Abstracts
The objective of this study was to evaluate bacterial prevalence in 31 root canals of human deciduous teeth with necrotic pulp and periapical lesions using bacterial culture. After crown access, the material was collected using absorbent paper points for microbiological evaluation and determination of colony forming units (CFU). Anaerobic microorganisms were found in 96.7% of the samples, black-pigmented bacilli in 35.5%, aerobic microorganisms in 93.5%, streptococci in 96.7%, and S. mutans in 48.4%. We concluded that in human deciduous teeth root canals with necrotic pulp and periapical lesions the infection is polymicrobial, with a large number of microorganisms and a predominance of streptococci and anaerobic microorganisms.
Tooth; deciduous; Bacteriological techniques; Periapical abscess
O objetivo deste estudo foi avaliar, por meio de cultura bacteriológica, a prevalência de microorganismos em 31 canais radiculares de dentes decíduos humanos com necrose pulpar e lesão periapical. O material, colhido dos canais radiculares após a realização da cirurgia de acesso, foi submetido ao processamento microbiológico para a determinação das unidades formadoras de colônia de microorganismos. Os resultados mostram que os microorganismos anaeróbios foram quantificados em 96,7% dos casos, os bacilos pigmentados de negro (BPB) em 35,5%, os aeróbios em 93,5%, os estreptococos em 96,7% e os S. mutans em 48,4%. Assim, pôde-se concluir que a infecção em canais radiculares de dentes decíduos humanos portadores de necrose pulpar e lesão periapical é polimicrobiana, com grande quantidade de microorganismos e maior prevalência de estreptococos e microorganismos anaeróbios.
Dente decíduo; Técnicas bacteriológicas; Abscesso periapical
PEDIATRIC DENTISTRY
Prevalence of microorganisms in root canals of human deciduous teeth with necrotic pulp and chronic periapical lesions
Prevalência de microrganismos em canais radiculares de dentes decíduos de humanos com necrose pulpar e lesão periapical crônica
Luciana Cunha PazelliI; Aldevina Campos de FreitasII; Izabel Yoko ItoIII; Maria Cristina Monteiro de Souza-GugelminIII; Alexandra Sárzyla MedeirosI; Paulo Nelson-FilhoII
IGraduate Students, Department of Pediatric Dentistry, School of Dentistry of Ribeirão Preto
IIProfessors, Department of Pediatric Dentistry, School of Dentistry of Ribeirão Preto
IIIProfessors, School of Pharmaceutical Sciences of Ribeirão Preto University of São Paulo
ABSTRACT
The objective of this study was to evaluate bacterial prevalence in 31 root canals of human deciduous teeth with necrotic pulp and periapical lesions using bacterial culture. After crown access, the material was collected using absorbent paper points for microbiological evaluation and determination of colony forming units (CFU). Anaerobic microorganisms were found in 96.7% of the samples, black-pigmented bacilli in 35.5%, aerobic microorganisms in 93.5%, streptococci in 96.7%, and S. mutans in 48.4%. We concluded that in human deciduous teeth root canals with necrotic pulp and periapical lesions the infection is polymicrobial, with a large number of microorganisms and a predominance of streptococci and anaerobic microorganisms.
Descritors: Tooth, deciduous; Bacteriological techniques; Periapical abscess.
RESUMO
O objetivo deste estudo foi avaliar, por meio de cultura bacteriológica, a prevalência de microorganismos em 31 canais radiculares de dentes decíduos humanos com necrose pulpar e lesão periapical. O material, colhido dos canais radiculares após a realização da cirurgia de acesso, foi submetido ao processamento microbiológico para a determinação das unidades formadoras de colônia de microorganismos. Os resultados mostram que os microorganismos anaeróbios foram quantificados em 96,7% dos casos, os bacilos pigmentados de negro (BPB) em 35,5%, os aeróbios em 93,5%, os estreptococos em 96,7% e os S. mutans em 48,4%. Assim, pôde-se concluir que a infecção em canais radiculares de dentes decíduos humanos portadores de necrose pulpar e lesão periapical é polimicrobiana, com grande quantidade de microorganismos e maior prevalência de estreptococos e microorganismos anaeróbios.
Descritores: Dente decíduo; Técnicas bacteriológicas; Abscesso periapical.
INTRODUCTION
Although oral health education and prevention are priorities in contemporary dentistry, pulp changes in deciduous teeth are very frequent. Thus, curative dentistry should simultaneously evolve and improve concepts and therapeutic procedures.
Despite the controversy, many authors report the need to treat root canals of deciduous teeth with pulp necrosis5,6,12 due to the propagation of microorganisms throughout the entire root canal system, including the lumen, lateral, accessory and secondary canals, dentinal tubules, ramifications of the apical delta, apical foramen, areas of apical cementum resorption and periapical biofilm7,11.
The success of endodontic treatment depends on many factors and the reduction or elimination of bacterial infection9,21 is the most important one. However, for this to occur, it is important to identify which microorganisms are present. Little research has been done to identify which bacterial species are present3,4,6,10,13,20,21 in deciduous teeth with pulp necrosis and periapical infection.
Therefore, the aim of this study was to evaluate the bacterial profile in root canals of human deciduous teeth with necrotic pulp and periapical lesions.
MATERIAL AND METHODS
Clinical procedures
The research protocol was approved by the Institutions Ethics Committee (#2000.1.488.58.7).
Fifteen 3- to 7-year-old children, of both sexes, seen at the Pediatric Dental Clinic, School of Dentistry of Ribeirão Preto (University of São Paulo) were selected. None of the children had been treated with antibiotic for at least 3 months. A total of 31 root canals from 18 deciduous teeth (maxillary incisors and canines, and maxillary and mandibular molars) with necrotic pulp and radiographically visible radiolucent areas in the region of the bone furcation and/or the periapical region suggesting chronic periapical lesion were used. The teeth had carious lesions, some with the pulp chamber exposed to the oral cavity. However, it was possible to isolate the surgical field with a rubber dam and restore the tooth. They also had intact roots or less than 2/3 of physiological root resorption, no periodontal pocket and no previous root canal intervention. Among the teeth treated, 19 root canals from 13 teeth had fistulae.
After antisepsis of the oral cavity by rinsing for 1 min with 5.0 ml of 0.12% chlorhexidine digluconate (Periogard, Colgate-Palmolive Ind. Brasileira, Osasco, SP, Brazil), local anesthesia was applied with 2.0% mepivacaine (DFL Ind. e Comércio Ltda., Rio de Janeiro, RJ, Brazil). A rubber dam was placed and 1.0% chlorhexidine digluconate was used for antisepsis.
All carious tissue was removed with curettes and low-speed spherical burs, and antisepsis was performed again. High-speed spherical diamond burs, cooled with air and water, were used for surgical access of the root canals. Compensatory wearing was carried out with a high-speed Endo-Z stainless steel bur (Les Fils dAugust, Maillefer, Ballaigues, Switzerland), cooled with air and water, and followed by irrigation/suction with sterile saline (School of Pharmaceutical Sciences of Ribeirão Preto, University of São Paulo, SP, Brazil).
Absorbent paper points (Tanari Industrial Ltda., Manaus, AM, Brazil) were used for collection of root canal samples. At the largest end of the paper points, "wings" made out of a stainless steel matrix for amalgam (0.5 mm) were adapted in order to prevent them from floating when placed in test tubes containing solution (reduced transport fluid - RTF). The different caliber paper points were placed individually in 12 x 75 mm tubes, which were then labeled and closed with cotton and sterilized in an autoclave at 120ºC, for 20 min.
Bacteriological samples were collected immediately after crown access by introducing 3-4 sequential sterile absorbent paper points, of a size visually compatible with the root canal diameter, 2-3 mm before the radiographic apex or the limit of the physiological root resorption, as determined by diagnostic radiographs.
After approximately 1 min, the paper points were removed from each root canal and placed in a test tube (13 x 100 mm) containing 2.0 ml of reduced transport fluid (RTF), prepared according to Syed and Loesche19, that is recommended for the maintenance of viable anaerobic microorganisms. The tube was closed hermetically and processed for microbiological analysis.
After samples collection, the root canals were treated for the immediate and progressive neutralization of septic/toxic content using K-files and copious irrigation/suction with 2.5% sodium hypochlorite followed by odontometry 1 mm before the radiographic apex or the limit of the physiological root resorption. Biomechanical preparation was carried out with sequential K-files and irrigation with 2.5% sodium hypochlorite. The canals were subsequently dried with sterile absorbent paper points and filled with EDTA (Odahcam Herpo Produtos Dentários Ltda., Rio de Janeiro, RJ, Brazil), and mixed for 3 min with a K-file to remove smear layer. The canals were irrigated and dried, and then filled with calcium hydroxide paste (Calen PMCC, S.S. White Artigos Dentários Ltda., Rio de Janeiro, RJ, Brazil) with a special syringe (ML, S.S. White Artigos Dentários Ltda., Brazil). The pulp chamber was sealed with zinc oxide/eugenol cement (IRM, Dentsply Indústria e Comércio Ltda., Petrópolis, RJ, Brazil). After 14-30 days, the intracanal dressing was removed and the canals were filled with Calen thickened with zinc oxide, as recommended by Silva and Leonardo15, and restored.
Microbiological processing
The tubes containing the samples were mixed with 4-6 sterile glass beads in a mixer (Mixtron-Toptronix, SP, Brazil) for 2 min at maximum speed. Subsequently, serial decimal dilutions up to 10-5 were made in Sorensen Phosphate Buffer (PBS) under laminar airflow. A volume of 0.05 ml of the pure samples and of each dilution was sown, with a sterile calibrated pipette, onto plates containing As (Blood agar, Difco, Detroit, USA), Ms (Mitis Salivarius agar, Difco, Detroit, USA) and Ask media (Blood agar supplemented with hemin and menadione, Sigma Chemical Co., St. Louis, USA). Plates containing SB20 agar (Bacitracin sucrose agar, Difco, Detroit, USA) received dilutions only up to 10-1. The samples were then distributed uniformly from the most diluted to the least diluted using a sterile glass L-shaped rod.
The Ask plates were incubated anaerobically using the Gas-Pak system, in hermetically sealed jars (Permution, Equipamentos e produtos químicos Ltda., Curitiba, Brazil) for 7-10 days. The As plates were incubated aerobically for 24-48 h, at 37ºC, and the Ms and SB20 plates were incubated for 3 days in microaerofilic conditions (candle jar system) in a hermetically sealed jar. After incubation, colonies were counted with a stereomicroscope (Nikon, Tokyo, Japan) under reflect light and the colony forming units (CFU/ml) were calculated.
From the SB20 agar plates, 3-4 colonies suspect of being mutans streptococci were isolated and identified according to Ito et al.8: fermentation of mannitol, sorbitol, raffinose and melibiose; hydrolisis of arginine and sculin; and sensitivity to bacitracin.
RESULTS
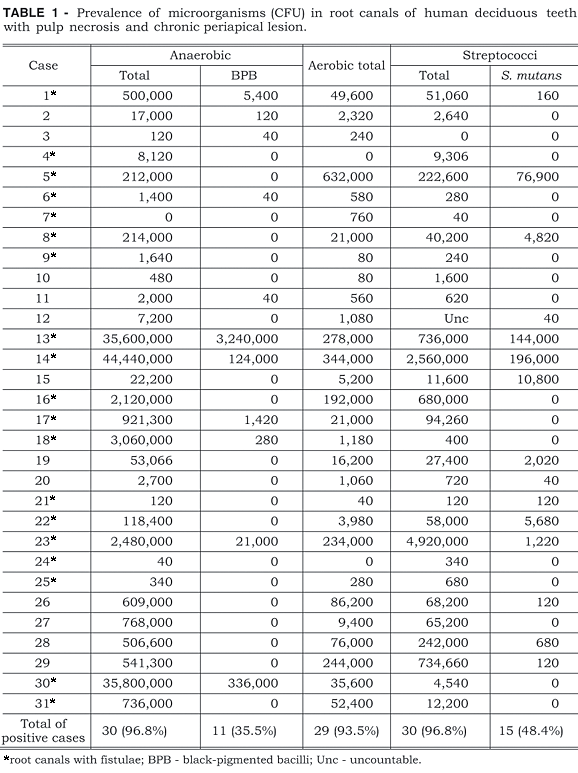
The CFU (colony forming units) of the samples from 31 root canals from deciduous teeth with necrotic pulp and radiographically visible periapical lesion are presented in Table 1.
The prevalence of anaerobic microorganisms was 96.8% (30 root canals), and black-pigmented bacillus (BPB) were found in 11 cases (35.5%). Aerobic microorganisms were present in 29 root canals (93.5%) with streptococci present in 30 canals (96.8%). Streptococcus mutans was quantified in 15 canals (48.4%) whereas Streptococcus sobrinus was not detected.
DISCUSSION
During the 1950s and 1960s, researchers isolated mainly aerobic and facultative bacterial species from root canals with necrotic pulp and periapical lesions due to the limitation of isolation techniques and microbial culture14. With scientific and technological evolution, anaerobic techniques have been developed and, in the 1980s, this concept was modified showing that in root canals of permanent teeth with necrotic pulp and periapical lesions there is a polymicrobial infection with predominance of strict anaerobic species1,17,22.
The microbiota is constituted of only a few species when compared to the total bacteria of the oral cavity. There are many factors that can influence the growth and development of these microorganisms in root canals, i.e., nutrient availability, low oxygen tension, bacteria interaction, as well as disintegrated pulp tissue and tissue fluids that are essential nutrient sources17.
In the present study, anaerobic microorganisms were detected in 96.8% of the samples. These results are in agreement with those of Toyoshima et al.21, Sato et al.13 and Faria6, who reported that in root canals of primary teeth with periapical lesions there is a polymicrobial infection with predominance of anaerobic microorganisms, similar to those of the microbiota of permanent teeth.
Black-pigmented bacilli (BPB) have frequently been isolated from root canals of permanent teeth with necrotic pulp. Sundqvist et al.18 reported their presence in 30% of the cases while Assed et al.1 verified by immunofluorescence that these microorganisms were found in 60% of the samples. In the present study in deciduous teeth, BPB were found in 11 cases (35.5%), a figure which is similar to those found in reports by Tomic-Karovic, Jelinek20 and Faria6 who detected these microorganisms in 36% and 30%, respectively, of the root canals of deciduous teeth with necrotic pulp. However, Toyoshima et al.21 quantified BPB in 44.4% of deciduous root canals in retreatment cases.
Studies of permanent teeth associate the presence of BPB with the development of abscesses18. We agree with Faria6 who did not observe this correlation when considering deciduous teeth. In the present study, 8 of the 19 root canals with fistulae had BPB. However, this microorganism was also detected in 3 cases in which there were no fistulae.
In this study, aerobic microorganisms were quantified in deciduous teeth with necrotic pulp and chronic periapical lesions in 29 root canals (93.5%). Although Sato et al.13 and Faria6 observed a higher prevalence of anaerobic microorganisms over aerobic microorganisms, in our study prevalence rates were similar (93.5% aerobic and 96.8% anaerobic). These results show that endodontic infections in deciduous teeth, similarly to those in permanent teeth, are polymicrobial with the development of microbial interactions.
The literature shows the presence of streptococci in 70%4, in 82%10, in 76%20 and in 85%6 of the root canals of deciduous teeth with pulp necrosis. In our study, streptococci were detected in 30 cases (96.8%). Streptococci and anaerobic microorganisms were the most prevalent bacteria in the deciduous teeth with necrotic pulp and periapical lesions.
Mutans streptococci were found in 15 root canals (48.4%); however, only S. mutans was found. S. sobrinus was not found in any case. Regarding the quantification of mutans streptococci in root canals of permanent teeth, many studies have detected this microorganism, which was found by Stobbering, Eggink16 in 3.05% of the cases, by Baumgartner, Falkler Jr.2 in 33.4%, and by Assed et al.1 in 52.0% of the cases. However, in deciduous teeth root canals only Faria6 reported the presence of mutans streptococci (30.0%) in which S. mutans was found in 25.0% and S. sobrinus in 5.0% of the cases. The variations in the prevalence of these microorganisms in different studies can be explained by the fact that in the cases in which the numbers were higher, some root canals could have been exposed directly to the oral cavity, enhancing the prevalence of S. mutans, as observed in our study.
According to the results of this study, anaerobic microorganisms, black-pigmented bacilli, aerobic bacteria, streptococci and mutans streptococci are components of root canals of deciduous teeth with periapical lesions. Because of the importance of microorganisms in the etiology of pulp and periapical changes, the lack of in vitro and in vivo studies evaluating the root canal microbiota of deciduous teeth with different degrees of pulp and periapical pathoses is not understandable. Thus, there is no consensus on which technique and material is better for the reduction and/or elimination of these microorganisms, so that the deciduous teeth can remain in function in the oral cavity until exfoliation. Therefore, we suggest further research on this subject.
CONCLUSIONS
According to the obtained results, we can conclude that, before biomechanical preparation, the root canals of deciduous teeth with necrotic pulp and chronic periapical lesion presented a high number of microorganisms and polymicrobial infection with the prevalence of anaerobic bacteria and streptococci.
Recebido para publicação em 20/02/03
Enviado para reformulação em 19/03/03
Aceito para publicação em 10/06/03
-
11. Assed S, Leonardo MR, Silva LAB, Ito IY. Prevalência de microorganismos em canais radiculares de dentes com necrose pulpar e reação periapical crônica imunofluorescência indireta efeito do preparo biomecânico e do curativo de demora pela cultura. Rev Bras Odontol 1996;53:24-8.
-
22. Baumgartner JC, Falkler Jr WA. Bacteria in the apical 5 mm of infected root canals. J Endod 1991;17:380-3.
-
33. Brook J, Grimm S, Kielich RB. Bacteriology of acute periapical abscess in children. J Endod 1981;7:378-80.
-
44. Cohen MM, Joress SM, Calisti LP, Mass B. Bacteriologic study of infected deciduous molars. Oral Surg Oral Med Oral Pathol 1960;3:1382-6.
-
55. Faraco Jr IM, Percinoto C. Avaliação de duas técnicas de pulpectomia em dentes decíduos. Rev Assoc Paul Cir Dent 1998;52:400-4.
-
66. Faria G. Prevalência de microorganismos em canais radiculares de dentes decíduos de humanos portadores de necrose pulpar e lesão periapical. Efeito de preparo biomecânico e do curativo de demora à base de hidróxido de cálcio [Dissertação de Mestrado]. Ribeirão Preto: Faculdade de Odontologia da USP; 2001.
-
77. Godoy VL. Distribuição de bactérias planctônicas, colônias bacterianas e biofilmes microbianos em dentes decíduos com pulpite e/ou necrose pulpar [Tese de Doutorado]. Bauru: Faculdade de Odontologia da USP; 1999.
-
88. Ito IY, Albuquerque Junior RF, Alonso-Verri R. Estreptococos: modificação na técnica de identificação das cepas isoladas da cavidade oral. In: 15ª Jornada Odontológica de Ribeirão Preto - USP: 1993; Ribeirão Preto. Anais. São Paulo; 1993. p. 5.
-
99. Leonardo MR, Silva LAB. Medicação tópica entre sessões, curativo de demora em biopulpectomia e necropulpectomias I e II. In: Leonardo MR, Leal JM. Endodontia: tratamento de canais radiculares. 3ª ed. São Paulo: Panamericana; 1998. p. 491-534.
-
1010. Marsh SJ, Largent MD. A bacteriological study of the pulp canals of infected primary molars. J Dent Child 1967; 34:460-70.
-
1111. Obson P. Pulp treatment of deciduous teeth. Factors affecting diagnosis and treatment. Br Dent J 1970;128:232-8.
-
1212. Ribeiro RA, Corrêa MSNP, Costa LRRS. Tratamento pulpar de dentes decíduos. In: Corrêa MSNP. Odontopediatria na primeira infância. São Paulo: Santos; 1998. p. 473-95.
-
1313. Sato T, Hoshino E, Uematsu H, Noda T. Predominant obligate anaerobes in necrotic pulps of human deciduous teeth. Microb Ecol Health Dis 1993;6:269-75.
-
1414. Shovelton DS. The presence and distribution of microorganisms within non-vital teeth. Br Dent J 1964;117:101-7.
-
1515. Silva LAB, Leonardo MR. Qual a orientação para o tratamento endodôntico dos dentes decíduos? Rev Assoc Paul Cir Dent 1995;49:385.
-
1616. Stobbering EE, Eggink CO. The value of the bacteriological culture in endodontics. II. The bacteriological flora of endodontic specimens. Int Endod J 1982;15:87-93.
-
1717. Sundqvist G. Ecology of root canal flora. J Endod 1992; 18:427-30.
-
1818. Sundqvist G, Johansson E, Sjögren U. Prevalence of black-pigmented bacteroides species in root canal infections. J Endod 1989;15:13-9.
-
1919. Syed SA, Loesche WJ. Survival of human dental plaque flora in various transport media. Appl Microbiol 1972;24:638-44.
-
2020. Tomic-Karovic K, Jelinek E. Comparative study of the bacterial flora in the surroundings, the root canals and sockets of deciduous molars. Int Dent J 1971;21:375-88.
-
2121. Toyoshima Y, Fukushima H, Inoue JI, Sasaki Y, Yamamoto K, Katao H, et al. A bacteriological study of periapical pathosis on deciduous teeth. JPN J Pedod 1988;26:449-58.
-
2222. Tronstad L. Recent development in endodontic research. Scand J Dent Res 1992;100:52-9.
Publication Dates
-
Publication in this collection
16 Apr 2004 -
Date of issue
Dec 2003
History
-
Accepted
10 June 2003 -
Received
20 Feb 2003 -
Reviewed
19 Mar 2003