ABSTRACT
Screening promising L. thermophiles with high productivity, high efficiency and strong adaptability are very important in lactic acid industry. For this purpose, 80MeV/u carbon ions were applied to irradiate L. thermophiles. After high-throughput screening, a mutant, named SRZ50, was obtained. Different carbon sources or nitrogen sources were provided to investigate carbon or nitrogen source utilization between mutant SRZ50 and wild type, and different fermentation periods were also chose to study fermentation characteristic between mutant SRZ50 and wild type. The results showed that mutant SRZ50 exhibited the enhanced L-(+)-lactic acid production from wild type. When glucose or fructose was the sole carbon source, the L(+)-lactic acid production by mutant SRZ50 was both the highest, respectively, 23.16 ± 0.72 g/L or 23.24 ± 0.66 g/L, which had a significant increase from that of wild type (P<0.01), following obvious increase in biomass (P<0.05). When yeast powder was the sole nitrogen source, it can promote mutant SRZ50 to accumulate the highest L-(+)-lactic acid accumulation, which also had a significant increase from that of wild type (P<0.01). Under different fermentation periods, it was obtained that mutant SRZ50 all exhibited significant increase in L-(+)-lactic acid accumulation from wild type. In conclusion, a mutant strain with improved production profiles for L-(+)-lactic acid, was obtained, indicating that heavy ions can be an efficient tool to improve metabolic product accumulations in microbes.
Key words:
L. thermophilus; mutant; heavy ion irradiation; L-(+)-lactic acid production; carbon source; fermentation characteristic
INTRODUCTION
Recently, fermentative production of L-(+)-lactic acid has attracted a great deal of attention due to its widespread application, mainly in food, chemical, cosmetic, pharmaceutical, and plastics industries (Abdel-Rahman et al., 2013Abdel-Rahman, M.A., Tashiro, Y., Sonomoto, K. 2013. Recent advances in lactic acid production by microbial fermentation processes. Biotechnology Advances, 31(6), 877-902.; Pal & Dey, 2013Pal, P., Dey, P. 2013. Process intensification in lactic acid production by three stage membrane integrated hybrid reactor system. Chemical Engineering and Processing, 64, 1-9.; Qin et al., 2009Qin, J.Y., Zhao, B., Wang, X.W., Wang, L.M., Yu, B., Ma, Y.H., Ma, C.Q., Tang, H.Z., Sun, J.B., Xu, P. 2009. Non-Sterilized Fermentative Production of Polymer-Grade L-Lactic Acid by a Newly Isolated Thermophilic Strain Bacillus sp 2-6. Plos One, 4(2), e4359.). The world lactic acid production is expected to reach 259,000 metric tons by the year 2012 (Martinez et al., 2013Martinez, F.A.C., Balciunas, E.M., Salgado, J.M., Gonzalez, J.M.D., Converti, A., Oliveira, R.P.D. 2013. Lactic acid properties, applications and production: A review. Trends in Food Science & Technology, 30(1), 70-83.), and the industrial demand for L-(+)-lactic acid is continuously increasing. Several microorganisms, classified into bacteria, fungi, cyanobacteria, yeast, and algae, have been proved to produce lactic acid (Abdel-Rahman et al., 2013). Especially, L. thermophiles, as an important microbial cell factory, has several potential advantages to L-(+)-lactic acid production that may help for the reduction of costs in lactic acid fermentation as follows: (ⅰ): thermal fermentation (≥50℃), resulting in low energy consumption (Ou et al., 2011Ou, M.S., Ingram, L.O., Shanmugam, K.T. 2011. l(+)-Lactic acid production from non-food carbohydrates by thermotolerant Bacillus coagulans. Journal of Industrial Microbiology & Biotechnology, 38(5), 599-605.); (ⅱ): low byproduct levels, through homofermentative process, L. thermophiles can produce L-(+)-lactic acid as the major end product; (ⅲ): high optical purity.
Any significant enhancement of L-(+)-lactic acid strains will offer great opportunity for industrial production. For this purpose, different mutagenesis methods were applied to modify production ability for L-(+)-lactic acid in different microorganism strains. One such example is, Bacillus subtilis MUR1, a UV induced mutant of Bacillus subtilis 1A304 (U105MU331), can produce 99.3 or 183.2 g/L of L-(+)-lactic acid in 12 or 52 h (Gao et al., 2012Gao, T., Wong, Y.K., Ng, C., Ho, K.P. 2012. L-lactic acid production by Bacillus subtilis MUR1. Bioresource Technology, 121, 105-110.). An over-producing mutant Rhizopus oryzae R1021 induced by compound mutation, was screened, and can produce 79.4 g/L L-(+)-lactic acid after 60 h fermentation in flasks containing 120 g/L corn starch, which was 52% higher than that of wild type strain (Bai et al., 2003Bai, D.M., Jia, M.Z., Zhao, X.M., Ban, R., Shen, F., Li, X.G., Xu, S.M. 2003. L(+)-lactic acid production by pellet-form Rhizopus oryzae R1021 in a stirred tank fermentor. Chemical Engineering Science, 58(3-6), 785-791.). After low-energy ion beam implantation, a high-yield mutant, named Rhizopus oryzae RQ4015, was also obtained, which can produce 83 g/L L-(+)-lactic acid with mixed carbon containing xylose (25 g/L) and glucose (75 g/L) (Wang et al., 2009Wang, P., Li, J., Wang, L., Tang, M.L., Yu, Z.L., Zheng, Z.M. 2009. l(+)-Lactic acid production by co-fermentation of glucose and xylose with Rhizopus oryzae obtained by low-energy ion beam irradiation. Journal of Industrial Microbiology & Biotechnology, 36(11), 1363-1368.).
Presently, heavy-ion irradiation, as a novel breeding method, which has been a highly efficient tool for creating different kinds of new mutants (Inthima et al., 2014Inthima, P., Otani, M., Hirano, T., Hayashi, Y., Abe, T., Nakano, M., Supaibulwatana, K. 2014. Mutagenic effects of heavy-ion beam irradiation on in vitro nodal segments of Artemisia annua L. Plant Cell Tissue and Organ Culture, 119(1), 131-139.; Ishikawa et al., 2012Ishikawa, S., Ishimaru, Y., Igura, M., Kuramata, M., Abe, T., Senoura, T., Hase, Y., Arao, T., Nishizawa, N.K., Nakanishi, H. 2012. Ion-beam irradiation, gene identification, and marker-assisted breeding in the development of low-cadmium rice. Proceedings of the National Academy of Sciences of the United States of America, 109(47), 19166-19171.), has drawn extensive attention of biologists and breeding experts. Compared with low linear energy transfer (LET) radiations such as electrons, protons, and X-rays, heavy ions can cause higher relative biological effectiveness (RBE), higher mutation rate and a broader mutation spectrum due to its high LET (Shikazono et al., 2002Shikazono, N., Tanaka, A., Kitayama, S., Watanabe, H., Tano, S. 2002. LET dependence of lethality in Arabidopsis thaliana irradiated by heavy ions. Radiation and Environmental Biophysics, 41(2), 159-162.; Tanaka et al., 2010Tanaka, A., Shikazono, N., Hase, Y. 2010. Studies on Biological Effects of Ion Beams on Lethality, Molecular Nature of Mutation, Mutation Rate, and Spectrum of Mutation Phenotype for Mutation Breeding in Higher Plants. Journal of Radiation Research, 51(3), 223-233.), which can produce dense ionization along their trajectories, resulting in complex and irreparable clustered damage in DNA (Hamada, 2009Hamada, N. 2009. Recent Insights into the Biological Action of Heavy-Ion Radiation. Journal of Radiation Research, 50(1), 1-9.), and deletions, translocations or rearrangements in the genome of live body, which can generate thousands of mutants (Hu et al., 2013Hu, G.R., Fan, Y., Zhang, L., Yuan, C., Wang, J.F., Li, W.J., Hu, Q., Li, F.L. 2013. Enhanced Lipid Productivity and Photosynthesis Efficiency in a Desmodesmus sp Mutant Induced by Heavy Carbon Ions. Plos One, 8(4).).
In china, Heavy Ion Research Facility of Lanzhou (HIRFL) and Cooling storage Ring (CSR) have been constructed as national key research facilities for basic research as well as the applications of heavy-ion physics and nuclear techniques. The HIRFL-CSR complex comprises a super-conducting Electron Cyclotron Resonance (ECR) ion source, the Sector Focused Cyclotron (energy constant K = 69), a main Sector-Separated Cyclotron (energy constant K = 450), the Cooling Storage Ring system which is connected to the HIRFL as an injector, the Radioactive Ion Beam Line in Lanzhou (RIBLL) and several terminals for different experiments (Li, 2007Li, Q. 2007. Biomedical research with heavy ions at the IMP accelerators. Advances in Space Research, 40(4), 455-460.; Zhan et al., 2010Zhan, W.L., Xu, H.S., Xiao, G.Q., Xia, J.W., Zhao, H.W., Yuan, Y.J., Grp, H.-C. 2010. Progress in HIRFL-CSR. Nuclear Physics A, 834(1-4), 694c-700c.). The HIRFL -CSR can provide several kinds of ion beams from protons to uranium with energy varying from 2800 MeV/u to 1000 MeV/u (Zhou et al., 2014Zhou, X., Lu, X.H., Li, X.H., Xin, Z.J., Xie, J.R., Zhao, M.R., Wang, L., Du, W.Y., Liang, J.P. 2014. Radiation induces acid tolerance of Clostridium tyrobutyricum and enhances bioproduction of butyric acid through a metabolic switch. Biotechnology for Biofuels, 7, 22). In recent years, great progress has been made in generating different kinds of mutants with high productivity of microbes via heavy ion irradiations (Hu et al., 2016Hu, W., Chen, J.H., Wang, S.Y., Liu, J., Song, Y., Wu, Q.F., Li, W.J. 2016. Changes in the physiological properties and kinetics of citric acid accumulation via carbon ion irradiation mutagenesis of Aspergillus niger. Journal of Zhejiang University-Science B, 17(4), 262-270.; Hu et al., 2014; Li et al., 2016; Wang et al., 2015Wang, S.Y., Jiang, B.L., Zhou, X., Chen, J.H., Li, W.J., Liu, J., Hu, W., Xiao, G.Q., Dong, M.Y., Wang, Y.C. 2015. Study of a High-Yield Cellulase System Created by Heavy-Ion Irradiation-Induced Mutagenesis of Aspergillus niger and Mixed Fermentation with Trichoderma reesei. Plos One, 10(12), e0144233.).
To date, no studies of L. thermophilus for enhanced L-(+)-lactic acid accumulation by high-LET 12C6+ ion irradiation have been reported. In current study, we obtained one mutant of L. thermophilus induced by 80MeV/u 12C6+ ion beams from HIRFL. Different carbon or nitrogen sources were provided to investigate carbon or nitrogen source utilization between mutant SRZ50 and wild type. Under optimized medium compositions, the fermentation characteristics between mutant SRZ50 and wild type under different fermentation periods was also studied.
MATERIAL AND METHODS
Strain, media, and reagents
A parent L. thermophilus SR7, which can produce L-(+)-lactic acid, was obtained from X-ray irradiation and provided by biophysics lab of IMP, CAS (Wu et al., 2015Wu, Q.H., Chen, J.H., Zhang, Z., Li, W.J., Hu, W., Wei, Z.H., Liu, J., Wang, S.Y. 2015. Breeding study of Lactobacillus Thermophilics induced by X-ray for AL-lactic acid production. Science and Technology of Food Industry, 36(3), 116-118.) . The strain was maintained on MRS agar slants at 4℃. The MRS agar medium contained per litre of distilled water: glucose 20 g (Tianjin Da-Mao Co., Ltd., China), peptone 10 g (Beijing Shuang-Xuan Co., Ltd., China), beef extract 10 g (Beijing Shuang-Xuan Co., Ltd., China), yeast extract 5 g (Guangfu Chem Co., Ltd., China), sodium citrate 5 g (Tianjin Bei-Chen Co., Ltd., China), tween-80 1ml (Shanghai Da-Zong Co., Ltd., China), sodium acetate 5 g (Tianjin Ba-Shifu Co., Ltd., China), K2HPO4 2 g (Tianjin Heng-Xing Co., Ltd., China), MgSO4·7H2O 0.58 g (Tianjin Bei-Chen Co., Ltd., China), MnSO4·4H2O 0.25 g (Beijing Shuang-Xuan Co., Ltd., China), agar 15 g (Beijing Solarbio Co., Ltd., China); and pH 6.2-6.4 (Ramyasree & Dutta, 2013Ramyasree, S., Dutta, J.R. 2013. The effect of process parameters in enhancement of lipase production by co-culture of lactic acid bacteria and their mutagenesis study. Biocatalysis and Agricultural Biotechnology, 2(4), 393-398.). Fructose and xylose were bought from Tianjin Mi-Ou Co., Ltd., China. Urea,sodium nitrate, and maltose were purchased from Gansu Peng-Cheng Co., Ltd., China. Corn steep liquor was purchased from Tianjin Li-long Co., Ltd., China. Calcium carbonate was purchased from Tianjin Heng-Xing Co., Ltd., China. All of the chemical used were of analytical grade.
Irradiation
A parent L. thermophiles SR7 was incubated on the MRS agar slants at 50℃ for 24 h, and then the bacteria suspension from slants was diluted at 1×108 cells/mL with sterile saline water. Then, 1ml bacteria suspension was transferred into 35mm irradiation dish, respectively. The dishes were put into the sample holder in TR4 experiment terminal, and were irradiated by 80 Mev/u carbon ion beams with the LET of 40 Kev/u. The irradiation doses were 25, 50, 75, 100, 125, and 150 Gy, calculated from particle fluencies and liner energy transfer (LET), and there were three L. thermophiles samples for every dose treated. After irradiation, the mutagen-treated and untreated aliquots of L. thermophiles cells were properly diluted and spread on selective plate medium, followed by cultivated at 50 ℃ for 24 h, and total number of colonies in the mutagen-treated bacteria suspension (M) and untreated sample (N) were determined and the survival fraction of L. thermophiles cells after irradiation was calculated (Tu et al., 2016Tu, R., Jin, W., Wang, M., Han, S., Abomohra, A.E.-F., Wu, W.-M. 2016. Improving of lipid productivity of the biodiesel promising green microalga Chlorella pyrenoidosa via low-energy ion implantation. Journal of Applied Phycology, 28(4), 2159-2166.).
Survival rate (%) = (M/N)*100
Screening process
After irradiation, the mutagen-treated and untreated aliquots of L. thermophiles cells were properly diluted and spread on selective plate medium, followed by cultivated at 50 ℃ for 24 h. The colonies showing large halos (yellow) on selective plate medium were selected as the primary screening, and then cultivated in the submerged fermentation medium for L-(+)-lactic acid accumulation as the second round screening, followed by still cultivated at 50 ℃ for 72 h. Several mutant strains with the highest L-(+)-lactic acid concentration, obtained though the primary and second round screening, were further tested for their genetic stability for producing high L-(+)-lactic acid.
The medium compositions of different carbon sources or nitrogen sources
Both mutant SRZ50 strain and wild type strain were cultured for 24 h on MRS agar slant, and the cells of one loop, respectively, were inoculated into 10 ml seed fermentation medium contained in a test tube (18×180 mm) and still cultured at 50 ℃ for 18h. Then, 1ml seed fermentation broth of both mutant SRZ50 strain and wild type strain, respectively, were transferred to fermentation medium containing different carbon sources or different nitrogen sources for L-(+)-lactic acid accumulation, followed by still cultivated at 50 ℃ for 72 h. The compositions of seed fermentation medium were same as strain culture medium except agar. The compositions of different carbon source mediums contained per litre of distilled water: carbon source concentration 50 g (glucose, fructose, maltose or xylose, respectively), peptone 10 g, beef extract 10 g, yeast extract 5 g, sodium citrate 5 g, tween-80 1ml, calcium carbonate 25 g, sodium acetate 5 g, K2HPO4 2 g, MgSO4·7H2O 0.58 g, MnSO4·4H2O 0.25 g, and pH 7. When the effect of different nitrogen sources was examined, The compositions of mediums contained per litre of distilled water: glucose 50 g, nitrogen concentration 13 g (peptone, beef extract, yeast powder, urea, sodium nitrate, ammonium sulfate, ammonium citrate tribasic or corn steep liquor, respectively), sodium citrate 5 g, tween-80 1ml, calcium carbonate 25 g, sodium acetate 5 g, K2HPO4 2 g, MgSO4·7H2O 0.58 g, MnSO4·4H2O 0.25 g, and pH 7.
The analysis of fermentation characteristics between mutant SRZ50 and wild type
Under optimized medium compositions, both wild type strain and SRZ50 strain were tested to analyze fermentation characteristic of L-(+)-lactic acid accumulation. The tested fermentation medium compositions contained per litre of distilled water: glucose 50 g, yeast powder 30 g, sodium citrate 5 g, tween-80 1ml, calcium carbonate 25 g, sodium acetate 5 g, K2HPO4 2 g, MgSO4·7H2O 0.58 g, MnSO4·4H2O 0.25 g, and pH 7.
Analytical methods
During screening process, the concentration of L-(+)-lactic acid was tested by 0.01M marked sodium hydroxide. Under different carbon sources fermentation, the concentration of L-(+)-lactic acid was tested by EDTA titration method (Li et al., 2011Li, S., Zhu, Z., Gu, S., Liu, H., Wang, D. 2011. Mutation-Screening in l-(+)-Lactic Acid Producing Strains by Ion Implantation. Indian J Microbiol, 51(2), 138-143.). The purity was determined by High performance liquid chromatography (HPLC), 5μm C18 column (Lanzhou Zhongke-Kaidi chemical technology Co., Ltd, China) under following condition: mobile phase, 0.01mol/L H3PO4, flow rate, 0.7 mL/min. The detection was made in the UV range at 210 nm at a temperature of 25°C. Standard L-(+)-lactic acid was purchased from Solarbio (Beijing Solarbio Science & Technology Co., Ltd, China). Biomass was also tested through OD600 by spectrophotometer. To assess the statistical significance of fermentation characteristic by different strains, the T-Test was employed. Figures were plotted with Origin 7.5 software. Mycelia morphologies were also observed by optical microscopy. Each experiment was repeated three times.
RESULTS AND DISCUSSION
Survival rates and mutants selection
After L. thermophilus SR7 cell suspension was irradiated at doses of 25, 50, 75, 100, 125, and 150 Gy by 80 MeV/u carbon ion beams, and the dose rate was about 20-25 Gy per minute, respectively. The lethality of L. thermophilus SR7 was determined by colony formation assay. As showed in Fig 1, the relationship between the survival rates of L. thermophilus SR7 and the irradiation dose of 12C6+ ion beam exhibits a “saddle shape”, which is different form “shoulder type” or “straight” survival curve from traditional irradiation means on live body, such as ultraviolet rays or gamma-rays irradiation. It was demonstrated that medium-energy 12C6+ ion beam irradiation have similar action mechanism to low-energy ion implantation (Yang et al., 2013Yang, Y.N., Liu, C.L., Wang, Y.K., Xue, J.M. 2013. Mutation effects of C2+ ion irradiation on the greasy Nitzschia sp. Mutation Research-Fundamental and Molecular Mechanisms of Mutagenesis, 751, 24-28.). In order to explain this phenomenon, Yu proposed relative hypothesis: in the first “down” stage, energy deposition and momentum transfer of ions causes a series of ionization, which can damage the cell by etching of the cell wall, destruction of the cell framework, perforation of the membrane, DNA breaks and the oxidation of the cell membrane, etc. In this stage, the damage degree to cell activity increases rapidly with the increase of the irradiation dose. When the dose increases to a certain value, the unclear repair mechanism in cell is activated, causing a temporary rise of the survival rate in cell. When the dose further increases, the irradiation damage becomes irreparable. Thus, the survival rate goes on declining (He et al., 2011He, Q.T., Li, N., Chen, X.C., Ye, Q., Bai, J.X., Xiong, J.A., Ying, H.J. 2011. Mutation breeding of nuclease p1 production in Penicillium citrinum by low-energy ion beam implantation. Korean Journal of Chemical Engineering, 28(2), 544-549.; Zhang & Yu, 2009Zhang, N., Yu, L. 2009. Mutation Breeding of beta-carotene Producing Strain B. trispora by Low Energy Ion Implantation. Plasma Science & Technology, 11(1), 110-115.).
After irradiation, the survivors of L. thermophilus may contain different kinds of mutants. However, a very small scale of survivors may have improved ability in lactic acid accumulation (Zhou et al., 2013Zhou, X., Xin, Z.J., Lu, X.H., Yang, X.P., Zhao, M.R., Wang, L., Liang, J.P. 2013. High efficiency degradation crude oil by a novel mutant irradiated from Dietzia strain by C-12(6+) heavy ion using response surface methodology. Bioresource Technology, 137, 386-393.). Thus, feasible high-throughput screening methods will be need, and some promising mutants may be obtained quickly (Murai et al., 2013Murai, K., Nishiura, A., Kazama, Y., Abe, T. 2013. A large-scale mutant panel in wheat developed using heavy-ion beam mutagenesis and its application to genetic research. Nuclear Instruments & Methods in Physics Research Section B-Beam Interactions with Materials and Atoms, 314, 59-62.; Shikazono et al., 2005Shikazono, N., Suzuki, C., Kitamura, S., Watanabe, H., Tano, S., Tanaka, A. 2005. Analysis of mutations induced by carbon ions in Arabidopsis thaliana. Journal of Experimental Botany, 56(412), 587-596.). Generally, the more L-(+)-lactic acid concentrations were yielded, the larger the diameters of transparent halos formed (Bai et al., 2004Bai, D.M., Zhao, X.M., Li, X.G., Xu, S.M. 2004. Strain improvement of Rhizopus oryzae for over-production Of L(+)-lactic acid and metabolic flux analysis of mutants. Biochemical Engineering Journal, 18(1), 41-48.). However, it was also found that some mutant strains with larger diameters hales showed low L-(+)-lactic acid concentration (Li et al., 2011Li, S., Zhu, Z., Gu, S., Liu, H., Wang, D. 2011. Mutation-Screening in l-(+)-Lactic Acid Producing Strains by Ion Implantation. Indian J Microbiol, 51(2), 138-143.). Hence, in order to select high-yield lactic acid mutants, combining diameters of transparent halos and fermentation experiments as accurate selection project may be the best choice. In this study, depending on the determination of transparent hale diameters for the primary screening, the L-(+)-lactic acid yield of 59 strains under different doses irradiated by heavy 12C6+ ion beams were obtained (dates not shown). Depending on secondary screening, five mutants exhibited better L-(+)-lactic acid production than wild type, especially SRZ50, SRZ10, SRZ48 exhibited significant increase in L-(+)-lactic acid production from that of wild type.
Genetic stability for mutants
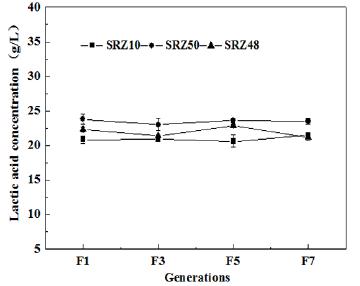
The consecutive generation experiments determining the genetic stability of SRZ50, SRZ10, SRZ48 were performed to examine whether mutants could inherit a steady L-(+)-lactic acid production. The experiment results of consecutive seven generations among three mutants were shown in Fig 3. It was accepted that SRZ50, SRZ10, SRZ48 all had equal performances in L-(+)-lactic acid production, which implied that all the mutants had remarkably steady capacities in L-lactic acid production. Especially, SRZ50 had the highest L-(+)-lactic acid production.
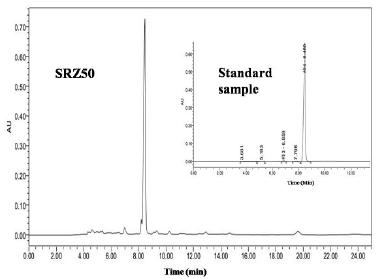
HPLC was also employed to determine the L-(+)-lactic acid purity in fermentation broth of SRZ50. As showed in Fig. 4, the ridge was appeared during 8.00-8.50 min of fermentation sample, which was in accordance with the ridge appearance time of the standard L-(+)-lactic acid. By comparing to the standard sample and the fermentation sample, it was obtained that there was no miscellaneous peak of other organic acid, which indicated SRZ50 mainly produced L-(+)-lactic acid.
L-(+)-lactic acid production by different carbon source using selected mutant strain SRZ50 and wild type
The regulation of carbon utilization by microorganism plays an important role in studying morphological development and metabolic product accumulation (Khaliq et al., 2009Khaliq, S., Akhtar, K., Ghauri, M.A., Iqbal, R., Khalid, A.M., Muddassar, M. 2009. Change in colony morphology and kinetics of tylosin production after UV and gamma irradiation mutagenesis of Streptomyces fradiae NRRL-2702. Microbiological Research, 164(4), 469-477.). Glucose, maltose, sucrose, molasses, cheese whey or some agricultural sub-products had been successfully applied in lactic acid fermentation (Bulut et al., 2004Bulut, S., Elibol, M., Ozer, D. 2004. Effect of different carbon sources on L(+)-lactic acid production by Rhizopus oryzae. Biochemical Engineering Journal, 21(1), 33-37.). However, different carbon sources can result in different concentrations of product accumulation. For example, when using sucrose or fructose as the sole carbon source, enhanced L-(+)-lactic acid production (24.8 ± 0.8 g/L) by Bacillus coagulans was obtained from sucrose, however, only (6.9 ± 0.2 g/L) L-(+)-lactic acid were produced from fructose (Xu & Xu, 2014Xu, K., Xu, P. 2014. Efficient production of L-lactic acid using co-feeding strategy based on cane molasses/glucose carbon sources. Bioresource Technology, 153, 23-29.). Hence, it was necessary to investigate carbon adaptability between mutant and wild type. In present study, we have investigated the effects of different carbon sources on the L-(+)-lactic acid production and biomass in submerged fermentation by mutant SRZ50 and wild type strain. The results showed that both mutant SRZ50 and wild type can grow in all carbon sources tested (Fig 5). Especially, when glucose or fructose was the sole carbon source, the L-(+)-lactic acid production by mutant SRZ50 was both the highest, respectively, 23.16 ± 0.72 g/L or 23.24 ± 0.66 g/L, which had a significant increase from that of wild type (P<0.01). However, when maltose or xylose was the sole carbon source, there was no significant difference in the L-(+)-lactic acid production from that of wild type (p>0.05). Of course, there was no significant difference in utilizing glucose or fructose or maltose as a sole carbon source to produce L-(+)-lactic acid by strain SRZ50 (P>0.05), while the xylose was the sole carbon source, the yield of L-(+)-lactic acid had an obvious decrease (p<0.05). These results demonstrated that mutant SRZ50 exhibited more promising fermentation characteristic of producing L-(+)-lactic acid than wild type using glucose, fructose or maltose, respectively.
L-(+)-lactic acid production by different carbon sources using selected mutant strain SRZ50 and wild type. **P<0.01 and *P<0.05 (lactic acid production of T-Test by mutant SRZ50 and wild type). Each experiment was repeated three times and each experiment had three independent replicates.
Biomass was an important fermentation parameter for submerged fermentation. In this study, under different carbon sources, mutant SRZ50 exhibited different biomass accumulation (Fig 6). When glucose or fructose was the sole carbon source, there was a significant increase in biomass by mutant SRZ50 from that of wild type (P<0.05). However, when maltose or xylose was a sole carbon source, there was no significant difference between mutant SRZ50 and wild type (P>0.05).
Biomass accumulation by different carbon sources using selected mutant strain SRZ50 and wild type. **P<0.01 and *P<0.05 (Biomass production of T-Test by mutant SRZ50 and wild type). Each experiment was repeated three times and each experiment had three independent replicates.
L-(+)-lactic acid production by different nitrogen source using selected mutant strain SRZ50 and wild type
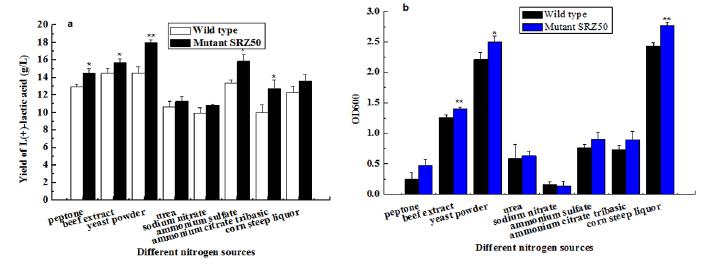
Nitrogen substrates play an important role in product economic production of lactic acid (Altaf et al., 2007Altaf, M., Venkateshwar, M., Srijana, M., Reddy, G. 2007. An economic approach for L-(+) lactic acid fermentation by Lactobacillus amylophilus GV6 using inexpensive carbon and nitrogen sources. Journal of Applied Microbiology, 103(2), 372-380.). Different organic nitrogen (yeast extract, peptone, corn-steep) and inorganic nitrogen (Urea, (NH4)2SO4) have been applied to investigate nitrogen adaptability by different lactic acid producing strains (Hujanen & Linko, 1996Hujanen, M., Linko, Y.Y. 1996. Effect of temperature and various nitrogen sources on L(+) lactic acid production by Lactobacillus casei. Applied Microbiology and Biotechnology, 45(3), 307-313.; Nancib et al., 2001Nancib, N., Nancib, A., Boudjelal, A., Benslimane, C., Blanchard, F., Boudrant, J. 2001. The effect of supplementation by different nitrogen sources on the production of lactic acid from date juice by Lactobacillus casei subsp rhamnosus. Bioresource Technology, 78(2), 149-153.). Among them, yeast powder has been proved to be a major factor to affect lactic acid production and biomass accumulation significantly. In this study, eight nitrogen sources, such as yeast powder, peptone, ammonium sulfate and so on, has been studied, in which yeast powder can promote both wild type and mutant SRZ50 to accumulate the highest L-(+)-lactic acid, respectively 18.02 ± 0.32 g/L and 14.54 ± 0.7 g/L (Fig 7a). Meanwhile, when peptone, beef extract, yeast powder, ammonium sulfate or ammonium citrate tribasic was the nitrogen source, mutant SRZ50 exhibited significant increase than wild type in yield of L-(+)-lactic acid (p<0.05). When the other nitrogen sources were supplied, mutant SRZ50 exhibited enhanced increase in yield of L-(+)-lactic acid. Different nitrogen sources can also result in significant difference in biomass accumulation. When beef extract, yeast powder or corn liquor were the only nitrogen source, mutant SRZ50 can accumulate significant increase in biomass (p<0.05) (Fig 7b). These results can also demonstrate that mutant SRZ50 exhibited more promising fermentation characteristic of producing L-(+)-lactic acid than wild type using different nitrogen sources.
(a). L-(+)-lactic acid production by different nitrogen sources using selected mutant strain SRZ50 and wild type. (b). Biomass accumulation by different nitrogen sources. **P<0.01 and *P<0.05 (lactic acid production or biomass production of T-Test by mutant SRZ50 and wild type). Each experiment was repeated three times and each experiment had three independent replicates.
In addition, according to the radio of L-(+)-lactic acid production and biomass, sodium nitrate had higher efficiency than other nitrogen source in producing L-(+)-lactic acid. Meanwhile, sodium nitrate could be considered as a feasible and inexpensive alternative nitrogen source. Therefore, it may be worthy of using a combination of organic (yeast extract) and inorganic (sodium nitrate) nitrogen sources for L-(+)-lactic acid accumulation in mutant SRZ50 in the future. Similar studies for combination of organic and inorganic have been also previously done by Nancib et al (Nancib et al., 2001Nancib, N., Nancib, A., Boudjelal, A., Benslimane, C., Blanchard, F., Boudrant, J. 2001. The effect of supplementation by different nitrogen sources on the production of lactic acid from date juice by Lactobacillus casei subsp rhamnosus. Bioresource Technology, 78(2), 149-153.) and Arasaratnan et al (Arasaratnam et al., 1996Arasaratnam, V., Senthuran, A., Balasubramaniam, K. 1996. Supplementation of whey with glucose and different nitrogen sources for lactic acid production by Lactobacillus delbrueckii. Enzyme and Microbial Technology, 19(7), 482-486.).
Analysis of fermentation characteristics between mutant SRZ50 and wild type
Depending on above optimized carbon source and nitrogen source, different fermentation time was chose to investigate fermentation characteristic between mutant SRZ50 and wild type (Fig 8). The results showed that mutant SRZ50 all exhibited significant increase in lactic acid accumulation from wild type under different fermentation time (p<0.05). In the early phase of submerged fermentation (8-32h), both mutant SRZ50 and wild type can accumulate L-(+)-lactic acid quickly. From 32h to 72h, there were only slight fluctuation on lactic acid production between mutant SRZ50 and wild type. Consequently, it was ascertained that mutant SRZ50 possess a stable enhancement of L-(+)- lactic acid production.
Different fermentation periods for L-(+)-lactic acid production between mutant SRZ50 and wild type. Each experiment was repeated three times and each experiment had three independent replicates.
CONCLUSION
Recently, heavy ion beams has been proved to be a novel powerful mutagen, which can induce high mutation frequency and a broad spectrum of phenotypes in plants (Kazama et al., 2011Kazama, Y., Hirano, T., Saito, H., Liu, Y., Ohbu, S., Hayashi, Y., Abe, T. 2011. Characterization of highly efficient heavy-ion mutagenesis in Arabidopsis thaliana. Bmc Plant Biology, 11,161; Tanaka et al., 2010Tanaka, A., Shikazono, N., Hase, Y. 2010. Studies on Biological Effects of Ion Beams on Lethality, Molecular Nature of Mutation, Mutation Rate, and Spectrum of Mutation Phenotype for Mutation Breeding in Higher Plants. Journal of Radiation Research, 51(3), 223-233.). In china, only the HIRFL can provide high LET heavy ion beams to carry out breeding of microbes, such as yeast, fungus, and bacteria. In present study, after irradiated by carbon ions, we successfully obtained one high-yield L-(+)-lactic acid mutant. Meanwhile, it was also clearly demonstrated that L-(+)-lactic acid production was affected by the carbon sources used in the fermentation process. Glucose, fructose or maltose was suitable carbon sources for lactic acid production both mutant SRZ50 and wild type. Especially, when glucose or fructose was the sole carbon source, there was significant increase in L-(+)-lactic acid production by mutant SRZ50 from that of wild type. When yeast powder was the sole nitrogen source, it can promote mutant SRZ50 to accumulate the highest L(+) lactic acid accumulation, which had a significant increase from that of wild type (P<0.01). Under different fermentation periods, it was obtained that mutant SRZ50 exhibited significant increase in L-(+)-lactic acid accumulation from wild type. All these results implied that mutant SRZ50 displayed enhanced L-(+) lactic acid production from wild type.
ACKNOWLEDGE
We thank master Qinghua Wu due to the experiment work, Wei Hu, and Shuyang Wang due to drafting and reviewing the work. The authors are grateful to all the staff of Heavy Ion Research Facility in Lanzhou (HIRFL) for providing the carbon beams. The work is supported by Agriculture Science Technology Achievement Transformation Fund (2013GB24910680).
REFERENCES
- Abdel-Rahman, M.A., Tashiro, Y., Sonomoto, K. 2013. Recent advances in lactic acid production by microbial fermentation processes. Biotechnology Advances, 31(6), 877-902.
- Altaf, M., Venkateshwar, M., Srijana, M., Reddy, G. 2007. An economic approach for L-(+) lactic acid fermentation by Lactobacillus amylophilus GV6 using inexpensive carbon and nitrogen sources. Journal of Applied Microbiology, 103(2), 372-380.
- Arasaratnam, V., Senthuran, A., Balasubramaniam, K. 1996. Supplementation of whey with glucose and different nitrogen sources for lactic acid production by Lactobacillus delbrueckii. Enzyme and Microbial Technology, 19(7), 482-486.
- Bai, D.M., Jia, M.Z., Zhao, X.M., Ban, R., Shen, F., Li, X.G., Xu, S.M. 2003. L(+)-lactic acid production by pellet-form Rhizopus oryzae R1021 in a stirred tank fermentor. Chemical Engineering Science, 58(3-6), 785-791.
- Bai, D.M., Zhao, X.M., Li, X.G., Xu, S.M. 2004. Strain improvement of Rhizopus oryzae for over-production Of L(+)-lactic acid and metabolic flux analysis of mutants. Biochemical Engineering Journal, 18(1), 41-48.
- Bulut, S., Elibol, M., Ozer, D. 2004. Effect of different carbon sources on L(+)-lactic acid production by Rhizopus oryzae. Biochemical Engineering Journal, 21(1), 33-37.
- Gao, T., Wong, Y.K., Ng, C., Ho, K.P. 2012. L-lactic acid production by Bacillus subtilis MUR1. Bioresource Technology, 121, 105-110.
- Hamada, N. 2009. Recent Insights into the Biological Action of Heavy-Ion Radiation. Journal of Radiation Research, 50(1), 1-9.
- He, Q.T., Li, N., Chen, X.C., Ye, Q., Bai, J.X., Xiong, J.A., Ying, H.J. 2011. Mutation breeding of nuclease p1 production in Penicillium citrinum by low-energy ion beam implantation. Korean Journal of Chemical Engineering, 28(2), 544-549.
- Hu, G.R., Fan, Y., Zhang, L., Yuan, C., Wang, J.F., Li, W.J., Hu, Q., Li, F.L. 2013. Enhanced Lipid Productivity and Photosynthesis Efficiency in a Desmodesmus sp Mutant Induced by Heavy Carbon Ions. Plos One, 8(4).
- Hu, W., Chen, J.H., Wang, S.Y., Liu, J., Song, Y., Wu, Q.F., Li, W.J. 2016. Changes in the physiological properties and kinetics of citric acid accumulation via carbon ion irradiation mutagenesis of Aspergillus niger. Journal of Zhejiang University-Science B, 17(4), 262-270.
- Hu, W., Liu, J., Chen, J.H., Wang, S.Y., Lu, D., Wu, Q.H., Li, W.J. 2014. A mutation of Aspergillus niger for hyper-production of citric acid from corn meal hydrolysate in a bioreactor. Journal of Zhejiang University-Science B, 15(11), 1006-1010.
- Hujanen, M., Linko, Y.Y. 1996. Effect of temperature and various nitrogen sources on L(+) lactic acid production by Lactobacillus casei. Applied Microbiology and Biotechnology, 45(3), 307-313.
- Inthima, P., Otani, M., Hirano, T., Hayashi, Y., Abe, T., Nakano, M., Supaibulwatana, K. 2014. Mutagenic effects of heavy-ion beam irradiation on in vitro nodal segments of Artemisia annua L. Plant Cell Tissue and Organ Culture, 119(1), 131-139.
- Ishikawa, S., Ishimaru, Y., Igura, M., Kuramata, M., Abe, T., Senoura, T., Hase, Y., Arao, T., Nishizawa, N.K., Nakanishi, H. 2012. Ion-beam irradiation, gene identification, and marker-assisted breeding in the development of low-cadmium rice. Proceedings of the National Academy of Sciences of the United States of America, 109(47), 19166-19171.
- Kazama, Y., Hirano, T., Saito, H., Liu, Y., Ohbu, S., Hayashi, Y., Abe, T. 2011. Characterization of highly efficient heavy-ion mutagenesis in Arabidopsis thaliana. Bmc Plant Biology, 11,161
- Khaliq, S., Akhtar, K., Ghauri, M.A., Iqbal, R., Khalid, A.M., Muddassar, M. 2009. Change in colony morphology and kinetics of tylosin production after UV and gamma irradiation mutagenesis of Streptomyces fradiae NRRL-2702. Microbiological Research, 164(4), 469-477.
- Li, Q. 2007. Biomedical research with heavy ions at the IMP accelerators. Advances in Space Research, 40(4), 455-460.
- Li, S., Zhu, Z., Gu, S., Liu, H., Wang, D. 2011. Mutation-Screening in l-(+)-Lactic Acid Producing Strains by Ion Implantation. Indian J Microbiol, 51(2), 138-143.
- Li, Z.Z., Chen, X.J., Li, Z.L., Li, D.M., Wang, Y., Gao, H.L., Cao, L., Hou, Y.Z., Li, S.B., Liang, J.P. 2016. Strain improvement of Trichoderma viride for increased cellulase production by irradiation of electron and C-12(6+)-ion beams. Biotechnology Letters, 38(6), 983-989.
- Martinez, F.A.C., Balciunas, E.M., Salgado, J.M., Gonzalez, J.M.D., Converti, A., Oliveira, R.P.D. 2013. Lactic acid properties, applications and production: A review. Trends in Food Science & Technology, 30(1), 70-83.
- Murai, K., Nishiura, A., Kazama, Y., Abe, T. 2013. A large-scale mutant panel in wheat developed using heavy-ion beam mutagenesis and its application to genetic research. Nuclear Instruments & Methods in Physics Research Section B-Beam Interactions with Materials and Atoms, 314, 59-62.
- Nancib, N., Nancib, A., Boudjelal, A., Benslimane, C., Blanchard, F., Boudrant, J. 2001. The effect of supplementation by different nitrogen sources on the production of lactic acid from date juice by Lactobacillus casei subsp rhamnosus. Bioresource Technology, 78(2), 149-153.
- Ou, M.S., Ingram, L.O., Shanmugam, K.T. 2011. l(+)-Lactic acid production from non-food carbohydrates by thermotolerant Bacillus coagulans. Journal of Industrial Microbiology & Biotechnology, 38(5), 599-605.
- Pal, P., Dey, P. 2013. Process intensification in lactic acid production by three stage membrane integrated hybrid reactor system. Chemical Engineering and Processing, 64, 1-9.
- Qin, J.Y., Zhao, B., Wang, X.W., Wang, L.M., Yu, B., Ma, Y.H., Ma, C.Q., Tang, H.Z., Sun, J.B., Xu, P. 2009. Non-Sterilized Fermentative Production of Polymer-Grade L-Lactic Acid by a Newly Isolated Thermophilic Strain Bacillus sp 2-6. Plos One, 4(2), e4359.
- Ramyasree, S., Dutta, J.R. 2013. The effect of process parameters in enhancement of lipase production by co-culture of lactic acid bacteria and their mutagenesis study. Biocatalysis and Agricultural Biotechnology, 2(4), 393-398.
- Shikazono, N., Suzuki, C., Kitamura, S., Watanabe, H., Tano, S., Tanaka, A. 2005. Analysis of mutations induced by carbon ions in Arabidopsis thaliana. Journal of Experimental Botany, 56(412), 587-596.
- Shikazono, N., Tanaka, A., Kitayama, S., Watanabe, H., Tano, S. 2002. LET dependence of lethality in Arabidopsis thaliana irradiated by heavy ions. Radiation and Environmental Biophysics, 41(2), 159-162.
- Tanaka, A., Shikazono, N., Hase, Y. 2010. Studies on Biological Effects of Ion Beams on Lethality, Molecular Nature of Mutation, Mutation Rate, and Spectrum of Mutation Phenotype for Mutation Breeding in Higher Plants. Journal of Radiation Research, 51(3), 223-233.
- Tu, R., Jin, W., Wang, M., Han, S., Abomohra, A.E.-F., Wu, W.-M. 2016. Improving of lipid productivity of the biodiesel promising green microalga Chlorella pyrenoidosa via low-energy ion implantation. Journal of Applied Phycology, 28(4), 2159-2166.
- Wang, P., Li, J., Wang, L., Tang, M.L., Yu, Z.L., Zheng, Z.M. 2009. l(+)-Lactic acid production by co-fermentation of glucose and xylose with Rhizopus oryzae obtained by low-energy ion beam irradiation. Journal of Industrial Microbiology & Biotechnology, 36(11), 1363-1368.
- Wang, S.Y., Jiang, B.L., Zhou, X., Chen, J.H., Li, W.J., Liu, J., Hu, W., Xiao, G.Q., Dong, M.Y., Wang, Y.C. 2015. Study of a High-Yield Cellulase System Created by Heavy-Ion Irradiation-Induced Mutagenesis of Aspergillus niger and Mixed Fermentation with Trichoderma reesei. Plos One, 10(12), e0144233.
- Wu, Q.H., Chen, J.H., Zhang, Z., Li, W.J., Hu, W., Wei, Z.H., Liu, J., Wang, S.Y. 2015. Breeding study of Lactobacillus Thermophilics induced by X-ray for AL-lactic acid production. Science and Technology of Food Industry, 36(3), 116-118.
- Xu, K., Xu, P. 2014. Efficient production of L-lactic acid using co-feeding strategy based on cane molasses/glucose carbon sources. Bioresource Technology, 153, 23-29.
- Yang, Y.N., Liu, C.L., Wang, Y.K., Xue, J.M. 2013. Mutation effects of C2+ ion irradiation on the greasy Nitzschia sp. Mutation Research-Fundamental and Molecular Mechanisms of Mutagenesis, 751, 24-28.
- Zhan, W.L., Xu, H.S., Xiao, G.Q., Xia, J.W., Zhao, H.W., Yuan, Y.J., Grp, H.-C. 2010. Progress in HIRFL-CSR. Nuclear Physics A, 834(1-4), 694c-700c.
- Zhang, N., Yu, L. 2009. Mutation Breeding of beta-carotene Producing Strain B. trispora by Low Energy Ion Implantation. Plasma Science & Technology, 11(1), 110-115.
- Zhou, X., Lu, X.H., Li, X.H., Xin, Z.J., Xie, J.R., Zhao, M.R., Wang, L., Du, W.Y., Liang, J.P. 2014. Radiation induces acid tolerance of Clostridium tyrobutyricum and enhances bioproduction of butyric acid through a metabolic switch. Biotechnology for Biofuels, 7, 22
- Zhou, X., Xin, Z.J., Lu, X.H., Yang, X.P., Zhao, M.R., Wang, L., Liang, J.P. 2013. High efficiency degradation crude oil by a novel mutant irradiated from Dietzia strain by C-12(6+) heavy ion using response surface methodology. Bioresource Technology, 137, 386-393.
Publication Dates
-
Publication in this collection
2017
History
-
Received
03 Feb 2016 -
Accepted
14 July 2016