Abstract
ZnO cross-like structures has been synthesized by a cetyltrimethylammonioum hydroxide (CTAOH)-assisted hydrothermal method at low temperature (60ºC) as a function of the CTAOH concentration. The structure and morphology of the structures ZnO cross-like were studied by means of x-ray diffraction (XRD), high resolution transmission electron microscopy (HRTEM), and scanning electron microscopy (SEM).
Zinc oxide; cross-like nanostructures; assisted hydrothermal method
Growth and characterization of ZnO cross-like structures by hydrothermal method
Lopez-Romero, S.
Instituto de Investigaciones en Materiales - Universidad Nacional Autónoma de México, Coyoacán 04510, México DF, México. e-mail: sebas@servidor.unam.mx
ABSTRACT
ZnO cross-like structures has been synthesized by a cetyltrimethylammonioum hydroxide (CTAOH)-assisted hydrothermal method at low temperature (60ºC) as a function of the CTAOH concentration. The structure and morphology of the structures ZnO cross-like were studied by means of x-ray diffraction (XRD), high resolution transmission electron microscopy (HRTEM), and scanning electron microscopy (SEM).
Keywords: Zinc oxide, cross-like nanostructures, assisted hydrothermal method.
1 INTRODUCTION
The low dimensional, binary semiconductor oxide ZnO has distinctive properties: optically, ZnO is a wide-band-gap (3.37eV) at room temperature, compound that is promising for short-wavelength optoelectronic applications [1]. Its large excitation energy (60meV) can ensure an efficient excitonic emission at room temperature and ultraviolet (UV) luminescence has been reported in disordered nanoparticles and thin films [2-8]. ZnO is a uniquely structured material: is a hexagonal wurtzite-structured oxide, where each anion (O-2 ) is surrounded by four cations (Zn+2) at the corners of a tetrahedron and vice versa. Since the Wurtzite no possess an centre of symmetry, the crystal exhibits crystallographic polarity whose polar axis is the c-axis; ZnO have two polar-surfaces {0001}, and it belongs to the C64v space group.With this properties ZnO has been synthesized in a great diversity of nanostructures, including nanosprings [9], nanorings [10], nanobows [11], nanojunctions arrays [12,13], nanobelts [14], nanocombs [15] and nanopropeller arrays [16] synthesized efficiently due to the high ionicity of the polar surfaces [9,10].
In this work, using a cetyltrimethylammonioun hydroxide-assisted hydrothermal method, ZnO cross-like structures were synthesized as a function of CTAOH concentration. The structural and morphological properties of this structures as-grown have been analyzed.
2 EXPERIMENTAL
2.1 Preparation of cross-like structures
The chemicals precursors used in this study were analytical reagent grade (Sigma-Aldrich) and used without further purification.
Zinc acetate [Zn(CH3COO)2] was used as precursor and cetyltrimethylammonioum hydroxide (CTAOH) as surfactant and catalyst. Initially, 0.01M of zinc acetate was dissolved in ethyl alcohol and magnetically stirred at 60ºC for 1h. The concentration of [Zn(CH3COO)2] was maintained constant for all reactions. Then, cetyltrimethylammonioun hydroxide was mixed into the solution with Zn2+/CTAOH molar ratios of 1/1.6, 1/1.2, 1/0.98 and 1/0.94, and then refluxed at 60ºC for 2h. It was observed that in all reactions a white ZnO powder precipitated at the flask bottom. Finally, the white powder was thoroughly washed with deionized water and allowed to dry in air at room temperature.
The reaction mechanisms proposed for hydrothermal synthesis is already reported by J. Zhung and coworkers [17]. In this model of reaction is well established that the growth unit correspond to the anions [Zn(OH)4]2- . In the context of this model, the following chemical reactions are expected to occur in this case:
CTAOH, 60ºC
In reaction (1) Zn2+ ions are combined with OH- radicals in the alcoholic solution to form a Zn(OH)2 colloid through the reaction Zn2+ +2OH- → Zn(OH)2. Later, in the hydrothermal process, the Zn(OH)2 is separated into Zn2+ ions and OH- radicals according to reaction (2). Then, ZnO nuclei are formed according reaction (3), when the concentration of Zn2+ ions and OH- radicals reaches a supersaturation grade. Finally, the growth unites of [Zn(OH)4]2- radicals are obtained through the reaction (4). The dissolution-nucleation cycle according to reactions (5) and (6) respectively produces:
3 CHARACTERIZATION
The x-ray diffraction (XRD) pattern of the cross-like structures was obtained with a x-ray diffractometer (SIEMENS D 5000) using the CuKα (1.5406Ǻ) radiation, with a scanning speed of 10 per min. at 35 KV and 30mA. The morphology of the sample was studied using a JEM5600-LV scanning electron microscope (SEM). The single-crystal structure of the ZnO cross-like structures was analyzed using a JEOL FEG 2010 fast transmission electron microscope (TEM) with a 2.1Ǻ resolution (point to point).
4 RESULTS AND DISCUSSION
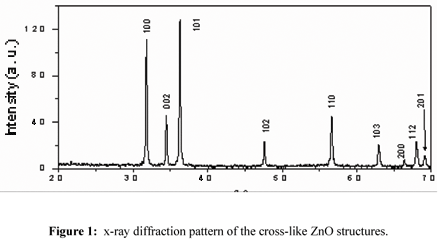
Figure 1 shows the XRD pattern of the cross-like ZnO structures prepared by the CTAOH-assisted hydrothermal method. The XRD patterns of the samples prepared with different CTAOH concentration are similar for the four CTAOH concentration used and confirm that all of them correspond to ZnO system with Wurtzite structure. All diffraction peaks of the samples were indexed to the hexagonal phase of ZnO having lattice parameters a = 3.249 and c = 5.206Ǻ (JCPD file No 36-1451). The patterns do not shown the presence of Zn or any other phase, indicating that the growth direction of the ZnO cross-like was along c-axis orientation [18,19]. A schematic sketch of an ZnO hexagonal crystal is shown in reference [20]. Figure 2a shows the typical low magnification SEM images of a individual cross-like ZnO structure. She consisted of four petals emerging from a common point in four directions, with an angle between the petals of 90ºC. The petals sizes are between 0.5-1µm in wide, and 7-8mµ in length. The same cross-like structures were obtained for all CTAOH concentration used. The Fig. 2b. shows a panoramic view of many cross-like structures, it can be observed that all the cross-like structures have the same form and similar dimensions.
Based on the crystal growth dynamics and experimental results which showed that the morphology and property of ZnO structures could be affected by experimental parameters such as: temperature, time, surfactant and solvent type, etc. an formation mechanism of the cross-like can be proposed.
ZnO is an polar crystal that present anisotropic growth habitat because various crystal facets have different growth rates [21]. This anisotropic growth of ZnO structures is along the fastest growth rate of [0001] direction and the lowest rate of [000-1] direction [1-23]. In all crystal growth process there are nuclei and growth and the surfactant roll (CTAOH) is promote the anisotropic growth of crystals and stabilize the interfaces of the nuclei and retard oxidation and aggregation process [24]. Also, in the hydrothermal method the CTAOH function as catalyst and promote the generation of Zn+2 ions and OH- radicals by means of the reaction (2). In the initial stage of the hydrothermal process, ZnO seed crystallites can be formed as product of the reaction between Zn+2 and OH-, after a certain time , precipitation starts to take place in the solution and some amount of seed crystallites aggregate and three-dimensional cluster would be formed, which can act as nuclei for ZnO structures growth. In addition, on the nuclei surface are formed active sites so as its distribution determine the shape of the final crystal. It is believed that the active sites are basically controlled by the PH value (the concentration of OH-). In this experiment, the value PH used was of 7.5 which is low and therefore there are few active sites around the ZnO nuclei were formed (4). When the number of active sites on the ZnO nuclei surface is bigger, the ZnO obtained structures are flower-like. This results are in concordance with the results obtained by J. Liu et al [25].
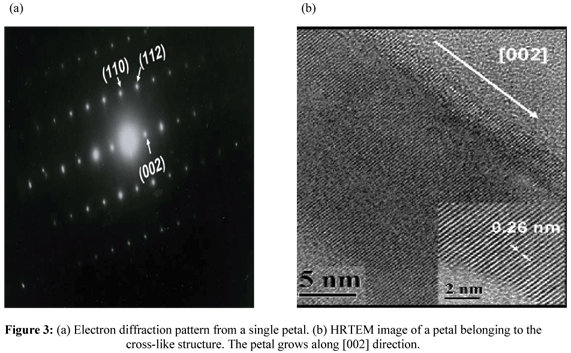
Figures 3(a) and 3(b) are the electron diffraction pattern and HRTEM images of a petal belonging to the cross-like structures respectively. These results indicate a good crystalline quality of the obtained material, which is consistent with the x-ray results shown in figure 1. The HRTEM image reveals that the interplanar spacing in the crystalline petal is 0.26nm, which corresponds to the distance between two (002) planes of the hexagonal ZnO phase, indicating the preferential growth along the [002] direction (c-axes).
5 CONCLUSIONS
In this work ZnO cross-like structures were synthesized by the CTAOH-assisted hydrothermal method as a function of the CTAOH concentration. The cross-like structures obtained consist of four petals emergin from a common point. An model for ZnO cross-like growth process is suggested, which indicate that the active sites on the ZnO nuclei is proportional to the value PH and that the distribution of active sites determine the form of the final crystal. The ZnO petals are highly crystalline and present hexagonal wurtzite phase. The CTAOH concentration have no effect on the structure and morphology of the material obtained in this range of CTAOH used but promote the anisotropic growth of ZnO structures and accelerate the generation of Zn+2 ions and OH- radicals. The HRTEM showed that the growth direction of the ZnO crystals was along [0002] direction (c axes).
6 ACKNOWLEDGEMENTS
The author wish to thank L. Baños and A. tejeda (IIM) for his support in carrying out x-ray study, Omar Novelo Peralta (IIM), Carlos Flores (IIM) and Luis Rendón (IF) for their support in electron microscopy characterization. The authors are also thankfull to the Central Microscopy facilities of the Institute of Physics, UNAM, for providing the microscope tools used in this work.
7 REFERENCES
Data de envio: 04/09/08 Data de aceite: 18/09/09
- [1] HUANG, M.H., MAO, S., FEICK, H. et al., "Room temperature ultraviolet nanowire nanolasers", Science, v. 292, pp. 1897, 2001.
- [2] CHOI, K.S., LICHTENEGGER, H.C., STUCKY, G.D. et al., "Electrochemical synthesis of nanostructured ZnO films utilizing self-assembly of surfactant molecules at solid-liquid interfaces", Journal of American Chemical Society, v. 124, pp. 2402-2403, July 2002.
- [3] ZHANG, J., SUN, L.D., YIN, J.L. et al., "Control of ZnO morphology via a simple solution route", Chemical of Materials, v.14, pp. 4172-4176, 2002.
- [4] HU, J.Q, BANDO, Y, ZHAN, J.H. et al., "Two-dimensional micrometer-sized single-crystalline ZnO thin nanosheets", Applied Physics Letters, v. 83, pp. 4414-4418, 2003.
- [5] LAO, J.Y., WEN, J.G., REN, Z.F. et al., "Hierarchical ZnO nanostructures", Nano Letters, v. 2, pp. 1287-1291, 2002.
- [6] GAO, P.X., WANG, Z.L., "Mesoporous polyhedral cajes and shells formed by textured self-assembly of ZnO nanocrystals", Journal of The American Chemical Society, v. 125, pp. 11299-11305, 2003.
- [7] WANG, X.D., SUMMERS, C.J., WANG, Z.L. et al., "Large-scale hexagonal-patterned growth of aligned ZnO nanorods for nano- optoelectronics and nanosensor arrays", Nano Letters, v. 4, pp. 423-426, 2004.
- [8] GAO, P.X., DING, Y, MAI, W.J. et al., "Conversion of zinc oxide nanobelts into superlaticce-structured nanohelices", Science, v. 309, pp. 1700-1704, 2005.
- [9] JIECHAO, GE,TANG, B., ZHUO, L., SHI, Z. et al.,"A rapid hydrothermal route to sisal-like 3D ZnO nanostructures via the assembly of CTA+ and Zn(OH<)2− 4: growth mechanism and photoluminescence properties", Nanotechnology, v. 17, pp. 1316-1320, 2006.
- [10] HUANG, M.H., WU, Y.Y., FEICK, H. et al., "Catalytic growth of zinc oxide nanowires by vapor transport", Advanced Materials, v. 13, pp. 113-116, 2001.
- [11] HAN, X.H., WANG, G.Z., ZHOU, L. et al., "Crystal orientation-ordered ZnO nanorod bundles on hexagonal heads of ZnO microcones: epitaxial growth and self-atraction", Chemical Communications, v.2, pp. 212-214, 2006.
- [12] PAN, Z.W., DAI, Z.R., WANG, Z.L. et al., "Nanobelts of semiconductig oxides", Science, v. 291, pp. 1947-1950, 2001.
- [13] WANG, Z.L., KONG, X.Y., ZUO, J.M. et al., "Induced growth of asymmetric nanocantilever arrays on polar surfaces", Physical Review Letters, v. 91, pp. 185502-185504, 2003.
- [14] KONG, X.Y., WANG, Z.L., "Spontaneous polarization-induced nanohelixes, nanospring, and nanorings of piezoelectric nanobelts", Nano Letters, v. 3, pp. 1625-1631, 2003.
- [15] KONG, X.Y., DING, Y., YANG, R. et al., "Single-crystal nanorings formed by epitaxial self-coiling of polar nanobelts", Science, v. 303, pp. 1348, 2004.
- [16] HUGHES, W., WANG, Z.L., "Formation of piezoelectric single-crystal nanorings and nanobows", Journal of American Chemical Societ, v. 126, pp. 6703-6709, 2004.
- [17] ZHANG, J., SUN, L.D., LIAO, C.S., et al., "A simple route towards tubular ZnO", Chemical communication, v. 3, pp. 262-263, 2002.
- [18] ZHANG, B.P., BIUH, N.T., SEGAWA, Y., WAKATSUKI, K., USAMI, N., "Optical properties of ZnO rods formed by metalorganic chemical vapor deposition", Applied Physics Letters, v. 83, pp. 1635-1637, 2003.
- [19] ZHAO, Q.X., WILLANDER, M., "Optical recombination of ZnO nanowires grown on sapphire and Si substrates", Applied Physics Letters, v. 83, pp. 165-167, 2003.
- [20] WANG, G.Z., MA, N.G., DENG, C.J. et al., "Large-scale synthesis of aligned hexagonal ZnO nanorods using chemical vapor deposition", Materials Letters, v. 58, pp. 2195-2198, 2004.
- [21] YONGHONG, N., FEI, G., HONGJIANG, L., et al., "A novel aqueus-phase route to prepare flower-shaped PbS micron crystals", Journal of crystal growth, v. 262, pp 399-402, 2004.
- [22] PAN, Z.W., DAI, Z.R, WANG, Z.L., "Nanobelts of semiconductor oxides", Science, v. 291, 2001.
- [23] YAMAMOTO, K., NAGASAGUA, K., OHMORI, T., "Preparation and characterization of ZnO nanowires", Physica E, v. 24, pp 129-132, 2004.
- [24] ZHONG LIN WANG, "Zinc oxide nanostructures: growth, properties and applications", Journal of Physics: Condensed Matter, v16, R829-R858, 2004.
- [25] JINPING, L., XINTANG, H., YUANYUAN, L., et al., "Large-scale synthesis of flower-like structures by a surfactant free and low temperature process", Materials Chemistry and physics, 2005.
Publication Dates
-
Publication in this collection
18 Dec 2009 -
Date of issue
2009
History
-
Accepted
18 Sept 2009 -
Received
04 Sept 2008