Abstract:
The aim of this study is to explain the biological response and rhizofiltration capacity of Pistia stratiotes, which is an aquatic macrophyte, in alleviating heavy metal stress. In our study, Pistia species were exposed to Nickel (Ni) at different concentrations over seven days. The potential of the aquatic macrophytes in accumulating heavy metals in the water and in removing them from the environment was evaluated by determining the bio-concentration factors (BCF). Selected biological parameters in the leaves of Pistia stratiotes, including metal accumulation, photosynthetic pigment amount, lipid peroxidation activity, and growth rates were measured at the end of the seven-day period. The highest amount of Ni accumulation in P. stratiotes occurred at a concentration of 20 mg l-1. The amount of chlorophyll a (chl a) in P. stratiotes reached a value of 0.087 mg g-1 at the Ni concentration of 20 mg l-1. The relative growth rate (RGR) of P. stratiotes showed a negative correlation with the increases in the concentration levels of the metal. Malondialdehyde (MDA) levels increased to 8.214 nmol/g at the concentration of 20 mg l-1, depending on Ni concentration and time. In our study, the use of P. stratiotes has been determined to be an appropriate plant as an effective Ni accumulator to be utilized with the purpose of rhizofiltration.
Keywords:
Heavy metal; Bioconcentration factor; Pistia stratiotes; Rhizofiltration
INTRODUCTION
The environmental conditions have reached a critical level in the world generally due to the rapid development of industrial activities. Environmental pollution due to the toxic metals has been on the rise since the industrial revolution, leading to serious ecological problems [11 Leblebici Z, Kar M. Accumulation of Heavy Metals in Vegetables Irrigated with Different Water Sources and Their Daily Intake in Nevsehir. J Agr Sci and Tech. 2018; 20: 401-415.,22 Leblebici Z, Kar M, Yalçın V. Comparative Study of Cd, Pb, and Ni removal potential by Salvinia natans (L.) All. and Lemna minor L.: Interactions with Growth Parameters. Rom Biotechnol Lett. 2018; 23: 13235-13248.]. Several studies were conducted over the last two decades on macrophytes removing the heavy metals from the polluted water [33 Leblebici Z, Aksoy A, Duman F. Influence of nutrient addition on growth and accumulation of cadmium and copper in Lemna gibba. Chem Speciat Bioavailab. 2010; 22: 157–164.
4 Leblebici Z, Aksoy A. Growth and heavy metal accumulation capacity of Lemna minor and Spirodela polyrhiza (Lemnaceae): Interactions with nutrient enrichment. Water Air Soil Pollut. 2011; 214: 175-184.-55 Leblebici Z, Aksoy A, Duman F. Influence of salinity on the growth and heavy metal accumulation capacity of Spirodela polyrrhiza (Lemnaceae). Turkish J Biol. 2011; 35: 215–220.]. These macrophytes include the water ferns (Salvinia molesta, Salvinia auriculata, Salvinia minima), water lettuce (Pistia stratiotes), water hyacinth (Eichhornia azurea, Eichhornia diversifolia, Eichhornia paniculata), and duckweed (Lemna minor) [33 Leblebici Z, Aksoy A, Duman F. Influence of nutrient addition on growth and accumulation of cadmium and copper in Lemna gibba. Chem Speciat Bioavailab. 2010; 22: 157–164.,66 Deng H, Ye ZH, Wong MH. Accumulation of lead, zinc, copper and cadmium by 12 wetland plants species thriving in metal contaminated sites in China. Environ Pollut. 2004; 132: 29–40.].
There are several chemical methods available to remove heavy metals from the polluted areas, including electrolysis, reverse osmosis, and adsorption. However, these processes are expensive when they are used for large volumes of water. Therefore, phytoremediation is preferred because of its high efficiency and relatively lower cost. Despite various advantages of the phytoremediation method, not so many species have been studied so far and the appropriate species have not been fully identified, yet [77 Hume NP, Fleming MS, Horne AJ. Denitrification Potential and Carbon Quality of Four Aquatic Plants in Wetland Microcosms. Soil Sci Soc Am J. 2002; 66:1706–1712.,88 Duman F, Leblebici Z, Aksoy A. Growth and bioaccumulation characteristics of watercress (Nasturtium officinale R. BR.) exposed to cadmium, cobalt and chromium. Chem Speciat Bioavailab. 2009; 21: 257–265.].
The ideal plants to be used for remediation of the areas polluted by heavy metals must have relatively larger root systems that can grow rapidly, has a large biomass, and can uptake and accumulate the pollutants. Therefore, the plants to be used for the remediation of the water polluted by the heavy metals need to be carefully selected.
The principle aim of our study is to determine the rhizofiltration capacity of the Pistia stratiotes plant, to determine the amount of Ni accumulated in the plant, and to examine the physiological responses of the plant to the heavy metal stress.
MATERIAL AND METHODS
Sample Material and Preparation for the Experiment Stage
Pistia stratiotes is a freely floating water plant from the monocotyledon clade and it belongs to the family of Araceae. Its rosette-leaves are flossy and thick, slightly lighter green in color. The plant spreads densely in the shallow areas of rivers, lakes, and marshes. They can also be grown in open-top aquariums and in garden ponds. Pistia is a plant which does not have high-level requirements and it can grow easily even in moderate light and can generally reach larger sizes under bright light. The optimum growth temperature of the Pistia plant is between 22-30°C. The plant can grow up to 5-6 cm in diameter in the aquarium conditions while, in nature, it can achieve diameters of up to 20 cm [99 Schmitz DC, Schardt JD, Leslie AG, Dray FA, Osborne JA, Nelson BV. The ecological impact and management history of three invasive alien aquatic plants in Florida McKnight, B.N. eds. Biological Pollution–the Control and Impact of Invasive Exotic Species. Indiana Acad Sci. 1993; 261.].
Pistia stratiotes plant was brought from Adana and raised in the culture medium in the laboratory. After taking the plants out of the culture medium and washing them using distilled water, they were placed in the plant growth cabinet providing photoperiodic lighting, that is16 hours of light and 8 hours of dark, at 23oC temperature and at a humidity level of 70%. Nickel chloride (NiCl26H2O) was used to expose the plants to a heavy metal. The plants were kept in 400 mL beakers in the growth cabinet and Ni was applied to the plants at different concentrations (1-5-10-20 mg l-1) with 10% Hoagland solution over duration of seven days [44 Leblebici Z, Aksoy A. Growth and heavy metal accumulation capacity of Lemna minor and Spirodela polyrhiza (Lemnaceae): Interactions with nutrient enrichment. Water Air Soil Pollut. 2011; 214: 175-184.]. A control group without heavy metal exposure was used for each test group during the study and each trial was repeated three times.
Determining the Levels of Nickel
At the end of the period of exposure to the heavy metal, the plants were taken out from the growth cabinet. They were washed using double-distilled water and dried at 80o C. Samples weighing 0.5 g were taken and dissolved in acid in a microwave device using 10 ml of HNO3. After this dissolving procedure, the samples were immersed in double-distilled water to have a volume of 10 ml and then the amount of Ni was determined with inductively coupled plasma mass spectroscopy (ICP-MS). Peach leaf (SRM-1547 NIST) was used as a reference material for the analytical procedures performed. While the standard value for Ni was defined to be 0.04±0.01 μg l–1 for SRM-1547, the concentration of Ni was found out to be 0.03±0.01 μg l-1 using ICP-MS. Tests were repeated three times for each sample and results were recorded. All the chemicals used in this study conformed to the standards of analytical purity (Merck, Darmstadt, Germany).
Bioconcentration factor (BCF) was calculated as follows [1010 Rahmani GNH, Sternberg SPK. Bioremoval of lead from water using Lemna minor, Bioresour Technol. 1999; 70: 225–230.].
Determining the Relative Growth Rate (RGR) and the Quantity of Photosynthetic Pigments
To determine the relative growth rate, the harvested samples were placed on a blotting paper and left there for the water to be drained over a period of five minutes. Then, they were desiccated at 80OC over a period of 24 hours. After weighing the samples, the relative growth rates were calculated using the equation below.
Relative Growth Rate (RGR) calculation using Hunt's equation:
R: Relative Growth Rate (RGR) (gg-1 d-1); W1 and W2: initial and final dry weights; T2-T1: the time of the start and end of the experiment [1111 Hunt R. Plant growth analysis. Studies in biology, Edward Arnold Ltd.: London; 1978.].
The fresh weight was used to determine the amounts of the photosynthetic pigments. After applying the procedures, a 100 mg of plant sample was taken and immersed in 80% acetone to make a volume of 10 ml. The sample was then centrifuged over a period of 10 minutes at 10,000x g. The quantities of the photosynthetic pigments (chlorophyll a and carotenoids) were determined at 450, 645, and 663 nm using spectrophotometer [1212 Witham FH, Blaydes DF, Devlin RM. Experiments in plant physiology, New York; 1973.].
Determining the Rhizofiltration Potential (RP) of the Plant
The rhizofiltration potential (RP) (mgheavy metal m-2 year-2) of the harvested plants represents the ratio of the total amount of heavy metal, to which the plant was exposed, to the amount of heavy metal found out at the end of the experiment. RP was calculated using the below modified formula by Neugschwandtner et al. [1313 Neugschwandtner RW, Tlustoš P, Komárek M, Száková J. Phytoextraction of Pb and Cd from a contaminated agricultural soil using different EDTA application regimes: Laboratory versus field scale measures of efficiency. Geoderma. 2008; 144: 446–454.].
(3)
Fonkou et al. [1414 Fonkou T, Agendia P, Kengne I, Akoa A, Nya J. Potentials of water lettuce (Pistia stratiotes) in domestic sewage treatment with macrophytic lagoon systems in Cameroon. Proc Int Symposium Environ Pollut Control Waste Manage. Tunis, 2002. 709–714.] defined that Mleaves denoted dry biomass yield of the leaves (g); Mtotal denoted total dry biomass yield (g) (leaves and the roots); Cleaves denoted the amount of heavy metal concentration in the leaves (μg g-1); and Mplant denoted the mean yield of P. straiotes (g DW m-2 year-1).
Measuring Lipid Peroxidation
After the experiment, a 500 mg plant sample was taken and homogenized in 3 ml of 20% TCA (w/v) in 0.5% TBA. The homogenate was incubated at 95°C over a period of 30 minutes and then, the reaction was stopped using ice. The samples were centrifuged at 10,000 x g for 10 minutes and the absorbance of the resulting supernatant was measured at 532 nm and 600 nm with a spectrophotometer. The amount of MDA was calculated using the values obtained at 532 nm and 600 nm [1515 Heath RL, Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968; 125: 189–198.].
Statistical Analysis
The obtained data were expressed as standard errors (SE) and mean values. One-way analysis of variance (ANOVA) was used to confirm the validity of the data and the results. The Duncan test was used to determine the significant difference between the procedures. Statistical significance was accepted to be p≤0.05. All statistical analyzes were performed using SPSS 17.0 software package.
RESULTS AND DISCUSSION
Bioaccumulation and toxicity of nickel
In our study, the accumulation of nickel in plant leaves was determined. On the 7th day of the heavy metal exposure, the maximum Ni accumulation (1820.18 µg g-1) was observed in P. stratiotes samples exposed to Ni at a concentration of 20 mg l-1, as shown in Figure 1. The Ni accumulation on the leaves of P. stratiotes and the Ni concentrations applied to the plant were positively correlated with each other (R=0.919, P≤0.01). It is demonstrated clearly in Figure 1 that the bioaccumulation of metals in the plants increases not only by time but also with the increasing levels of metal concentration, which they were exposed to. In our study, a high level of heavy metal accumulation was detected in the plant over the period of seven days.
Ni accumulation by P. stratiotes at the end of the seven days of exposure to various concentrations of Ni (1-5-10-20 mg l-1). All values are presented with the standard deviation as ± SD. ANOVA significance level was accepted to be p≤0.05.
The accumulation potential of aquatic plants in removing heavy metals from the environment has been examined in several studies using different methods [1616 Ugya A, Yunusa T, Sabiu I, Salisu MT. The Use of Pistia stratiotes To Remove Some Heavy Metals From Romi Stream: A Case Study Of Kaduna Refinery And Petrochemical Company Polluted Stream. IOSR J Environ Sci Toxicol Food Technol. 2015; 9: 48-51.
17 Nurhayati P, Sapta A, Siti K, Iqbal RF. Water Lettuce (Pistia stratiotes, L.) Potency as One of Eco-friendly Phytoextraction Absorbers of Zinc Heavy Metal to Solve Industrial Waste Problem in Indonesia. IPCBEE. 2012; 41: 151-156.
18 Vesely T, Pavel T, Jirina S. The Use of Water Lettuce (Pistia Stratiotes L.) for Rhizofiltration of a Highly Polluted Solution by Cadmium and Lead. Int J Phytoremediation. 2011; 13:859–872.-1919 Odjegba VJ, Fasidi IO. Accumulation of Trace Elements by Pistia stratiotes: Implications for phytoremediation. Ecotoxicol. 2004; 13: 637–646]. The researchers have proved that the aquatic plants are able to accumulate various heavy metals at high concentrations. As shown in Figure 1, P. stratiotes showed higher rates of accumulation of Ni, depending on the increases in concentrations exposed, over the period of seven days in our study.
The initial metal concentration, to which the plant is exposed, is a parameter required to determine the metal concentration that accumulates during the whole period of exposure [2020 Naumann B, Eberius M, Appenroth KJ. Growth rate based dose-response relationships and EC-values of ten heavy metals using the duckweed growth inhibition test (ISO 20079) with Lemna minor L. clone St., J Plant Physiol. 2007; 164:1656–64.]. Zayed et al. [2121 Zayed A, Gowthaman S, Terry N. Phytoaccumulation of Trace Elements by Wetland Plants: I. Duckweed. J Environ Qual. 1998; 27: 715–721.] report that only plants with more than 1,000 BCFs should be considered as good accumulators. Odjegba and Fasadi [1919 Odjegba VJ, Fasidi IO. Accumulation of Trace Elements by Pistia stratiotes: Implications for phytoremediation. Ecotoxicol. 2004; 13: 637–646] discovered that P. stratiotes was a very efficient copper accumulator. As shown in Figure 2, our study results confirm that P. stratiotes is is a good accumulator for Ni and that it has a high potential for remediating water contaminated with Ni.
The levels of the bioconcentration factor (BCF) of P. stratiotes exposed to Ni over a period of seven days. The vertical bars indicate SD, n = 3.
The effect of Ni exposure on the growth rate of P. stratiotes and on the amount of photosynthetic pigments
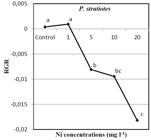
With the increased concentrations of the heavy metal, to which the plant was exposed, the relative growth rate of the plant decreased (Figure 3) and the accumulated heavy metal caused a darker coloring on the leaves. As shown in Figure 3, at the end of the 7th day, the highest decrease in RGR of P. stratiotes was observed with the samples exposed to a Ni concentration of 20 mg l-1. A significant effect on the growth parameters was observed, like the chlorosis of the leaves, in the plants exposed to Ni concentrations of 10 and 20 mg l-1. A significant negative correlation was determined between the RGR values and the concentrations of Ni applied to the P. stratiotes (R=-0.898, p ≤ 0.01).
The relative growth rates at the end of the period of Ni application to the P. stratiotes. All values are presented with the standard deviation as ± SD. ANOVA significance level was accepted to be p≤0.05.
Usually, the first and the most obvious response of the plants to heavy metal stress is the change in the growth rate. This present study has shown that the lower doses of Ni (1 mg l-1), which is a trace element, have increased the growth rate of P. stratiotes slightly. However, the higher doses of Ni have led to adverse effects on the growth of the plant. Ugya et al. [1616 Ugya A, Yunusa T, Sabiu I, Salisu MT. The Use of Pistia stratiotes To Remove Some Heavy Metals From Romi Stream: A Case Study Of Kaduna Refinery And Petrochemical Company Polluted Stream. IOSR J Environ Sci Toxicol Food Technol. 2015; 9: 48-51.] have similarly reported that the heavy metals, which are the trace elements, accelerate the growth at relatively lower doses, but they cause toxic effects at higher doses.
It is known that the toxic metals produce reactive oxygen species (ROS) that destroy the cell walls and cell membranes of the plants under stress depending on the concentration of exposure. The RGR values may be reduced at higher concentrations of exposure due to the effects of ROS [2222 Srivastava S, Mishra S, Tripathi RD, Dwivedi S, Trivedi PK, Tandon PK. Phytochelatins and Antioxidant Systems Respond Differentially during Arsenite and Arsenate Stress in Hydrilla verticillata (L.f.) Royle. Environ Sci Technol. 2007; 41: 2930–2936.]. Our study results are fully supportive of these findings.
As shown in Figure 4, the amount of chlorophyll in P. stratiotes showed a negative correlation with the applied concentration levels of Ni. When P. stratiotes leaves were exposed to 1 mg l-1 or higher concentrations of Ni, a reduction in the amount of chlorophyll pigment was observed. Figure 4 shows that, at the end of the 7th day, the lowest amount of chlorophyll pigment was found out to be 0.79 mg g -1 in the samples exposed to a concentration of 20 mg l -1, compared to the control sample (1.52 mg g -1) (R=-0.908, p≤0.01).
The effects of Ni on chlorophyll a (a) and carotenoid (b) levels in the P. stratiotes plant. All values are presented with the standard deviation as ± SD. ANOVA significance level was accepted to be p≤0.05.
Figure 4 also shows that the carotenoid levels decreased in the plant depending on the Ni concentrations exposed to and by time. The minimum level of carotenoid was found out to be 0.59 mg g-1 in the leaves of P. stratiotes in the samples exposed to a Ni concentration of 20 mg l-1 (R=-0.912, p≤0.01). As shown in Figure 4, the photosynthetic pigment levels in the plants exposed to Ni were determined to be lower compared to the control sample for all concentrations of exposure.
Vesely et al. [1818 Vesely T, Pavel T, Jirina S. The Use of Water Lettuce (Pistia Stratiotes L.) for Rhizofiltration of a Highly Polluted Solution by Cadmium and Lead. Int J Phytoremediation. 2011; 13:859–872.] detected a decrease in the amount of the photosynthetic pigment in the leaves of P. stratiotes. Our study results support this finding as it has been found out that the chlorophyll content of P. stratiotes decreases by the higher concentrations of exposure.
The photosynthetic pigments (chlorophyll and carotenoids) in green plants are important components of the photosynthesis system. A significant change in the amount of pigments can lead to the adverse consequences on the entire metabolism of the plant, through the degradation of membrane lipids and the reactive oxygen species, decreases in the intake of nutrients (such as Mn, Cu, Fe, and P), or degradation of chlorophyll [2323 Srivastava M, Ma LQ, Rathinasabapathi B, Srivastava P. Effects of selenium on arsenic uptake in arsenic hyperaccumulator Pteris vittata L. Bioresour Technol. 2009; 100: 1115–1121.].
The rhizofiltration capacity of P. stratiotes
The rhizofiltration potential (RP) is an indicator of the actual uptake of the heavy metal from the environment. The RP values calculated for P. stratiotes after heavy metal exposure in the experiment are presented in Table 1. P. stratiotes's rhizofiltration potential showed an increase as the Ni concentrations increased over the entire experiment. At the end of the experiment, the RP of the plant was determined to be 512 mg Ni m-2 year-1 at the lowest level of Ni exposure. At the Ni exposure level of 5 mg L -1, the RP was 834 mg Ni m -2 year -1. At the end of the experiment, the RP was observed to be 1274 mg Ni m-2 yıl-1 at a Ni exposure level of 10 mg L-1 and it was 6210 mg Ni m-2 year-1 at a Ni exposure level of 20 mg L-1. Vesely et al. [1818 Vesely T, Pavel T, Jirina S. The Use of Water Lettuce (Pistia Stratiotes L.) for Rhizofiltration of a Highly Polluted Solution by Cadmium and Lead. Int J Phytoremediation. 2011; 13:859–872.] found out that the rhizofiltration potential of the plant, exposed to Pb, continued to increase until the fourth day, and then it decreased. The results of our study, using Ni exposure, support these findings.
Effects of nickel on MDA levels
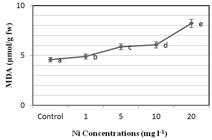
Figure 5 shows that the levels of MDA in the P. stratiotes plant are positively correlated with the levels of exposure to Ni. Although the level of MDA was not significantly different at the Ni exposure level of 1 mg l-1, compared to the control sample; the MDA levels proportionally increased as the concentrations of Ni applied to the plant increased. Moreover, as shown in Figure 5, the MDA content at the concentration level of 20 mg l-1 was significantly different from that of the control sample (p ≤ 0.05). Figure 5 shows that the levels of MDA increased both by time and with the increased Ni concentrations at the end of the seven-day period, reaching a maximum level of 8.214 nmol/g with exposure to a concentration of 20 mg l-1 (R=0.729, p≤0.01).
The effects of Ni on lipid peroxidation in P stratiotes. All values are presented with the standard deviation as “± SD”. ANOVA significance level was accepted to be p≤0.05.
The MDA content suggests information on the antioxidant defense mechanism of a given plant. In the literature, it is reported that the plants containing high levels of MDA are under severe stress [2424 Shri M et al. Effect of arsenic on growth, oxidative stress, and antioxidant system in rice seedlings. Ecotoxicol Environ Saf. 2009; 72: 1102–1110.]. With the increases in the levels of heavy metal accumulation; the duration of exposure increases, the activities of the heavy metal enzymes are changed, the membrane permeability is affected, and ion leaks occur; damaging the plant [2525 Chakrabarty D et al. Comparative transcriptome analysis of arsenate and arsenite stresses in rice seedlings. Chemosphere. 2009; 74: 688–702.]. This study supports the findings in the literature which states that the increases in the concentration and duration of exposure cause increased levels of MDA.
CONCLUSION
To conclude,
-
P. stratiotes showed an effective accumulation potential, especially with exposure to Ni at high concentrations.
-
Water, the elixir of life existing in our cells, is very important as a universal solvent that carries nutrients and waste matter. In the recent years, the water pollution has become a major problem. The spread of heavy metals such as Ni in the environment increases the degradation of living and non-living organisms.
-
The phytoremediation technology aims to alleviate the pollution via the uptake of the heavy metals by plants. This technology has remarkable advantageous aspects compared to the conventional methods currently in use. To be successful with this method, one of the most important requirements is to select the appropriate plants, which accumulate the metals. For this reason, the research on the phytoremediation capacity of the plants is a critical job in order to solve this environmental problem.
-
P. stratiotesis is an important plant in the remediation of domestic and industrial wastewater and in the restoration of the mine sites, which are no longer exploited.
-
P. stratiotes shows most of the characteristics of the ideal plant species performing phytoremediation using their roots (rhizofiltration).
-
The results of our study reveal that P. stratiotes plant accumulates Ni in high quantities and has a high rhizofiltration capacity.
Acknowledgements
This study was supported by Nevsehir Hacı Bektaş Veli University Research Fund. Project number: NEÜLÜP15F8.
HIGHLIGHTS
-
• Pistia stratiotes as an effective Ni accumulator
-
• Rhizofiltration capacity of Pistia stratiotes hight
-
• Biological response of Pistia stratiotes sensitive
-
• Physiological responses of the plant to the heavy metal stress very successufull
-
Funding: This research received no external funding
REFERENCES
-
1Leblebici Z, Kar M. Accumulation of Heavy Metals in Vegetables Irrigated with Different Water Sources and Their Daily Intake in Nevsehir. J Agr Sci and Tech 2018; 20: 401-415.
-
2Leblebici Z, Kar M, Yalçın V. Comparative Study of Cd, Pb, and Ni removal potential by Salvinia natans (L.) All. and Lemna minor L.: Interactions with Growth Parameters. Rom Biotechnol Lett 2018; 23: 13235-13248.
-
3Leblebici Z, Aksoy A, Duman F. Influence of nutrient addition on growth and accumulation of cadmium and copper in Lemna gibba. Chem Speciat Bioavailab 2010; 22: 157–164.
-
4Leblebici Z, Aksoy A. Growth and heavy metal accumulation capacity of Lemna minor and Spirodela polyrhiza (Lemnaceae): Interactions with nutrient enrichment. Water Air Soil Pollut 2011; 214: 175-184.
-
5Leblebici Z, Aksoy A, Duman F. Influence of salinity on the growth and heavy metal accumulation capacity of Spirodela polyrrhiza (Lemnaceae). Turkish J Biol 2011; 35: 215–220.
-
6Deng H, Ye ZH, Wong MH. Accumulation of lead, zinc, copper and cadmium by 12 wetland plants species thriving in metal contaminated sites in China. Environ Pollut. 2004; 132: 29–40.
-
7Hume NP, Fleming MS, Horne AJ. Denitrification Potential and Carbon Quality of Four Aquatic Plants in Wetland Microcosms. Soil Sci Soc Am J. 2002; 66:1706–1712.
-
8Duman F, Leblebici Z, Aksoy A. Growth and bioaccumulation characteristics of watercress (Nasturtium officinale R. BR.) exposed to cadmium, cobalt and chromium. Chem Speciat Bioavailab 2009; 21: 257–265.
-
9Schmitz DC, Schardt JD, Leslie AG, Dray FA, Osborne JA, Nelson BV. The ecological impact and management history of three invasive alien aquatic plants in Florida McKnight, B.N. eds. Biological Pollution–the Control and Impact of Invasive Exotic Species. Indiana Acad Sci. 1993; 261.
-
10Rahmani GNH, Sternberg SPK. Bioremoval of lead from water using Lemna minor, Bioresour Technol 1999; 70: 225–230.
-
11Hunt R. Plant growth analysis. Studies in biology, Edward Arnold Ltd.: London; 1978.
-
12Witham FH, Blaydes DF, Devlin RM. Experiments in plant physiology, New York; 1973.
-
13Neugschwandtner RW, Tlustoš P, Komárek M, Száková J. Phytoextraction of Pb and Cd from a contaminated agricultural soil using different EDTA application regimes: Laboratory versus field scale measures of efficiency. Geoderma 2008; 144: 446–454.
-
14Fonkou T, Agendia P, Kengne I, Akoa A, Nya J. Potentials of water lettuce (Pistia stratiotes) in domestic sewage treatment with macrophytic lagoon systems in Cameroon. Proc Int Symposium Environ Pollut Control Waste Manage. Tunis, 2002. 709–714.
-
15Heath RL, Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 1968; 125: 189–198.
-
16Ugya A, Yunusa T, Sabiu I, Salisu MT. The Use of Pistia stratiotes To Remove Some Heavy Metals From Romi Stream: A Case Study Of Kaduna Refinery And Petrochemical Company Polluted Stream. IOSR J Environ Sci Toxicol Food Technol. 2015; 9: 48-51.
-
17Nurhayati P, Sapta A, Siti K, Iqbal RF. Water Lettuce (Pistia stratiotes, L.) Potency as One of Eco-friendly Phytoextraction Absorbers of Zinc Heavy Metal to Solve Industrial Waste Problem in Indonesia. IPCBEE. 2012; 41: 151-156.
-
18Vesely T, Pavel T, Jirina S. The Use of Water Lettuce (Pistia Stratiotes L.) for Rhizofiltration of a Highly Polluted Solution by Cadmium and Lead. Int J Phytoremediation 2011; 13:859–872.
-
19Odjegba VJ, Fasidi IO. Accumulation of Trace Elements by Pistia stratiotes: Implications for phytoremediation. Ecotoxicol 2004; 13: 637–646
-
20Naumann B, Eberius M, Appenroth KJ. Growth rate based dose-response relationships and EC-values of ten heavy metals using the duckweed growth inhibition test (ISO 20079) with Lemna minor L. clone St., J Plant Physiol 2007; 164:1656–64.
-
21Zayed A, Gowthaman S, Terry N. Phytoaccumulation of Trace Elements by Wetland Plants: I. Duckweed. J Environ Qual 1998; 27: 715–721.
-
22Srivastava S, Mishra S, Tripathi RD, Dwivedi S, Trivedi PK, Tandon PK. Phytochelatins and Antioxidant Systems Respond Differentially during Arsenite and Arsenate Stress in Hydrilla verticillata (L.f.) Royle. Environ Sci Technol 2007; 41: 2930–2936.
-
23Srivastava M, Ma LQ, Rathinasabapathi B, Srivastava P. Effects of selenium on arsenic uptake in arsenic hyperaccumulator Pteris vittata L. Bioresour Technol 2009; 100: 1115–1121.
-
24Shri M et al. Effect of arsenic on growth, oxidative stress, and antioxidant system in rice seedlings. Ecotoxicol Environ Saf 2009; 72: 1102–1110.
-
25Chakrabarty D et al. Comparative transcriptome analysis of arsenate and arsenite stresses in rice seedlings. Chemosphere. 2009; 74: 688–702.
Publication Dates
-
Publication in this collection
25 Nov 2019 -
Date of issue
2019
History
-
Received
18 Sept 2018 -
Accepted
08 July 2019