5 mg/kg, SS + GM 10.0 mg/kg were 21.8%, 28.8%, 25.4%, and 20%, respectively.
Since GM (2.5 mg/kg, 5.0 mg/kg, 7.5 mg/kg, and 10.0 mg/kg) decrease (P<0.05) the intensity of elevation of glycemia promoted by soluble starch, the experiments described in the Figure 3A were repeated again with lower (0.1 mg/kg and 1.0 mg/kg) and higher (100.0 mg/kg) doses of GM.
As shown in Figure 3B, the dose of 100.0 mg/kg, but not the doses of 1.0 mg/kg, and 0.1 mg/kg, decrease (P<0.05) the elevation of glycemia promoted by soluble starch. The percent of reduction in the elevation of glycemia after the administration of soluble starch in the groups SS + GM 0.1 mg/kg, SS + GM 1.0 mg/kg, and SS + GM 100.0 mg/kg in comparison with SS + acarbose (100%) were 0%, 0%, and 29.90%, respectively.
DISCUSSION
An effective strategy for pre diabetes and type 2 diabetes treatments is the inhibition of intestinal α-glucosidase. In this context, acarbose inhibits α-glucosidase from brush border of the small intestine and slows carbohydrate digestion retarding intestinal absorption and thereby reducing postprandial hyperglycemia1818. Neuser D, Benson A, Bruckner A, Goldberg RB, Hoogwerf BJ, Petzinna D. Safety and tolerability of acarbose in the treatment of type 1 and type 2 diabetes mellitus. Clin Drug Investig. 2005;25(9):579-87.. However, the undigested carbohydrates are fermented by colonic bacteria causing abdominal distention, flatulence, meteorism and diarrhea. Due to this side effect it is common the abandonment of treatment1919. Choi CW, Choi YH, Cha MR, Yoo DS, Kim YS, Yon GH, Hong KS, Kim YH, Ryu SY. Yeast α-glucosidase inhibition by isoflavones from plants of leguminosae as an in vitro alternative to acarbose. J Agric Food Chem. 2010; 58(18): 9988-93..
Considering that in Brazil there is not another antidiabetic drug with ability to slow intestinal absorption, the possibility of using new compounds instead acarbose has been investigated.
In fact there are many in vitro studies showing inhibition of α-glucosidase by isoflavones from plants2020. Ademiluyi AO1, Oboh G. Soybean phenolic-rich extracts inhibit key-enzymes linked to type 2 diabetes (α-amylase and α-glucosidase) and hypertension (angiotensin I converting enzyme) in vitro. Exp Toxicol Pathol. 2013;65(3): 305-9.
21. Benalla W, Bellahcen S, Bnouham M. Antidiabetic medicinal plants as a source of alpha glucosidase inhibitors. Curr Diabetes Rev. 2010; 6(4): 247-54.-2222. Galende SB, Neto OCO, Santos LF, Peicher MV, Souza HM, Bazotte RB. Glucose administration inhibits the hepatic activation of gluconeogenesis promoted by insulin-induced hypoglycemia. Braz Arch Biol Technol. 2009;52(4): 849-54.. However, there is absence of in vivo studies demonstrating antihyperglycemic properties of these compounds.
Furthermore, as we previously demonstrated2323. Galletto R, Siqueira VLD, Ferreira EB, Oliveira AJB, Bazotte RB. Absence of antidiabetic and hypolipidemic effect of Gymnema sylvestre in non-diabetic and alloxan-diabetic rats. Braz Arch Biol Technol. 2004; 47 (4): 545-51.- 25 non-diabetic rats represents a suitable pre-clinical model to investigate the impact of oral carbohydrates on post prandial glycemia.
Thus, by using this rat model we investigate if dried extracts of GM could reduce the elevation of glycemia promoted by oral ingestion of soluble starch.
To the best of our knowledge we demonstrate for the first time the antihyperglycemic properties of GM from in vivo experiments, i.e., the oral administration of GM (2.5 mg/kg, 5.0 mg/kg, 7.5 mg/kg, 10.0 mg/kg and 100.0 mg/kg) decrease (P<0.05) the intensity of elevation of glycemia promoted by soluble starch (Fig. 3A and 3B).
Thus, in agreement with several studies that demonstrated antidiabetic properties to GM77. Malarde L, Groussard C, Lefeuvre-Orfila L, Vincent S, Efstathiou T, Gratas-Delamarche A. Fermented soy permeate reduces cytokine level and oxidative stress in streptozotocin-induced diabetic rats. J Med Food. 2015;18(1):67-75.
8. Mueller NT, Odegaard AO, Gross MD, Koh WP, Yu MC, Yuan JM, et al. Soy intake and risk of type 2 diabetes in Chinese Singaporeans [corrected]. Eur J Nutr. 2012;51(8):1033-40.
9. Talaei M, Pan A. Role of phytoestrogens in prevention and management of type 2 diabetes. World J Diabetes. 2015;6(2):271-83.
10. Zimmermann C, Cederroth CR, Bourgoin L, Foti M, Nef S. Prevention of diabetes in db/db mice by dietary soy is independent of isoflavone levels. Endocrinology. 2012;153(11):5200-11.
11. Gilbert ER, Liu D. Anti-diabetic functions of soy isoflavone genistein: mechanisms underlying its effects on pancreatic beta-cell function. Food Funct. 2013;4(2):200-12.
12. Curtis PJ, Sampson M, Potter J, Dhatariya K, Kroon PA, Cassidy A. Chronic ingestion of flavan-3-ols and isoflavones improves insulin sensitivity and lipoprotein status and attenuates estimated 10-year CVD risk in medicated postmenopausal women with type 2 diabetes: a 1-year, double-blind, randomized, controlled trial. Diabetes Care. 2012;35(2):226-32.-1313. Genovese MI, Davila J, Lajolo FM. Isoflavones in Processed Soybean Products from Ecuador. Braz Arch Bio Technol. 2006;.49 (5): 853-59. we can conclude that isoflavones from dried extracts of GM show acute antihyperglycemic effect.
The difference from our results and several studies showing antidiabetic properties for GM is the fact that those evaluations were done after chronic treatment and our evaluation involve acute effects of GM on post prandial glycemia elevation after an oral overload of soluble starch.
Thus we concluded that the antidiabetic potential of GM also include acute effects preventing the elevation of post prandial glycemia.
Acknowledgments
This study received funding from Program of Support for Excellence Centers (PRONEX)/Araucaria Foundation.
-
1Mezei O, Banz WJ, Steger RW, Peluso MR, Winters TA, Shay N. Soy isoflavones exert antidiabetic and hypolipidemic effects through the PPAR pathways in obese Zucker rats and murine RAW 264.7 cells. J Nutr. 2003;133(5):1238-43.
-
2Ricketts ML, Moore DD, Banz WJ, Mezei O, Shay NF. Molecular mechanisms of action of the soy isoflavones includes activation of promiscuous nuclear receptors. A review. J Nutr Biochem. 2005;16(6):321-30.
-
3Babu PV, Liu D, Gilbert ER. Recent advances in understanding the anti-diabetic actions of dietary flavonoids. J Nutr Biochem. 2013;24(11):1777-89.
-
4Tsugane S, Sawada N. The JPHC study: design and some findings on the typical Japanese diet. Jpn J Clin Oncol. 2014;44(9):777-82.
-
5Wang Q, Ge X, Tian X, Zhang Y, Zhang J, Zhang P. Soy isoflavone: The multipurpose phytochemical (Review). Biomed Rep. 2013;1(5):697-701.
-
6Guo TL, Wang Y, Xiong T, Ling X, Zheng J. Genistein modulation of streptozotocin diabetes in male B6C3F1 mice can be induced by diet. Toxicol Appl Pharmacol. 2014;280(3):455-66.
-
7Malarde L, Groussard C, Lefeuvre-Orfila L, Vincent S, Efstathiou T, Gratas-Delamarche A. Fermented soy permeate reduces cytokine level and oxidative stress in streptozotocin-induced diabetic rats. J Med Food. 2015;18(1):67-75.
-
8Mueller NT, Odegaard AO, Gross MD, Koh WP, Yu MC, Yuan JM, et al. Soy intake and risk of type 2 diabetes in Chinese Singaporeans [corrected]. Eur J Nutr. 2012;51(8):1033-40.
-
9Talaei M, Pan A. Role of phytoestrogens in prevention and management of type 2 diabetes. World J Diabetes. 2015;6(2):271-83.
-
10Zimmermann C, Cederroth CR, Bourgoin L, Foti M, Nef S. Prevention of diabetes in db/db mice by dietary soy is independent of isoflavone levels. Endocrinology. 2012;153(11):5200-11.
-
11Gilbert ER, Liu D. Anti-diabetic functions of soy isoflavone genistein: mechanisms underlying its effects on pancreatic beta-cell function. Food Funct. 2013;4(2):200-12.
-
12Curtis PJ, Sampson M, Potter J, Dhatariya K, Kroon PA, Cassidy A. Chronic ingestion of flavan-3-ols and isoflavones improves insulin sensitivity and lipoprotein status and attenuates estimated 10-year CVD risk in medicated postmenopausal women with type 2 diabetes: a 1-year, double-blind, randomized, controlled trial. Diabetes Care. 2012;35(2):226-32.
-
13Genovese MI, Davila J, Lajolo FM. Isoflavones in Processed Soybean Products from Ecuador. Braz Arch Bio Technol. 2006;.49 (5): 853-59.
-
14Lee DS, Lee SH. Genistein, a soy isoflavone, is a potent alpha-glucosidase inhibitor. FEBS Lett. 2001;501(1):84-6.
-
15Bergmeyer HU, Bernt E. 1974. Determination of glucose with glucose oxidase and peroxidase. In Methods of Enzymatic Analysis, Bergmeyer HU (ed.). Academic Press: Weinhein, London; 1205-1215.
-
16Kim JH, Kang MJ, Choi HN, Jeong SM, Lee YM, Kim JI. Quercetin attenuates fasting and postprandial hyperglycemia in animal models of diabetes mellitus. Nutr Res Pract. 2011;5(2):107-11.
-
17DiNicolantonio JJ, Bhutani J, O'Keefe JH. Acarbose: safe and effective for lowering postprandial hyperglycaemia and improving cardiovascular outcomes. Open Heart. 2015;2(1):e000327.
-
18Neuser D, Benson A, Bruckner A, Goldberg RB, Hoogwerf BJ, Petzinna D. Safety and tolerability of acarbose in the treatment of type 1 and type 2 diabetes mellitus. Clin Drug Investig. 2005;25(9):579-87.
-
19Choi CW, Choi YH, Cha MR, Yoo DS, Kim YS, Yon GH, Hong KS, Kim YH, Ryu SY. Yeast α-glucosidase inhibition by isoflavones from plants of leguminosae as an in vitro alternative to acarbose. J Agric Food Chem. 2010; 58(18): 9988-93.
-
20Ademiluyi AO1, Oboh G. Soybean phenolic-rich extracts inhibit key-enzymes linked to type 2 diabetes (α-amylase and α-glucosidase) and hypertension (angiotensin I converting enzyme) in vitro. Exp Toxicol Pathol. 2013;65(3): 305-9.
-
21Benalla W, Bellahcen S, Bnouham M. Antidiabetic medicinal plants as a source of alpha glucosidase inhibitors. Curr Diabetes Rev. 2010; 6(4): 247-54.
-
22Galende SB, Neto OCO, Santos LF, Peicher MV, Souza HM, Bazotte RB. Glucose administration inhibits the hepatic activation of gluconeogenesis promoted by insulin-induced hypoglycemia. Braz Arch Biol Technol. 2009;52(4): 849-54.
-
23Galletto R, Siqueira VLD, Ferreira EB, Oliveira AJB, Bazotte RB. Absence of antidiabetic and hypolipidemic effect of Gymnema sylvestre in non-diabetic and alloxan-diabetic rats. Braz Arch Biol Technol. 2004; 47 (4): 545-51.
-
24Sybuia MF, Guilhermetti M, Mangolim CS, Bazotte RB, Matioli G. Impact of cyclodextrins on postprandial glycemia: evaluation in experimental animal model using the real-time continuous glucose monitoring system. J Med Food. 2015;18(6): 625-30.
Publication Dates
-
Publication in this collection
2016
History
-
Received
05 Feb 2016 -
Accepted
07 Apr 2016