Abstract
Abstract
Platelet-activating factor (PAF) is a potent proinflammatory mediator that is produced in increased amounts in the lungs of asthmatic humans and horses. The present pilot study, shows that mesenchymal stromal cells can modulate alveolar macrophage function in asthma, interfering in the activity of PAF, being another potential pathway for mesenchymal stromal cells benefits in asthma.
Keywords:
Asthma; Horses; Lung; Platelet-activating factor; Pulmonary inflammation
INTRODUCTION
Platelet-activating factor (PAF) is a potent proinflammatory mediator that is produced in increased amounts in the lungs of asthmatic humans [11 Gabrijelcic J, Acuña A, Profita M, Paternò A, Chung KF, Vignola AM, Rodríguez-Roisin R. Neutrophil airway influx by platelet-activating factor in asthma: role of adhesion molecules and LTB4 expression. Eur Respir J. 2003;22:290-7.,22 Patel PS, Kearney JF. CD36 and Platelet-Activating Factor receptor promote house dust mite allergy development. J Immunol. 2017;199:1184-95.], horses [33 Fairbairn SM, Marr KA, Lees P, Cunningham FM. Effects of platelet activating factor on the distribution of radiolabelled leucocytes and platelets in normal horses and symptomatic horses with chronic obstructive pulmonary disease. Res Vet Sci. 1996;61:107–13.,44 Michelotto-Jr PV, Muehlmann LA, Zanatta AL, Bieberbach EWR, Fernandes LC, Nishiyama A. Platelet activating factor and evidence of oxidative stress in the bronchoalveolar fluid of Thoroughbred colts during race training. J Vet Intern Med 2010;24:414–9.], and laboratory animals [55 Muehlmann LA, Zanatta AL, Farias CLA, Bieberbach EW, Mazzonetto AC, Michellotto-Jr PV, Fernandes LC, Nishiyama A. Dietary supplementation with soybean lecithin increases pulmonary PAF bioactivity in asthmatic rats. J Nutr Biochem. 2010;21:532–7.]. During pulmonary inflammation, PAF can be responsible for microvascular leakage [66 Jiang Y, Wen K, Zhou X, Schwegler-Berry D, Castranova V, He P. Three-dimensional localization and quantification of PAF-induced gap formation in intact venular microvessels. Am J Physiol Heart Circ Physiol. 2008;295:H898-H906.], bronchoconstriction, increased airway responsiveness and eosinophilic chemotaxis [77 Ishii S, Nagase T, Shindou H, Takizawa H, Ouchi Y, Shimizu T. Platelet-activating factor receptor develops airway hyperresponsiveness independently of airway inflammation in a murine asthma model. J Immunol. 2004;172:7095–102.], and increased mucus secretion [55 Muehlmann LA, Zanatta AL, Farias CLA, Bieberbach EW, Mazzonetto AC, Michellotto-Jr PV, Fernandes LC, Nishiyama A. Dietary supplementation with soybean lecithin increases pulmonary PAF bioactivity in asthmatic rats. J Nutr Biochem. 2010;21:532–7.]. Moreover, PAF can provoke a pulmonary influx of neutrophils mediated by leukotriene B4 [11 Gabrijelcic J, Acuña A, Profita M, Paternò A, Chung KF, Vignola AM, Rodríguez-Roisin R. Neutrophil airway influx by platelet-activating factor in asthma: role of adhesion molecules and LTB4 expression. Eur Respir J. 2003;22:290-7.], and increased phagocytic activity of neutrophils, which result in interleukin (IL)-8 secretion, leading to exacerbated neutrophil chemotaxis to the lungs [88 Au B, Teixeira MM, Collins PD, Williams TJ. Blockade of PAF receptors controls interleukin-8 production by regulating the activation of neutrophil CD11/CD18. Eur J Pharmacol. 2001;425:65-71.].
PAF has also been shown to be involved in the pathophysiology of equine asthma, causing pulmonary influx of eosinophils and neutrophils, and increased adhesion and activation of neutrophils, thus impairing pulmonary function [33 Fairbairn SM, Marr KA, Lees P, Cunningham FM. Effects of platelet activating factor on the distribution of radiolabelled leucocytes and platelets in normal horses and symptomatic horses with chronic obstructive pulmonary disease. Res Vet Sci. 1996;61:107–13.,99 Marr KA, Lees P, Cunningham FM. Antigen challenge increases adherence of circulating neutrophils in horses with chronic obstructive pulmonary disease. Equine Vet J 2002;34:65-70.]. Alveolar macrophages (AM) are one of the sources of PAF. Meanwhile, PAF can interfere with AM functions; it augments AM adhesion, phagocytosis, and oxidant production in vitro [1010 Muehlmann LA, Michelotto-Jr PV, Nunes EA, Grando FCC, Silva FT, Nishiyama A. PAF increases phagocytic capacity and superoxide anion production in equine alveolar macrophages and blood neutrophils. Res Vet Sci 2012;93:393-7.]. Our group has previously demonstrated the presence of PAF in the bronchoalveolar lavage fluid (BALF) of young Thoroughbred (TB) racehorses in advanced race training, which was associated with evidence of oxidative stress and interfered with AM phagocytosis and oxidant production [44 Michelotto-Jr PV, Muehlmann LA, Zanatta AL, Bieberbach EWR, Fernandes LC, Nishiyama A. Platelet activating factor and evidence of oxidative stress in the bronchoalveolar fluid of Thoroughbred colts during race training. J Vet Intern Med 2010;24:414–9.].
Our research group focuses on establishing cell therapies for asthmatic humans and horses, as many individuals do not respond to current treatments using corticosteroids. We have recently demonstrated the usability of autologous bone marrow-derived mononuclear cells (BMMNCs) in horses with severe asthma [1111 Barussi FC, Bastos FZ, Leite LM, Fragoso FYI, Senegaglia AC, Brofman PRS, Nishiyama A, Pimpão CT, Michelotto-Jr PV. Intratracheal therapy with autologous bone marrow-derived mononuclear cells reduces airway inflammation in horses with recurrent airway obstruction. Respir Physiol Neurobiol 2016;232:35-42.], a model used to investigate severe neutrophilic asthma [1212 Vargas A, Roux-Dalvai F, Droit A, Lavoie JP. Neutrophil-derived exossomes: A new mechanism contributing to airway smooth muscle remodeling. Am J Respir Cell Mol Biol. 2016;55:450-61.]. Intratracheal instillation of BMMNCs resulted in decreased respiratory effort and neutrophils in the BALF, increased expression of the anti-inflammatory IL-10, and maintenance of AM function [1111 Barussi FC, Bastos FZ, Leite LM, Fragoso FYI, Senegaglia AC, Brofman PRS, Nishiyama A, Pimpão CT, Michelotto-Jr PV. Intratracheal therapy with autologous bone marrow-derived mononuclear cells reduces airway inflammation in horses with recurrent airway obstruction. Respir Physiol Neurobiol 2016;232:35-42.]. However, for clinical perspectives of allogeneic and ready-to-use treatments in humans and horses, it is important to understand the effects of mesenchymal stromal cells (MSCs) on the inflammatory process and the immune response in asthma.
Therefore, considering that AM orchestrate the pulmonary immune response, we designed a preliminary study to comparatively investigate the effects of BMMNCs and bone marrow derived MSCs (BMMSCs) on AM function. In addition, we aimed to investigate whether their mechanism(s) of immunomodulation would involve PAF inhibition.
MATERIAL AND METHODS
The BMMNCs used in the present study had been previously obtained [1111 Barussi FC, Bastos FZ, Leite LM, Fragoso FYI, Senegaglia AC, Brofman PRS, Nishiyama A, Pimpão CT, Michelotto-Jr PV. Intratracheal therapy with autologous bone marrow-derived mononuclear cells reduces airway inflammation in horses with recurrent airway obstruction. Respir Physiol Neurobiol 2016;232:35-42.] and were maintained in liquid nitrogen for approximately 45 months. The cells had a viability of 74% as indicated by trypan blue staining. This finding substantially exceeds our previously reported observation that BMMNCs maintain acceptable viability after five months of frozen storage [1313 Bastos FZ, Barussi FCM, Santi TF, Vieira BP, Senegaglia AC, Cruz FF, Michelotto Jr PV. Collection, processing and freezing of equine bone marrow cells. Cryobiology. 2017;78:95-100.]. Moreover, the BMMSCs used in the present study were successfully cultured from these BMMNCs, using an established protocol [1414 Rebelatto CK, Aguiar AM, Moretao MP, Senegaglia AC, Hansen P, Barchiki F, Oliveira J, Martins J, Kuligovski C, Mansur F, Christofis A, Amaral VF, Brofman PS, Goldenberg S, Nakao LS, Correa A. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med. 2008;233:901-13.]. The study was approved by the Ethical Committee on the Animal Use of the Pontifical Catholic University of Paraná (PUCPR; approval number 01217).
Briefly, BALF of two clinically healthy TB horses was aseptically collected and processed as previously described [44 Michelotto-Jr PV, Muehlmann LA, Zanatta AL, Bieberbach EWR, Fernandes LC, Nishiyama A. Platelet activating factor and evidence of oxidative stress in the bronchoalveolar fluid of Thoroughbred colts during race training. J Vet Intern Med 2010;24:414–9.] to obtain AM, maintained in phosphate-buffered saline (PBS). BMMNCs and BMMSCs were in Iscove's Modified Dulbecco's Medium (IMDM, Sigma). Cells were plated in quadruplicate in 96-well plates (1 × 105 cells/well) and incubated at 37°C for 1 h. Then, the cells were washed and divided into four treatment groups: non-treated AM, AM+PAF (100 nM/well), AM+PAF+1×105 BMMNCs/well, and AM+PAF+1×105 BMMSCs/well. The cells were subjected to assays for cell adhesion, phagocytosis [44 Michelotto-Jr PV, Muehlmann LA, Zanatta AL, Bieberbach EWR, Fernandes LC, Nishiyama A. Platelet activating factor and evidence of oxidative stress in the bronchoalveolar fluid of Thoroughbred colts during race training. J Vet Intern Med 2010;24:414–9.,55 Muehlmann LA, Zanatta AL, Farias CLA, Bieberbach EW, Mazzonetto AC, Michellotto-Jr PV, Fernandes LC, Nishiyama A. Dietary supplementation with soybean lecithin increases pulmonary PAF bioactivity in asthmatic rats. J Nutr Biochem. 2010;21:532–7.,1010 Muehlmann LA, Michelotto-Jr PV, Nunes EA, Grando FCC, Silva FT, Nishiyama A. PAF increases phagocytic capacity and superoxide anion production in equine alveolar macrophages and blood neutrophils. Res Vet Sci 2012;93:393-7.], nitrite (Griess Reagent; ThermoFisher Scientific, Waltham, MA, USA), and oxidant production (CellROX® Deep Red; ThermoFisher Scientific) using an ELISA plate reader (VersaMax®; Molecular Devices, Sunnyvale, CA, USA) at the relevant wavelengths. Cell adhesion was determined to normalize the other AM functions assayed in each group [1010 Muehlmann LA, Michelotto-Jr PV, Nunes EA, Grando FCC, Silva FT, Nishiyama A. PAF increases phagocytic capacity and superoxide anion production in equine alveolar macrophages and blood neutrophils. Res Vet Sci 2012;93:393-7.]. The data were analyzed using one-way ANOVA followed by the Tukey post-hoc test, with p < 0.05 considered significant.
RESULTS AND DISCUSSION
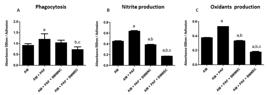
The results are shown in Figure 1. Consistently, PAF significantly increased phagocytosis as well as nitrite and oxidant production in the AM (p < 0.01). In the presence of BMMNCs, nitrite and oxidant production were significantly reduced to levels even lower than those in the AM group (p < 0.01); however, there was no difference in phagocytosis. Finally, in the presence of BMMSCs, all AM functions were significantly reduced, and the effects were significantly greater than those of BMMNCs (p < 0.0001).
Modulatory effect of BMMSCs on PAF-stimulated equine alveolar macrophages (AM). Non-treated AM (1 × 105 cells/well) were used as a control, PAF was used at 100 nM/well, BMMNCs or BMMSCs were additionally added at 1 × 105 cells/well. Prior to the treatments, AM were incubated with PAF at 37°C for 1 h. A. Phagocytosis of AM. aP = 0.002 vs. AM; bP = 0.002 vs. AM+PAF; cP = 0.001 vs. AM+PAF+BMMNCs. B. Nitrite production by AM. aP = 0.030 vs. AM; bP = 0.028 vs. AM+PAF; cP = 0.030 vs. AM+PAF+BMMNCs. C. Oxidant production by AM. aP < 0.0001 vs. AM; bP < 0.0001 vs. AM+PAF; cP < 0.0001 vs. AM+PAF+BMMNCs.
PAF can stimulate AM to eliminate inhaled particles from the airways [1010 Muehlmann LA, Michelotto-Jr PV, Nunes EA, Grando FCC, Silva FT, Nishiyama A. PAF increases phagocytic capacity and superoxide anion production in equine alveolar macrophages and blood neutrophils. Res Vet Sci 2012;93:393-7.]. However, in the case of pulmonary inflammation, PAF is produced uncontrolledly and in excess by the inflammatory cells because of tissue phospholipid oxidation due to oxidative stress [55 Muehlmann LA, Zanatta AL, Farias CLA, Bieberbach EW, Mazzonetto AC, Michellotto-Jr PV, Fernandes LC, Nishiyama A. Dietary supplementation with soybean lecithin increases pulmonary PAF bioactivity in asthmatic rats. J Nutr Biochem. 2010;21:532–7.]. The investigated TB horses, although clinically healthy, were in active race training and probably, the AM were in an active state, as previously demonstrated in a similar population [44 Michelotto-Jr PV, Muehlmann LA, Zanatta AL, Bieberbach EWR, Fernandes LC, Nishiyama A. Platelet activating factor and evidence of oxidative stress in the bronchoalveolar fluid of Thoroughbred colts during race training. J Vet Intern Med 2010;24:414–9.]. The BMMNCs modulated the airway immune response, reducing the production of oxygen and nitrogen reactive species by AM and maintaining AM phagocytosis in a non-activated status, in line with our previous observations, where AM phagocytosis decreased in asthmatic horses treated with BMMNCs [1111 Barussi FC, Bastos FZ, Leite LM, Fragoso FYI, Senegaglia AC, Brofman PRS, Nishiyama A, Pimpão CT, Michelotto-Jr PV. Intratracheal therapy with autologous bone marrow-derived mononuclear cells reduces airway inflammation in horses with recurrent airway obstruction. Respir Physiol Neurobiol 2016;232:35-42.]. The modulatory effects of BMMSCs on the Th1/Th2 airway immune response have been previously demonstrated in humans and involve positive interference with oxidative stress [1515 Nejad-Moghaddam A, Ajdary S, Tahmasbpour E, Rad FR, Panahi Y, Ghanei M. Immunomodulatory properties of mesenchymal stem cells can mitigate oxidative stress and inflammation process in human mustard lung. Biochem Genet. 2016;54:769-83.].
CONCLUSION
In conclusion, cell therapy immunomodulates equine AM activity, with BMMSCs showing better results than BMMNCs, and thus, has therapeutic potential for equine asthma.
Acknowledgments:
We are grateful for the scholarship from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Capes for supporting our research.
HIGHLIGHTS
-
• BMMNCs used in this study were viable after cryopreserved for 45 months.
-
• PAF stimulated alveolar macrophages’ actions.
-
• controlled by BMMNCs and BMMSCs.
-
• BMMSCs effects were significantly better than BMMNCs.
-
Funding: This work was supported by Fundação Araucária (Chamada Universal 05/2011, 22701 and 1058/2013).
REFERENCES
-
1Gabrijelcic J, Acuña A, Profita M, Paternò A, Chung KF, Vignola AM, Rodríguez-Roisin R. Neutrophil airway influx by platelet-activating factor in asthma: role of adhesion molecules and LTB4 expression. Eur Respir J. 2003;22:290-7.
-
2Patel PS, Kearney JF. CD36 and Platelet-Activating Factor receptor promote house dust mite allergy development. J Immunol. 2017;199:1184-95.
-
3Fairbairn SM, Marr KA, Lees P, Cunningham FM. Effects of platelet activating factor on the distribution of radiolabelled leucocytes and platelets in normal horses and symptomatic horses with chronic obstructive pulmonary disease. Res Vet Sci. 1996;61:107–13.
-
4Michelotto-Jr PV, Muehlmann LA, Zanatta AL, Bieberbach EWR, Fernandes LC, Nishiyama A. Platelet activating factor and evidence of oxidative stress in the bronchoalveolar fluid of Thoroughbred colts during race training. J Vet Intern Med 2010;24:414–9.
-
5Muehlmann LA, Zanatta AL, Farias CLA, Bieberbach EW, Mazzonetto AC, Michellotto-Jr PV, Fernandes LC, Nishiyama A. Dietary supplementation with soybean lecithin increases pulmonary PAF bioactivity in asthmatic rats. J Nutr Biochem. 2010;21:532–7.
-
6Jiang Y, Wen K, Zhou X, Schwegler-Berry D, Castranova V, He P. Three-dimensional localization and quantification of PAF-induced gap formation in intact venular microvessels. Am J Physiol Heart Circ Physiol. 2008;295:H898-H906.
-
7Ishii S, Nagase T, Shindou H, Takizawa H, Ouchi Y, Shimizu T. Platelet-activating factor receptor develops airway hyperresponsiveness independently of airway inflammation in a murine asthma model. J Immunol. 2004;172:7095–102.
-
8Au B, Teixeira MM, Collins PD, Williams TJ. Blockade of PAF receptors controls interleukin-8 production by regulating the activation of neutrophil CD11/CD18. Eur J Pharmacol. 2001;425:65-71.
-
9Marr KA, Lees P, Cunningham FM. Antigen challenge increases adherence of circulating neutrophils in horses with chronic obstructive pulmonary disease. Equine Vet J 2002;34:65-70.
-
10Muehlmann LA, Michelotto-Jr PV, Nunes EA, Grando FCC, Silva FT, Nishiyama A. PAF increases phagocytic capacity and superoxide anion production in equine alveolar macrophages and blood neutrophils. Res Vet Sci 2012;93:393-7.
-
11Barussi FC, Bastos FZ, Leite LM, Fragoso FYI, Senegaglia AC, Brofman PRS, Nishiyama A, Pimpão CT, Michelotto-Jr PV. Intratracheal therapy with autologous bone marrow-derived mononuclear cells reduces airway inflammation in horses with recurrent airway obstruction. Respir Physiol Neurobiol 2016;232:35-42.
-
12Vargas A, Roux-Dalvai F, Droit A, Lavoie JP. Neutrophil-derived exossomes: A new mechanism contributing to airway smooth muscle remodeling. Am J Respir Cell Mol Biol. 2016;55:450-61.
-
13Bastos FZ, Barussi FCM, Santi TF, Vieira BP, Senegaglia AC, Cruz FF, Michelotto Jr PV. Collection, processing and freezing of equine bone marrow cells. Cryobiology. 2017;78:95-100.
-
14Rebelatto CK, Aguiar AM, Moretao MP, Senegaglia AC, Hansen P, Barchiki F, Oliveira J, Martins J, Kuligovski C, Mansur F, Christofis A, Amaral VF, Brofman PS, Goldenberg S, Nakao LS, Correa A. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med. 2008;233:901-13.
-
15Nejad-Moghaddam A, Ajdary S, Tahmasbpour E, Rad FR, Panahi Y, Ghanei M. Immunomodulatory properties of mesenchymal stem cells can mitigate oxidative stress and inflammation process in human mustard lung. Biochem Genet. 2016;54:769-83.
Publication Dates
-
Publication in this collection
25 Nov 2019 -
Date of issue
2019
History
-
Received
17 July 2018 -
Accepted
22 July 2019