ABSTRACT
The aim of this study is the production, purification, and characterisation of thermostable raw starch hydrolyzing α-amylase produced by Bacillus mojavensis SO-10. The maximum production conditions of α-amylase were found at 36th hour, 35 °C and pH 7.0. We utilized three steps to purify the thermostable α-amylase and as a result, 34-fold and 18% yield were obtained. The molecular weight of purified α-amylase was determined as 73 kD. The Km and Vmax rates were detected as 0.010 mM and 3.38 µmol min−1, respectively. This purified α-amylase exhibited the highest activity at pH 5.0-6.0 and 70 ºC and showed stability over a wide variety of pH and temperature at 4.0-8.0, and 40-50 ºC, respectively. The thermostable purified α-amylase exhibited stability in the presence of denaturing agents and heavy metal ions. The purified enzyme hydrolyzed the raw starches of corn and wheat grains in the ratio of 36.7% and 39.2% respectively. The end-yields of soluble starch hydrolysis were analyzed by thin-layer chromatography (TLC). In addition, the usage of purified α-amylase in clarification of apple juice and domestic washing detergent industries were evaluated.
Key words:
α-Amylase; Raw starch; Thin-Layer Chromatography (TLC); Detergents; Apple Juice Clarification
INTRODUCTION
Amylase (EC 3.2.1.1, 1, 4-α-D-glucan-glucanohydrolase) is an extracellular enzyme that hydrolyzes starch and glycogen molecules 11 Gupta R, Gigras P, Mohapatra H, Goswami VK, Chauhan B, Microbial a-amylases: A biotechnological perspective. Process Biochem. 2003; 38: 1599-1616.. It breaks down the α-1, 4 bonds in starch molecules into glucose, maltose, maltotriose and α-limit dextrine 22 Roy I, Gupta MN, Hydrolysis of starch by a mixture of glucoamylse and pullulanase entrapped individually in calcium alginate beads. Enzyme Microb. Technol, 2004; 34: 26-32.,33 Balkan B, Ertan F. Production of a-Amylase from Penicillium chrysogenum Under Solid State Fermentation by Using Some Agricultural By-Product. Food Technol. Biotechnol. 2007; 45: 439-442.. Amylase is used primarily in the food industry for the preparation of maltose syrup and clarification of various drinks. In addition, amylase is generally used in other industries like biorefinery, paper, detergents, textiles and pharmaceuticals 44 Kumagai Y, Satoh T, Inoue A, Ojima T. Enzymatic properties and primary structures of two a-amylase isozymes from the Pacific abalone Haliotis discus hannai. Comp Biochem Physiol B: Biochem Mol Biol. 2013; 164: 80-88.
5 Wiseman A. The application of enzyme in industry. Handbook Enzyme Biotechnol. 1987; 3: 275-373.
6 Lama L, Nicolaus B, Di Donato P, Poli A, Oner ET, Finore I. A raw starch digesting a-amylase from the thermophilic Anoxybacillus amylolyticus. Purification and characterization. New Biotechnol. 2009; 25: 91-92.-77 Ozdemir S, Güven K, Baysal Z, Uyar F. Screening of various organic substrates and the development of a suitable low-cost fermentation medium for the production of a-amylase by Bacillus subtilis. Food Technol Biotechnol. 2009; 47: 364-369..
Starch-hydrolyzing enzymes as amylase play an important role in carbohydrate metabolisms. It is a well-known fact that α-amylases degrade the starches. They can also be utilized to form a variety of major products with various physical and chemical qualities for foods and pharmaceuticals 88 Ozdemir, S., Matpan, F., Guven, K., Baysal, Z., Production and characterisation of partially purified extracellular thermostable a-amylase by Bacillus subtilis in Submerged fermentation (SmF). Prep. Biochem. Biotechnol. 2011, 41, 365-381..
As native starch is not dissolve in water at low temperature, many conventional processes are carried out at high temperature and pressure for gelatinization of the raw starch. These processes require an extreme-energy input, so the production cost of starch-based products is increased. To decrease the starch processing cost, effective utilization of natural resources and viscosity problems, direct hydrolysis of starch at low temperature is desirable 99 Kelly CT, Tigue MM, Doyle EM, Fogarty WM. The raw starch degrading alkaline amylase of Bacillus sp. IMD 370. J Ind Microbiol 1995; 15:446-448.. The significance of enzymatic saccharification of raw starch without heating has become well recognized due to energy savings and low cost of starch processing 1010 Itkor P, Tsukagoshi N, Udaka S. Purification and properties of divalent cation-dependent raw starch digesting a-amylase from Bacillus sp. B. 1018. J Ferment Bioeng 1989; 68:247-251.
11 Singh H, Soni SK. Production of starch gel digesting amyloglucosidase by Aspergillus oryzae HS-3 in solid state fermentation. Process Biochem. 2001; 37:453-459.-1212 Shiau JR, Hung HC, Jeang CL. Improving the thermostability of raw starch digesting amylase from a Cytophaga sp. by site directed mutagenesis. Appl Environ Microbiol 2003; 69:2383-2385.. This has generated the discovery of some raw starch degrading enzymes (RSDE) that can directly hydrolize raw starch below the gelatinization temperature of starch 1313 Sun HY, Zhao PJ, Ge XY, Xia YJ, Hao ZK, Liu JW, Peng M. Recent advances in microbial raw starch degrading enzymes. Appl. Biochem. Biotechnol. 2010: 160; 988-1003.. In recent years, a worldwide interest has been focused on the raw starch digesting amylases, which would be of value to simplify the process of starch conversion.
The most widely utilized thermostable α-amylases are obtained from mesophilic and thermophilic microorganisms which are considered to be potential sources of thermostable α-amylases of industrial significance 1414 Ozdemir S, Matpan F, Okumus V, Dündar A, Ulutas MS, Kumru M. Isolation of a thermophilic Anoxybacillus flavithermus sp nov. and production of thermostable a-amylase under solid-state fermentation (SSF). Annals Microbiol. 2012; 62: 1367-1375.. They have been indicated from a large range of microorganisms from a few species of genus Bacillus and Streptomyces1515 Xie F, Quan S, Liu D, Ma H, Li F, Zhou F, Chen G. Purification and characterization of a novel a-amylase from a newly isolated Bacillus methylotrophicus strain P11-2. Process Biochem. 2014; 49: 47-53.. Bacillus is usually utilized for the commercial production of thermostable α-amylases.
The present research reports the production, purification and biochemical characterizations of an α-amylase obtained from Bacillus mojavensis SO-10. This purified α-amylase displayed attractive characteristics such as temperature and pH stability and chemical agents’ resistance. In addition, the purified amylase was utilized in some industrial starch processes like raw starch hydrolyzing, detergents and apple juice clarification.
MATERIALS AND METHODS
Culture conditions
Bacillus mojavensis SO-10 were grown in 250 mL glass bottle containing 50 mL Nutrient Broth (NB) media. The culture media’s pH was adjusted with 0.1 M HCl or NaOH to pH 7.0, and glass bottles were autoclaved at 121 ºC for 15 minutes. After being autoclaved, every bottle was inoculated with 0.5 mL (3.1×108 CFU/mL) of cell suspension. The fermentation media was cultured at 35 ºC on a shaker at 120 rpm.
Enzyme activity
α-Amylase activity was studied by determining the quantity of reducing sugars using 3, 5 dinitrosalicylic acid (DNS) process 1616 Bernfeld P. Enzymes carbohydrate metabolism. In Methods In Enzymol Academic Pres. 1955; 17: 149-158.. According to this process, 0.5% starch solution (200 µL) was incubated with 100 µL crude enzyme solution or 5 µL purified enzyme for 30 min at 70 °C. The reaction was stopped by adding DNS reagent and reaction solution was boiled for 5 min. After leaving it for cooling at room temperature, 3 mL of pure water was added to the reaction mixture and spectrophotometric determination was measured at 489 nm. One unit of α-amylase activity was determined as the quantity of enzyme that produced 1 mmol of reducing sugars maltose per minute.
Impact of incubation time on α-amylase production
The influence of various time courses on enzyme production was assayed at 35 ºC in NB at pH 6.0 and 35 ºC in a shaker at 120 rpm. A 2 mL of fermentation culture media was collected at various incubation times (0-96 h). Then, the media was centrifuged at 7.000 rpm for 10 min. The upper solution was tested for determination of α-amylase activity according to DNS process.
Influence of fermentation temperature and pH on enzyme production
The sterile NB fermentation media was incubated at different temperature between 20 to 55 ºC at pH 7.0 and 120 rpm in a shaker for 36 hours and enzyme assay was evaluated as mentioned above. The optimum fermentation media’s pH for production of α-amylase was examined at pH ranges of 4.0, 5.0, 6.0, 7.0, 8.0, 9.0, and 10.0 on optimum culture conditions. After incubation, fermentation media was centrifuged and the upper solution was tested for enzyme activity.
Purification of α-amylase
The crude enzyme was exposed to (NH4)2SO4 up to 80% (w/v) saturation and allowed to precipitate at 4 °C. The precipitates were centrifuged at 10.000 rpm for 15 min. The precipitate was dispersed in 0.1 M potassium phosphate buffer (pH 7.0), and dialyzed overnight at 4 °C. The dialyzed enzyme solution was applied to a DEAE (Diethylaminoethyl) cellulose (DE 32) column (flow rate 15 mL/ h).
Protein analysis on SDS-PAGE
The purity of α-amylase was determined on SDS-PAGE according to Laemmli process utilizing 10% polyacrylamide gel 1717 Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriphage T4. Nature. 1970; 277: 680-685..
Determination of kinetic parameters
The purified α-amylase was tested at different starch concentrations from 0.5% to 2% for determination of Km and Vmax. The Km and Vmax were calculated from the reciprocal plot of starch concentration (S) versus velocity (V).
Influence of temperature on purified α-amylase activity and stability
The purified enzyme activity at various temperatures was studied by incubating the reaction mixture at temperature ranging between 20 oC to 90 oC. The temperature stability of purified enzyme was also experimented by pre-incubating the enzyme between 40-70 oC for 30, 60, 90, 120, 150, and 180 min and the remaining purified α-amylase activity was determined.
Influence of pH and pH stability
The optimal pH of the enzyme was experimented by incubating the enzyme reaction solution at pH (3.0, 4.0, 5.0, 6.0, 7.0, 8.0, 9.0, 10.0, and 11.0). For measuring pH stability of purified α-amylase, the enzyme was pre-incubated between various pH ranges of 4.0 to 8.0 for 30-240 min. The purified α-amylase activity in standard reaction mixture was used as control.
Effects of inhibitors and different metal ions on purified enzyme activity
The effect of different metal ions such as MnCI2, ZnCI2, MgCI2, CuCI2, CaCI2, FeCI2, HgCI2 and various inhibitors such as EDTA (ethylenediaminetetraacetic acid), PMSF (phenylmethanesulfonyl fluoride), β-mercaptoethanol and DTT (dithiothreitol) on purified α-amylase was studied. The purified α-amylase was pre-incubated with 1.5 mM metal ions and 1, 5 and 10 mM inhibitors for 30 min at 37 °C and the standard α-amylase activity assay was carried out.
Thin layer chromatography (TLC)
The purified α-amylase was incubated with 1% soluble wheat starch to determine the end-products of hydrolyzed soluble starches at various incubation time (15, 30 and 240 min). 3 μL of end products of reaction were spotted on TLC plate (Merck). The end products of reaction were examined by pre-coated TLC plate with the solvent system of n-butanol/methanol/H2O (8:4:3, v/v/v). Spots were visualized by spraying a sulfuric acid/methanol solution (1:7, v/v), drying and heating at 95 ºC for 10 min.
Industrial applications of Purified α-amylase
Influence of detergents on purified α-amylase activity
Various detergent solutions (Omo, Tursil, and Alo) were prepared at 0.5% concentration. In this test, 50 µL detergent solution, 200 µL starch, and 10 µL α-amylase were mixed and incubated from 15 min to 120 min 8. The enzyme activity was assayed according to the DNS method as mentioned above.
Raw starch degrading
Wheat and corn raw starch grains were cleaned 3 times with distilled water to eliminate contaminants. The grains were dried and 1% of raw wheat and corn starch grain was suspended in 980 µL of 0.1M buffer (citric acid) at pH 6.0 and 10 µL of purified α-amylase was added. The purified α-amylase was incubated with wheat and corn raw starch grains at 50 oC for 4 h, and reaction solution was centrifuged. The upper solutions were examined to define released reduce sugars with DNS assay with maltose as the standard. The percentage of degrading of raw starch (Rh) was calculated by the following formula:
Rh (%) = (A1/A0) × 100,
where A1 refers to the quantity of reducing sugar in the upper after the degrading, and A0 refers to the quantity of raw starch before the degrading 1818 Mitsuiki S, Mukae K, Sakai M, Goto M, Hayashida S, Furukawa K. Comparative characterization of raw starch hydrolyzing a-amylases from various Bacillus strains. Enzyme Microb Technol. 2005; 37: 410-416.. After 4 h incubation, hydrolyzed starch of wheat and corn grains were cleaned with pure ethanol and dried. The dried starch grains were treated with iodine solution and photographed under the Olympus CX31 light microscope 400 times magnification.
The starch amounts of immature apple juices (green and red) and theirs clarification by purified enzyme
The detection of starch contents in immature apple juice was experimented according to the method defined by Carrín et al. 1919 Carrín ME, Ceci LN, Lozana JE. Characterization of starch in apple juice and its degradation by amylases. Food Chem. 2004; 87: 173-178., and the usage of purified alpha amylase in the apple juice clarification industry was assayed.
RESULTS AND DISCUSSION
Impact of time courses, temperature, and pH on α-amylase production
We tested the impact of fermentation course, temperature, and pH on α-amylase production. It is well known that short incubation time is important for cheap production of enzyme in fermentation industry. The production of α-amylase is demonstrated in Fig. 1. The production of the enzyme increased when the incubation time increased up to 36th. The maximum α-amylase production was achieved with 36th (Fig. 1a) and the enzyme production decreased after 36th. After this incubation period, there was a decrease in the production of α-amylase. This is because the cells have reached the decline phase and displayed low α-amylase synthesis. The decrease in the enzyme production may be due to consumption of the nutrients in the fermentation culture or due to accumulation of toxic by-products. The fermentation temperature and pH were also significant parameters, which clearly affect enzyme production. The fermentation temperature and pH were tested between 20 to 55 °C and 4.0 to 10.0 respectively in an incubator shaker to define the temperature and pH influences on α-amylase production. The highest α-amylase production was determined at 35 °C. When the fermentation temperature went up higher than 35 °C, α-amylase production diminished (Fig. 1b). The enzyme yield reduced 87.3%, while the fermentation temperature increased from 35 to 50 °C. The α-amylase activity found to be increasing as the fermentation pH rose from 4.0 to 7.0 (Fig. 1c). This was followed by a slight reduction in α-amylase activity above the 7.0. The highest enzyme activity was recorded at pH ranges of 6.0-7.0. The optimum fermentation conditions for α-amylase production were recorded at 36th hour, 35ºC and pH 6.0-7.0 by B. mojavensis SO-B11.
Purification of α-amylase
The enzyme purification steps were (NH4)2SO4 precipitation (80%), dialyzed and DEAE-cellulose ion-exchange chromatography, respectively. The purification fold and yield were determined as 34% and 18 % respectively completion of purification steps. The purification results are represented in Table 1. The α-amylase secreted by Geobacillus stearothermophilus was purified to homogeneity (65-fold and 46% yield) through a series of steps 2020 Aguloglu Fincan S, Enez B. Production, purification, and characterization of thermostable a-amylase from thermophilic Geobacillus stearothermophilus. Starch/Stärke. 2014; 66: 182-189.. Recently, Xie et al. 1515 Xie F, Quan S, Liu D, Ma H, Li F, Zhou F, Chen G. Purification and characterization of a novel a-amylase from a newly isolated Bacillus methylotrophicus strain P11-2. Process Biochem. 2014; 49: 47-53. have indicated 13.1 purification fold and 7.0% yield, Abdel-Fattah et al. 2121 Abdel-FattahYR, Soliman NA, El-Toukhy NM, El-Gendi H, Ahmed RS, Production, Purification and Characterization of Termostable a-amilase Produced by Bacillus licheniformis Isolate AI20. Journal Chemistry. 2012; 2013: 1-11. have pointed out 59.3 purification fold and 12.6 % yield, and Shukla and Singh 2222 Shukla RJ, Singh SP. Production optimization, purification and characterization of a-amylase from thermophilic Bacillus licheniformis TSI-14. Starch/Stärke. 2015; 67: 629-639. have reported 37 purification fold and 16.8% yield using chromatography methods.
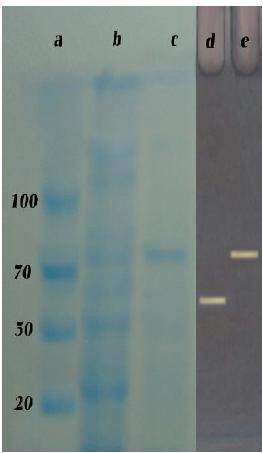
Molecular mass determination on SDS-PAGE
The molecular mass of the purified enzyme which appeared as a single band on SDS-PAGE was found to be 73 kDa (Fig. 2). Murakami et al. 2323 Murakami S, Nishimoto H, Toyama Y, Shimamoto E, Takenaka S, Kaulpiboon J, Prousoontorn M, Limpaseni T, Pongsawasdi P, Aoki K. Purification and characterization of two alkaline, thermotolerant a-amylases from Bacillus halodurans 38C-2-1 and expression of the cloned gene in Escherichia coli. Biosci Biotechnol Biochem. 2007; 71: 2393-2401. reported α-amylases having molecular masses of 105 and 75 kDa, respectively. Asoodeh et al. 2424 Asoodeh A, Chamani JK, Lazgian M. A novel thermostable, acidophilic a-amylase from a new thermophilic "Bacillus sp. Ferdowsicous" isolated from Ferdows hot mineral spring in Iran: Purification and biochemical characterization. Int J Biol Macromol. 2010; 46: 289-297. determined the molecular weight of α-amylase as 68.9 kDa. Michelin et al. 2525 Michele Michelin TM, Silva VM, Benassi SC, Peixoto-Nogueira LAB, Moraes JM, Leão JA, Terenzi HF, Polizeli Mde L. Purification and characterization of a thermostable a-amylase produced by the fungus Paecilomyces variotii. Carbohyd Res. 2010; 345: 2348-2353. demonstrated amylase as a single band of about 75 kDa by SDS-PAGE. Another α-amylase with molecular weight of 70 kDa was indicated by Zafar et al. 2626 Zafar A, Aftab MN, Ud Din Z, Aftab S, Iqbal I, Ul Haq I. Cloning, Purification and Characterization of a Highly Thermostable Amylase Gene of Thermotoga petrophila into Escherichia coli. Appl Environ Microbiol. 2015; 2: 1-18.. Different molecular masses of the α-amylases from various Bacillus sp. ranging from 42 to 150 kDa have been reported 2727 Pandey A. Nigam P., Soccol C.R. Soccol V.T. Singh D. & Mohan R. Advances in microbial amylases. Biotechnol Appl Biochem. 2000;31: 135-152..
Molecular mass determination on SDS-PAGE. Lane a: standard proteins; Lane b: after dialyze c: purified enzyme from DEAE cellulose; Native-PAGE (using iodine stain) Lane d: standard a-amylase; Lane e: purified enzyme from DEAE cellulose.
Determination of kinetic parameters
Kinetic studies of α-amylase were determined using soluble starch as substrate. The Km and Vmax values were estimated from a Lineweaver-Burk plot (Fig. 3). Values of V max and Km for the purified enzyme were 0,007964 mM and 2, 77 µmol min−1 respectively. Aguilar et al. 2828 Aguilar G, Morlon-Guyot J, Trejo-Aguilar B, Guyot JP. Purification and characterization of an extracellular a-amylase produced by Lactobacillus manihotivorans LMG 18010T, an amylolytic lactic acid bacterium. Enzyme Microb Technol. 2000; 27: 406-413. declared the Km value of α-amylase as 3.44 mg/mL. Aguloglu Fincan et al. 2929 Aguloglu Fincan S, Enez B, Ozdemir S, Matpan Bekler F. Purification and characterization of thermostable a-amylase from thermophilic Anoxybacillus flavithermus. Carbohydr Polym. 2014; 102: 144-150. determined Km and Vmax as 0.005 mM and 3.5 µmol min−1, respectively.
Impact of temperature on purified amylolytic activity and stability
The impact temperature on amylolytic activity is shown on Fig. 4a, and the residual activities of purified enzyme were found as 37.1%, 70.9%, 82.7%, 90.3% and 97.6% at 20, 30, 40, 50, and 60 °C, respectively. The purified amylase produced from B. mojavensis SO-B11 exhibited optimal activity at 70 °C. The results revealed that the relative amylolytic activity sharply declined from 82.3% to 24.4% with increasing temperature from 80 °C to 90 °C, respectively. The optimum temperature for α-amylase from Bacillus sp. ferdowsicou2424 Asoodeh A, Chamani JK, Lazgian M. A novel thermostable, acidophilic a-amylase from a new thermophilic "Bacillus sp. Ferdowsicous" isolated from Ferdows hot mineral spring in Iran: Purification and biochemical characterization. Int J Biol Macromol. 2010; 46: 289-297., Bacillus subtilis88 Ozdemir, S., Matpan, F., Guven, K., Baysal, Z., Production and characterisation of partially purified extracellular thermostable a-amylase by Bacillus subtilis in Submerged fermentation (SmF). Prep. Biochem. Biotechnol. 2011, 41, 365-381. and Anoxybacillus flavithermus1414 Ozdemir S, Matpan F, Okumus V, Dündar A, Ulutas MS, Kumru M. Isolation of a thermophilic Anoxybacillus flavithermus sp nov. and production of thermostable a-amylase under solid-state fermentation (SSF). Annals Microbiol. 2012; 62: 1367-1375. were found as 70, 60, and 70 °C, respectively. This reveals that the optimum temperature of purified amylase obtained from B. mojavensis SO-B11 showed well parallelism with these studies. Thermal stability of the purified enzyme produced by B. mojavensis was examined by incubating the enzyme at different temperatures (30-180 min). The results of thermal stability are represented in Fig. 4b. The purified enzyme was steady at temperature of 40 °C and 50 °C up to 180 min. The 87.1% and 85.7% of residual enzyme activity was retained after 30 min of incubation at the temperature of 60 °C and 70 °C, respectively. According to the results obtained from thermal stability of purified α-amylase, the enzyme can be applied in brewing and food processing.
Effect of pH on purified α-amylase activity and stability
The pH profile of the purified enzyme activity is depicted in Fig. 5a. As shown in Fig. 5a, the amylolytic activity is nearly the same at pH 5.0-7.0. The amylolytic activity sharply decreased from pH 4.0 to 3.0 and from pH 8.0 to 9.0. The purified α-amylase showed optimum activity at pH 5.0-6.0. Parallel results were found by some researchers such as Hamilton et al. 3030 Hamilton LM, Kelly CT, Fogarty WM. Production and properties of the starch-digesting a-amylase of Bacillus sp. IMD 435. Process Biochem. 1999; 35: 27-31., Sarikaya and Gürgün 3131 Sarikaya E, Gürgün S. Increase of the a-Amylase Yield by Some Bacillus Strains. Turkish J Biol. 2000; 24: 299-308. and Kikani and Singh 3232 Kikani BA., Singh SP. The stability and thermodynamic parameters of a very thermostable and calcium-independent a-amylase from a newly isolated bacterium, anoxybacillus beppuensis TSSC-1. Process Biochem. 2012; 47: 1791-1798.. The retained enzyme activity was about 96.2% at pH 7.0. The purified enzyme pH stability experimental results are depicted in Fig. 5b. The amylolytic activities did not significantly change between pH ranges of 4.0-8.0 for 120 min. The retained α-amylase activities obtained from B. mojavensis SO-B11 were defined as 84.1%, 86.1%, 89.2%, 88.2% and 83.8% at pH 4.0, 5.0, 6.0, 7.0, and 8.0 respectively for 120 min. These results indicated that purified novel enzyme can be utilized in starch saccharification and detergent industries.
Effect of Different Cations and Inhibitors on Purified α-Amylase Activity
The influences of Zn2+, Mg2+, Hg2+, Mn2+, Ca2+, Cu2+ and Fe2+ and different inhibitors on purified α-amylase activity are depicted in Table 2. When compared with the control group, the enzyme activities in presence of Zn2+, Mg2+, Hg2+, Mn2+, Ca2+, Cu2+, and Fe2+ were determined as 82%, 86%, 38%, 119%, 121%, 79%, and 94% respectively. The purified enzyme was activated by Ca2+ and Mn2+, but slightly inhibited by Fe2+, Zn2+, Mg2+, and Cu2+. Lin et al. 3333 Lin LL, Chyau CC, Hsu WH. Production and properties of a raw-strach degrading amylase from thermophilic and alkaliphilic Bacillus sp. TS-23. Biotechnol Appl Biochem. 1998; 28: 61-68.suggested that enzyme was activated with Ca2+, while it was inhibited in the presence of Hg2+. The effect of various metal ions on purified α-amylase activity was reported by Mamo and Gessesse 3434 Mamo G, Gessesse A. Purification and characterization of two rawstarch-digesting thermostable a-amylases from a thermophilic Bacillus. Enzyme Microb Technol. 1999; 25: 433-438., Aguloglu Fincan and Enez 2020 Aguloglu Fincan S, Enez B. Production, purification, and characterization of thermostable a-amylase from thermophilic Geobacillus stearothermophilus. Starch/Stärke. 2014; 66: 182-189., and Liu et al. 3535 Liu J., Weiguang X, Abdullahi A, Fan WQA, Dingyuan F, Jianjun Z. Purification and Partial Characterization of an Acidic a-Amylase from a Newly Isolated Bacillus subtilis ZJ-1 that may be Applied to Feed Enzyme. Prep Biochem Biotechnol. 2015; 45: 259-267.. Our results are very close to their studies. The highest inhibitory effect was obtained with EDTA. This inhibition can reveal that the purified α-amylase produced by B. mojavensis SO-B11 is a metalloenzyme. Our results completely match with the results obtained by other investigations on the α-amylase 1515 Xie F, Quan S, Liu D, Ma H, Li F, Zhou F, Chen G. Purification and characterization of a novel a-amylase from a newly isolated Bacillus methylotrophicus strain P11-2. Process Biochem. 2014; 49: 47-53.,3636 Chakraborty S, Khopade A, Kokare C, Mahadik K, Chopade B. Isolation and characterization of novel a-amylase from marine Streptomyces sp. D1. J Mol Catal B: Enzym. 2009; 58: 17-23.,3737 Hmidet N, Bayoudh A, Berrin JG, Kanoun S, Juge N, Nasri M. Purification and biochemical characterization of a novel a-amylase from Bacillus licheniformis NH1 Cloning, nucleotide sequence and expression of amyN gene in Escherichia coli. Process Biochem. 2008; 43: 499-510.. PMSF and β-mercaptoethanol did not show important influence on the purified α-amylase activity. This can make it obvious that serine residues and disulfide bonds are not essential for the amylolytic activity 3838 Shafiei M., Ziaee AA, Amoozegar MA. Purification and biochemical characterization of a novel SDS and surfactant stable, raw starch digesting, and halophilic a-amylase from a moderately halophilic bacterium, Nesterenkonia sp. strain. Process Biochem. 2010; 45: 694-699..
Determination of the end products of soluble starch by TLC
The purified α-amylase was incubated with soluble starch for 15, 30 and 240 min. It is seen from the Fig. 6 that the purified α-amylase produced by Bacillus mojavensis SO-10 hydrolyzed the soluble starch. There were various end products after hydrolysis of soluble starch by amylase. Maltose (G2), maltotriose (G3), maltotetraose (G4), and maltooligosaccharides occurred after 15 min and glucose (G1), maltose (G2), maltotriose (G3), maltotetraose (G4), and maltooligosaccharides after 30 min. After 240 min, whole soluble starch hydrolyzed into (G1). Parallel finding was described by Takasaki 39. These results described that purified α-amylase can be used in starch liquefying industry or food industries because of the end products of (G1), (G2), (G3), (G4), and maltooligosaccharides.
End products of soluble starch by Thin-layer Chromatography TLC. Lane A, glucose (G1), maltose (G2), maltotriose (G3) and maltotetraose (G4); Lane B-D, reaction time for 15 min, 30 min and 240 min, respectively.
Industrial applications of purified α-amylase
The capable of raw starch hydrolyzing of corn and wheat by purified α-amylase
Purified α-amylase capable of hydrolyzing raw starch was examined by defining the percentages of hydrolyzing of corn and wheat starch grains. After 4 h of incubation, the purified α-amylase hydrolyzed the raw wheat and corn grains in the ratio of 36.7% and 39.2% respectively. Parallel results were reported by Božić et al. 4040 Božić N, Ruiz J, Santίn JL, Vujćić Z. Production and properties of the highly efficient raw starch digesting a-amylase from a Bacillus licheniformis ATCC 9945. Biochem Eng J. 2011; 53: 203-209.and Hayashida et al. 4141 Hayashida S, Teramoto Y, Inoue T. Production and characteristics of raw potato-starch-digesting a-amylase from Bacillus subtilis 65. Appl Environ Microbiol. 1988; 54: 1516-1522.. Considering the literature, this is the first study to hydrolyze the raw starch by α-amylase obtained from B. mojavensis SO-B11. It is too important to degrade the raw starch grains under the gelatinization temperature of starch due to the economic approach. α-Amylase produced by B. mojavensis SO-B11 can be utilized for hydrolyzing of raw wheat and corn starch in various industries such as food, fermentation and gelatinization of starch. The hydrolyzed raw corn and wheat starch granules were monitored with Olympus CX31 light microscope (Fig. 7). The rate of corn and wheat starch grains decreased after 4 h of degrading, while the structure of starches grains were moderately damaged.
Raw starch hydrolyzing. (A) Corn starch before hydrolyzing; (B) Corn starch after hydrolyzing; (C) Wheat starch before hydrolyzing; (D) Wheat starch after hydrolyzing.
Influence of some commercial detergents and surfactants on purified enzyme activity
The influence of some surfactants and trading domestic washing detergents on the purified B. mojavensis α-amylase was experimented. As demonstrated in Fig. 8, the purified B. mojavensis α-amylase exhibited significant stability with surfactants and domestic washing detergents such as Omo (44.7%), Tursil (51.9%), Alo (63.1%), SDS (60.9%), Tween-10 (97.2%), Tween-40 (99.5) and Triton X-100 (98.2%) for 60 min and Omo (41.3%), Tursil (53.2%), Alo (73.8%), SDS (69.7%), Tween-10 (102.9%), Tween-40 (99.5%) and Triton X-100 (101.3%) for 120 min. Parallel findings were indicated by Goyal et al. 4242 Goyal N, Gupta JK, Soni SK. A novel raw starch digesting thermostable a-amylase from Bacillus sp. I-3 and its use in the direct hydrolysis of raw potato starch. Enzyme Microb Technol. 2005; 37: 723-734.and Pathak and Rekadwad 4343 Pathak AP, Rekadwad BN. Isolation of thermophilic Bacillus sp. strain EF_TYK1-5 and production of industrially important thermostable a-amylase using suspended solids for fermentation. J Sci Ind Res. 2013; 72: 685-689..
The starch amounts of immature apple juices (green and red) and theirs clarification by purified enzyme
Immature starch amounts of red and green apple were tested by using process of Carrín et al. 1919 Carrín ME, Ceci LN, Lozana JE. Characterization of starch in apple juice and its degradation by amylases. Food Chem. 2004; 87: 173-178.. The starch amounts of immature red apple were determined as 0.59 g/L for soluble starch and 3.87 g/L for insoluble starch and the starch amounts of immature green apple were determined as 0.52 g/L for soluble starch and 3.68 g/L for insoluble starch. Digesting of soluble starch of immature red and green apple was experimented for 30, 45, and, 60 min. The degrading percentages of soluble starch contents of red apple and green apple were found as 61% and 58%, 74% and 71%, 85%, and 80% for 30, 45, and 60 min, respectively. The finding results demonstrated that purified α-amylase should be applied in apple juice industry.
CONCLUSIONS
A thermo and pH stable α-amylase were produced, purified, characterized and utilized for various starch industries. The amylolytic activity did not meaningfully change between pH ranges of 4.0-8.0 and temperature degrees of 40, 50, and 60 °C for 120 min. The purified enzyme exhibited stability in the presence of surfactants and metal ions. The purified enzyme obtained from B. mojavensis SO-B11 was capable of hydrolyzing wheat and corn raw starch granules. G1, G2, G3, G4, and maltooligosaccharides were obtained after TLC analysis. In addition, the newly purified alpha amylase should be used in domestic washing detergents and apple juice clarification industries.
ACKNOWLEDGEMENTS
This study was supported by Scientific Research Projects Unit of Siirt University (Project code: BAP-2011-SIUFED-F3), Turkey.
REFERENCES
-
1Gupta R, Gigras P, Mohapatra H, Goswami VK, Chauhan B, Microbial a-amylases: A biotechnological perspective. Process Biochem. 2003; 38: 1599-1616.
-
2Roy I, Gupta MN, Hydrolysis of starch by a mixture of glucoamylse and pullulanase entrapped individually in calcium alginate beads. Enzyme Microb. Technol, 2004; 34: 26-32.
-
3Balkan B, Ertan F. Production of a-Amylase from Penicillium chrysogenum Under Solid State Fermentation by Using Some Agricultural By-Product. Food Technol. Biotechnol. 2007; 45: 439-442.
-
4Kumagai Y, Satoh T, Inoue A, Ojima T. Enzymatic properties and primary structures of two a-amylase isozymes from the Pacific abalone Haliotis discus hannai. Comp Biochem Physiol B: Biochem Mol Biol. 2013; 164: 80-88.
-
5Wiseman A. The application of enzyme in industry. Handbook Enzyme Biotechnol. 1987; 3: 275-373.
-
6Lama L, Nicolaus B, Di Donato P, Poli A, Oner ET, Finore I. A raw starch digesting a-amylase from the thermophilic Anoxybacillus amylolyticus. Purification and characterization. New Biotechnol. 2009; 25: 91-92.
-
7Ozdemir S, Güven K, Baysal Z, Uyar F. Screening of various organic substrates and the development of a suitable low-cost fermentation medium for the production of a-amylase by Bacillus subtilis. Food Technol Biotechnol. 2009; 47: 364-369.
-
8Ozdemir, S., Matpan, F., Guven, K., Baysal, Z., Production and characterisation of partially purified extracellular thermostable a-amylase by Bacillus subtilis in Submerged fermentation (SmF). Prep. Biochem. Biotechnol. 2011, 41, 365-381.
-
9Kelly CT, Tigue MM, Doyle EM, Fogarty WM. The raw starch degrading alkaline amylase of Bacillus sp. IMD 370. J Ind Microbiol 1995; 15:446-448.
-
10Itkor P, Tsukagoshi N, Udaka S. Purification and properties of divalent cation-dependent raw starch digesting a-amylase from Bacillus sp. B. 1018. J Ferment Bioeng 1989; 68:247-251.
-
11Singh H, Soni SK. Production of starch gel digesting amyloglucosidase by Aspergillus oryzae HS-3 in solid state fermentation. Process Biochem. 2001; 37:453-459.
-
12Shiau JR, Hung HC, Jeang CL. Improving the thermostability of raw starch digesting amylase from a Cytophaga sp. by site directed mutagenesis. Appl Environ Microbiol 2003; 69:2383-2385.
-
13Sun HY, Zhao PJ, Ge XY, Xia YJ, Hao ZK, Liu JW, Peng M. Recent advances in microbial raw starch degrading enzymes. Appl. Biochem. Biotechnol. 2010: 160; 988-1003.
-
14Ozdemir S, Matpan F, Okumus V, Dündar A, Ulutas MS, Kumru M. Isolation of a thermophilic Anoxybacillus flavithermus sp nov. and production of thermostable a-amylase under solid-state fermentation (SSF). Annals Microbiol. 2012; 62: 1367-1375.
-
15Xie F, Quan S, Liu D, Ma H, Li F, Zhou F, Chen G. Purification and characterization of a novel a-amylase from a newly isolated Bacillus methylotrophicus strain P11-2. Process Biochem. 2014; 49: 47-53.
-
16Bernfeld P. Enzymes carbohydrate metabolism. In Methods In Enzymol Academic Pres. 1955; 17: 149-158.
-
17Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriphage T4. Nature. 1970; 277: 680-685.
-
18Mitsuiki S, Mukae K, Sakai M, Goto M, Hayashida S, Furukawa K. Comparative characterization of raw starch hydrolyzing a-amylases from various Bacillus strains. Enzyme Microb Technol. 2005; 37: 410-416.
-
19Carrín ME, Ceci LN, Lozana JE. Characterization of starch in apple juice and its degradation by amylases. Food Chem. 2004; 87: 173-178.
-
20Aguloglu Fincan S, Enez B. Production, purification, and characterization of thermostable a-amylase from thermophilic Geobacillus stearothermophilus. Starch/Stärke. 2014; 66: 182-189.
-
21Abdel-FattahYR, Soliman NA, El-Toukhy NM, El-Gendi H, Ahmed RS, Production, Purification and Characterization of Termostable a-amilase Produced by Bacillus licheniformis Isolate AI20. Journal Chemistry. 2012; 2013: 1-11.
-
22Shukla RJ, Singh SP. Production optimization, purification and characterization of a-amylase from thermophilic Bacillus licheniformis TSI-14. Starch/Stärke. 2015; 67: 629-639.
-
23Murakami S, Nishimoto H, Toyama Y, Shimamoto E, Takenaka S, Kaulpiboon J, Prousoontorn M, Limpaseni T, Pongsawasdi P, Aoki K. Purification and characterization of two alkaline, thermotolerant a-amylases from Bacillus halodurans 38C-2-1 and expression of the cloned gene in Escherichia coli. Biosci Biotechnol Biochem. 2007; 71: 2393-2401.
-
24Asoodeh A, Chamani JK, Lazgian M. A novel thermostable, acidophilic a-amylase from a new thermophilic "Bacillus sp. Ferdowsicous" isolated from Ferdows hot mineral spring in Iran: Purification and biochemical characterization. Int J Biol Macromol. 2010; 46: 289-297.
-
25Michele Michelin TM, Silva VM, Benassi SC, Peixoto-Nogueira LAB, Moraes JM, Leão JA, Terenzi HF, Polizeli Mde L. Purification and characterization of a thermostable a-amylase produced by the fungus Paecilomyces variotii. Carbohyd Res. 2010; 345: 2348-2353.
-
26Zafar A, Aftab MN, Ud Din Z, Aftab S, Iqbal I, Ul Haq I. Cloning, Purification and Characterization of a Highly Thermostable Amylase Gene of Thermotoga petrophila into Escherichia coli. Appl Environ Microbiol. 2015; 2: 1-18.
-
27Pandey A. Nigam P., Soccol C.R. Soccol V.T. Singh D. & Mohan R. Advances in microbial amylases. Biotechnol Appl Biochem. 2000;31: 135-152.
-
28Aguilar G, Morlon-Guyot J, Trejo-Aguilar B, Guyot JP. Purification and characterization of an extracellular a-amylase produced by Lactobacillus manihotivorans LMG 18010T, an amylolytic lactic acid bacterium. Enzyme Microb Technol. 2000; 27: 406-413.
-
29Aguloglu Fincan S, Enez B, Ozdemir S, Matpan Bekler F. Purification and characterization of thermostable a-amylase from thermophilic Anoxybacillus flavithermus. Carbohydr Polym. 2014; 102: 144-150.
-
30Hamilton LM, Kelly CT, Fogarty WM. Production and properties of the starch-digesting a-amylase of Bacillus sp. IMD 435. Process Biochem. 1999; 35: 27-31.
-
31Sarikaya E, Gürgün S. Increase of the a-Amylase Yield by Some Bacillus Strains. Turkish J Biol. 2000; 24: 299-308.
-
32Kikani BA., Singh SP. The stability and thermodynamic parameters of a very thermostable and calcium-independent a-amylase from a newly isolated bacterium, anoxybacillus beppuensis TSSC-1. Process Biochem. 2012; 47: 1791-1798.
-
33Lin LL, Chyau CC, Hsu WH. Production and properties of a raw-strach degrading amylase from thermophilic and alkaliphilic Bacillus sp. TS-23. Biotechnol Appl Biochem. 1998; 28: 61-68.
-
34Mamo G, Gessesse A. Purification and characterization of two rawstarch-digesting thermostable a-amylases from a thermophilic Bacillus. Enzyme Microb Technol. 1999; 25: 433-438.
-
35Liu J., Weiguang X, Abdullahi A, Fan WQA, Dingyuan F, Jianjun Z. Purification and Partial Characterization of an Acidic a-Amylase from a Newly Isolated Bacillus subtilis ZJ-1 that may be Applied to Feed Enzyme. Prep Biochem Biotechnol. 2015; 45: 259-267.
-
36Chakraborty S, Khopade A, Kokare C, Mahadik K, Chopade B. Isolation and characterization of novel a-amylase from marine Streptomyces sp. D1. J Mol Catal B: Enzym. 2009; 58: 17-23.
-
37Hmidet N, Bayoudh A, Berrin JG, Kanoun S, Juge N, Nasri M. Purification and biochemical characterization of a novel a-amylase from Bacillus licheniformis NH1 Cloning, nucleotide sequence and expression of amyN gene in Escherichia coli. Process Biochem. 2008; 43: 499-510.
-
38Shafiei M., Ziaee AA, Amoozegar MA. Purification and biochemical characterization of a novel SDS and surfactant stable, raw starch digesting, and halophilic a-amylase from a moderately halophilic bacterium, Nesterenkonia sp. strain. Process Biochem. 2010; 45: 694-699.
-
39Takasaki Y. An amylase producing maltotriose from B. subtilis. Agric Biol Chem. 1985; 49: 1091-1097.
-
40Božić N, Ruiz J, Santίn JL, Vujćić Z. Production and properties of the highly efficient raw starch digesting a-amylase from a Bacillus licheniformis ATCC 9945. Biochem Eng J. 2011; 53: 203-209.
-
41Hayashida S, Teramoto Y, Inoue T. Production and characteristics of raw potato-starch-digesting a-amylase from Bacillus subtilis 65. Appl Environ Microbiol. 1988; 54: 1516-1522.
-
42Goyal N, Gupta JK, Soni SK. A novel raw starch digesting thermostable a-amylase from Bacillus sp. I-3 and its use in the direct hydrolysis of raw potato starch. Enzyme Microb Technol. 2005; 37: 723-734.
-
43Pathak AP, Rekadwad BN. Isolation of thermophilic Bacillus sp. strain EF_TYK1-5 and production of industrially important thermostable a-amylase using suspended solids for fermentation. J Sci Ind Res. 2013; 72: 685-689.
Publication Dates
-
Publication in this collection
2018
History
-
Received
03 Feb 2016 -
Accepted
14 July 2016