Abstracts
Protease production by thermophilic Bacillus sp strain SMIA-2 cultivated in liquid cultures containing trisodium citrate reached a maximum in 9h, with levels of 1.93U/mg protein. The microorganism utilized several carbon sources for the production of protease. Starch was the best substrate, followed by trisodium citrate, citric acid and sucrose. Among the various organic and inorganic nitrogen sources, ammonium nitrate was found to be the best. Studies on the protease characterization revealed that the optimum temperature of this enzyme was 60ºC. The enzyme was stable for 2h at 30ºC, while at 40ºC and 80ºC, 14% and 84% of the original activities were lost, respectively. The optimum pH of the enzyme was found to be 8.0. After incubation of crude enzyme solution for 24h at pH 5.5, 8.0 and 9.0, a decrease of about 51%, 18% and 66% of its original activity was observed respectively. A stronger inhibitory effect was observed in the presence of K+, Hg2+and Cu2+. Hg+ resulted in the complete loss of activity at 1mM concentrations. Activity was stimulated by Mn2+ and Ca+2, indicating that these ions had a functional role in the molecular structure of the enzyme.
protease; thermophilic bacterium; Bacillus sp.
A produção de protease pelo termofílico Bacillus sp cepa SMIA-2 cultivado em culturas líquidas contendo citrato trissódico alcançou o máximo em 9h, com níveis de 1,93U/mg de proteína. O microrganismo utilizou várias fontes de carbono para a produção da protease, sendo que o amido foi o melhor substrato seguido por citrato trissódico, ácido cítrico e sacarose. Entre as várias fontes de nitrogênio orgânico e inorgânico, o nitrato de amônio foi a melhor. Estudos sobre a caracterização da protease revelaram que a temperatura ótima desta enzima foi 60ºC. A enzima foi estável por 2h a 30ºC, enquanto a 40ºC and 80ºC, 14% e 84% da atividade original foram perdidas, respectivamente. O valor ótimo de pH encontrado para a enzima foi 8,0. Após a incubação da solução enzimática bruta por 24h a pH 5,5, 8,0, e 9,0 foi observado um decréscimo de em torno de 51%, 18% e 66% da sua atividade original, respectivamente. Um forte efeito inibitório foi observado na presença de K+, Hg2+, Cu2+. A presença de Hg+ resultou na perda completa da atividade da enzima na concentração de 1mM. A atividade foi estimulada pela presença do Mn2+ e Ca+2, indicando que estes íons tiveram um papel funcional na estrutura molecular da enzima.
protease; bactéria termofílica; Bacillus sp.
BIOTECHNOLOGY
Production and properties of an extracellular protease from thermophilic Bacillus sp
Produção e propriedades de uma protease extracelular de um Bacillus sp termofílico
Wellingta Cristina Almeida do Nascimento; Meire Lelis Leal Martins
Centro de Ciências e Tecnologias Agropecuárias, Universidade Estadual do Norte Fluminense Darcy Ribeiro, Campos dos Goytacazes, RJ, Brasil.
Correspondence Correspondence to: Meire Lelis Leal Martins Centro de Ciências e Tecnologias Agropecuárias Universidade Estadual do Norte Fluminense Darcy Ribeiro Av. Alberto Lamego, 2000 28013-600, Campos dos Goytacazes, RJ, Brasil Tel.: (+5522) 27261460 Fax: (+5522) 27263875 E-mail: meire@uenf.br
ABSTRACT
Protease production by thermophilic Bacillus sp strain SMIA-2 cultivated in liquid cultures containing trisodium citrate reached a maximum in 9h, with levels of 1.93U/mg protein. The microorganism utilized several carbon sources for the production of protease. Starch was the best substrate, followed by trisodium citrate, citric acid and sucrose. Among the various organic and inorganic nitrogen sources, ammonium nitrate was found to be the best. Studies on the protease characterization revealed that the optimum temperature of this enzyme was 60ºC. The enzyme was stable for 2h at 30ºC, while at 40ºC and 80ºC, 14% and 84% of the original activities were lost, respectively. The optimum pH of the enzyme was found to be 8.0. After incubation of crude enzyme solution for 24h at pH 5.5, 8.0 and 9.0, a decrease of about 51%, 18% and 66% of its original activity was observed respectively. A stronger inhibitory effect was observed in the presence of K+, Hg2+and Cu2+. Hg+ resulted in the complete loss of activity at 1mM concentrations. Activity was stimulated by Mn2+ and Ca+2, indicating that these ions had a functional role in the molecular structure of the enzyme.
Key words: protease, thermophilic bacterium, Bacillus sp.
RESUMO
A produção de protease pelo termofílico Bacillus sp cepa SMIA-2 cultivado em culturas líquidas contendo citrato trissódico alcançou o máximo em 9h, com níveis de 1,93U/mg de proteína. O microrganismo utilizou várias fontes de carbono para a produção da protease, sendo que o amido foi o melhor substrato seguido por citrato trissódico, ácido cítrico e sacarose. Entre as várias fontes de nitrogênio orgânico e inorgânico, o nitrato de amônio foi a melhor. Estudos sobre a caracterização da protease revelaram que a temperatura ótima desta enzima foi 60ºC. A enzima foi estável por 2h a 30ºC, enquanto a 40ºC and 80ºC, 14% e 84% da atividade original foram perdidas, respectivamente. O valor ótimo de pH encontrado para a enzima foi 8,0. Após a incubação da solução enzimática bruta por 24h a pH 5,5, 8,0, e 9,0 foi observado um decréscimo de em torno de 51%, 18% e 66% da sua atividade original, respectivamente. Um forte efeito inibitório foi observado na presença de K+, Hg2+, Cu2+. A presença de Hg+ resultou na perda completa da atividade da enzima na concentração de 1mM. A atividade foi estimulada pela presença do Mn2+ e Ca+2, indicando que estes íons tiveram um papel funcional na estrutura molecular da enzima.
Palavras-chave: protease, bactéria termofílica, Bacillus sp.
INTRODUCTION
Proteases [serine protease (EC. 3.4.21), cysteine (thiol) protease (EC 3.4.22), aspartic proteases (EC 3.4.23) and metallo-protease (EC 3.4.24)] constitute one of the most important groups of industrial enzymes, accounting for about 60% of the total enzyme market (12,19,23). Among the various proteases, bacterial proteases are the most significant, compared with animal and fungal proteases (24) and among bacteria, Bacillus sp are specific producers of extra-cellular proteases (17). These enzymes have wide industrial application, including pharmaceutical industry, leather industry, manufacture of protein hydrolizates, food industry and waste processing industry (13).
Thermostable proteases are advantageous in some applications because higher processing temperatures can be employed, resulting in faster reaction rates, increase in the solubility of nongaseous reactants and products, and reduced incidence of microbial contamination by mesophilic organisms. Proteases secreted from thermophilic bacteria are thus of particular interest and have become increasingly useful in a range of commercial applications (1,18,19,21,23).
Recently, a thermophilic Bacillus sp strain SMIA-2 was isolated from a soil sample collected in Campos dos Goytacazes city, Rio de Janeiro, Brazil (11,12). Phylogenetic analysis showed that this strain is a member of the Bacillus rRNA group 5. This group includes Bacillus stearothermophilus and other thermophilic Bacillus spp.
In this article, we describe the selection of medium components for the optimal production of extracellular protease by thermophilic Bacillus sp strain SMIA-2, along with some biochemical properties of the enzyme.
MATERIALS AND METHODS
Organism
The bacterial strain used in this studywas the thermophilic Bacillus sp strain SMIA-2, previously isolated from a soil sample collected in Campos dos Goytacazes City, Rio de Janeiro, Brazil (11,12).
Enzyme production
The culture medium used in this work for protease production contained (g/L of destilled water): MgSO4-0.5, K2HPO4-2.0, KCl-0.3, NH4NO3-10.0, peptone-1.0. Trisodium citrate-10.0. The pH was adjusted to 6.9-7.0 with 1.0 M NaOH and this basal medium was sterilized by autoclaving at 121ºC for 15 min. Peptone was sterilized separately and aseptically added to the flasks containing the liquid medium, after cooling. The above medium (50 mL in 250 mL Erlenmeyer flasks) was inoculated with 1mL of an overnight culture and incubated at 50ºC in a rotary shaker operated at 150 rpm for 12h. At time intervals, the turbidity of the cultures was determined by measuring the increase in optical density at 470 nm with a spectrophotometer Hitachi Model U-2000. Before assay, the cells were separed by centrifugation at 15.500g for 15 min and the clear supernatant was used as crude enzyme preparation.
Effect of culture conditions on enzyme production
The culture medium was supplemented with the following metal traces (g/L): CaCl2-2.2x10-3, ZnO-2.5x10-3, FeCl3.6H2O-2.7x10-2 , MnCl2.4H2O-1.0x 10-2, CuCl2.2H2O-8.5x10-4 , CoCl2.6H2O-2.4x10-3 , NiCl3.6H2O-2.5x10-4 , H3BO3-3.0x10-4 and Na2MoO4-1.0x10-3. The effects of carbon sources 1% (w/v) on enzyme secretion were investigated replacing trisodium citrate by glycerol, D(+) galactose, lactose, sucrose, maltose, starch, D(+) glucose, D(+) manose, L (+) arabinose, casein, D(+) xylose and citric acid. Different nitrogen sources including NH4NO3, peptone, yeast extract, meat extract, casein, (NH4)2SO4, (NH4)2HPO4, NH4Cl, KNO3, urea and ammonium citrate were employed in preliminary studies to determine growth and production of extracellular protease.
Enzyme assay
The activity of protease was assessed in triplicate by measuring the release of trichloroacetic-acid soluble peptides from 0.2% (w/v) azocasein in 50 mM HEPES/NaOH buffer (pH 7.5) at 50ºC for 10 min. The 1-mL reaction was terminated by the addition of 0.5 mL of 15% trichloroacetic acid and then centrifuged at 10.000g for 10 min, after cooling. One unit (U) enzyme activity was defined as the amount of enzyme required to produce an increase in absorbance at 420nm equal to 1.0 in 60 min (7).
Protein was measured by the method of Lowry, as modified by Petterson (14).
Effect of pH on activity and stability of protease
The optimum pH was determined with azocasein 1% (w/v) as substrate dissolved in different buffers (citrate phosphate, pH 5-6, sodium phosphate, pH 7.0, Tris-HCl, pH 8.0 and glycine NaOH, pH 9-13). The effect of pH on enzyme stability was determined by pre-incubating the enzyme without substrate at different pH values (5.5-9.0) for 24h at room temperature and measuring the residual activity at 60ºC.
Effect of temperature on activity and stability
The effect of temperature on the enzyme activity was determined by performing the standard assay procedure at pH 7.5 within a temperature range from 40 to 100ºC. Thermostability was determined by incubation of crude enzyme at temperatures ranging from 30-100ºC for 2h in a constant-temperature water bath. After treatment the residual enzyme activities were assayed.
Effect of metal ions on protease activity
The effect of different metal ions on protease activity was determined by the addition of the corresponding ion at a final concentration of 1.0 mM to the reaction mixture, and assayed under standard conditions. The enzyme assay was carried out in the presence of KCl, CaCl2, MgSO4, FeSO4, CoCl2, ZnCl2, MnSO4, HgCl2 , CuSO4 and NaCl.
RESULTS AND DISCUSSION
Effect of culture conditions on enzyme secretion
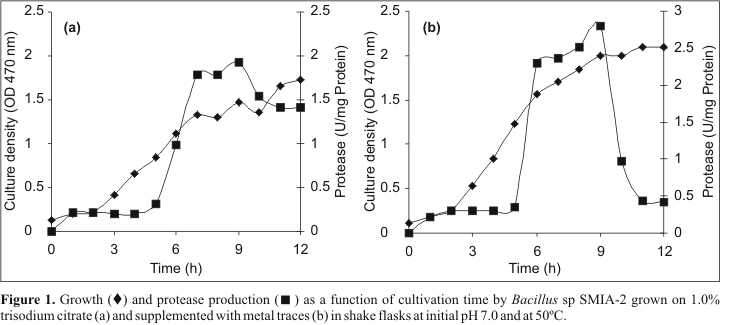
The growth pattern of Bacillus sp. SMI-2 and protease production was observed for 12 hours in liquid medium with 1% trisodium citrate as a carbon source in a 250 mL Erlenmeyer flask (Fig. 1a). Bacillus sp grew very fast and the formation of protease started from 5h of the growth and reached a maximum in 9 hours (1.93 U/mg Protein) and then began to fall. This suggests that protease production was directly linked to the culture being metabolically active. Ward (24) reported that Bacillus sp usually produce more protease during the late exponential phase. The function of this enzyme is obscure, but its production is correlated with the onset of a high rate of protein turnover during sporulation in certain bacilli.
The supplementation of the culture medium with a solution of metal traces improved substantially the growth of Bacillus sp SMIA-2 and the enzyme production (Fig. 1b), thereby indicating the requirements of some metal ions for protease production by this organism. These results corroborate the earlier findings of metal ions enhancing the activity of protease (7). Ferrero et al. (5) reported the use of trisodium citrate along with MgSO4, CaCl2, MnSO4 and ZnSO4 for protease production by Bacillus licheniformis MIR 29.
Although the addition of metal traces solution to the medium improved the growth of Bacillus sp SMIA-2 and the protease activity, a rapid loss of activity of this enzyme in the stationary phase culture was observed. These results are similar to the findings of Jansen et al. (7), who observed a marked decreased of proteinase activity in cultures of Thermus sp. Since Ca2+ has a significant stabilizing effect on protease, these authors suspected that chelators and phosphate were lowering the available Ca2+ in the culture. Thus, they modified the medium to remove chelators, lower the inorganic phosphate concentration and boost the Ca2+concentration without incurring a reduction in the growth rate. These changes resulted in an improvement in the proteinase activity half-life from 5.9 h to 11.7h.
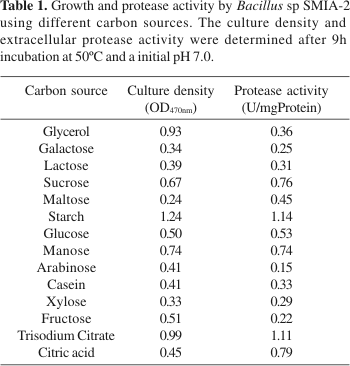
Bacillus sp SMIA-2 was capable of utilizing a wide range of carbon sources. However, the best carbon sources in the present study, for protease secretion were starch and trisodium citrate (Table 1). In a similar study Johnvesly and Nailk (8) showed that citric acid, soluble starch and trisodium citrate were the best carbon sources for protease production by Bacillus sp JB-99. According to these authors, culturing this organism in 1% glucose (w/v) repressed completely the synthesis of alkaline protease. However, in the present study glucose was found to be a relatively good carbon source for enzyme production since moderate amount of protease activity was detected.
The type of nitrogen sources also affected enzyme production. Among the various organic and inorganic nitrogen sources, the maximum enzyme activity (1.1U/mgProtein) was obtained when ammonium nitrate was used in the medium (Table 2). Moderate to good levels of enzyme activities were obtained when ammonium chloride, ammonium citrate and potassium nitrate were used as nitrogen sources. When various organic nitrogen sources were tested for protease production, it was found that protease formation by Bacillus sp SMIA-2 was repressed, although growth in some cases was stimulated. Similar results were obtained by Ponsare et al. (16) to Aeromonas hydrophila and by Banerjee et al. (3) to Bacillus brevis. On the other hand, Phadatare et al. (15) reported the enhancement of protease production in Conidiobolus coronatus by organic nitrogen sources like tryptone, peptone and yeast extract. Organic nitrogen sources have been found to be better nitrogen sources for growth and protease production in some organisms (2,15) and inorganic nitrogen sources (ammonium sulphate and potassium nitrate) gave better enzyme yields in other organisms (20).
Effect of pH on activity and stability of protease
A pH range between 5.5 and 9.0 was used to study the effect of pH on protease activity (Fig. 2). Optimum pH was found to be 8.0. At pH 6.5 only 29% of the maximum enzyme activity was obtained, increasing to 53% and 59% at pH 7.0 and 7.5, respectively. After incubation of crude enzyme solution at room temperature for 24h at pH values of 5.5, 8.0 and 9.0, it was observed a decrease of about 51%, 18% and 66% of its original activity, respectively. Sookkheo et al. (22) reported to three proteases, S, N and B from thermophilic Bacillus stearothermophilus TLS33, optimum pH values of 8.5, 7.5, and 7.0, respectively. The protease S was active over a very broad pH range, and about 60% of proteolytic activity was still detectable at pH 6 and 10 in the presence of 5 mM CaCl2. In contrast, proteases N and B retained relatively little activity above pH 9.0.
Effect of temperature on activity and stability of protease
The protease activities were assayed at different temperatures ranging from 30ºC-90ºC at a constant pH of 7.5 (Fig. 3). Enzyme activity increased with temperature within the range of 30ºC to 60ºC. A reduction in enzyme activity was observed at values above 60ºC. The optimum temperature of this protease was 60ºC which was similar to that described for other Bacillus proteases (3,6). The thermostability of the protease was examined by measuring the remaining activities at 60ºC, after incubation of the enzyme without substrate at various temperatures between 30 and 90ºC for 2h. Thermostability profile indicated that the enzyme was stable at 30ºC for 2h while at 40ºC and 80ºC, 14% and 84% of the original activities were lost, respectively. Protease from Bacillus sp. JB-99 retained 63% and 25% original activity after 1h heat treatment at 70 and 80ºC, however in the presence of 10mM Ca2+, the enzyme retained 83% and 74% of the original activity, respectively (8).
Effect of metal ions on protease activity
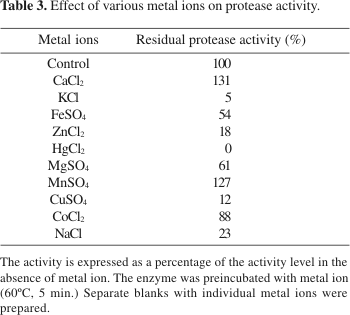
The effect of different metal ions on protease is shown in Table 3. A stronger inhibitory effect was observed in the presence of K+, Cu2+ and Zn2+. Hg2+ inhibited completely the enzyme at 1mM concentrations. The protease secreted by Brevibacillus (Bacillus) brevis was also inhibited by Hg2+, Zn2+ and Cu2+ (3). The inhibitory effect of heavy metal ions is well documented in the literature. It is known that the ions mercury, cadmium and lead react with the protein thiol groups (converting them to mercaptides), as well as with histidine and tryptophan residues. Moreover, by action of silver and mercury, the disulphide bonds were found to be hydrolytically degraded (9).
Protease activity was stimulated by Mn2+ and Ca+2. These results suggest that these metal ions apparently protected the enzyme against thermal denaturation and played a vital role in maintaining the active conformation of the enzyme at higher temperatures (4). Similar effects of Mn2+ on the activity of proteases were also observed by Rahman et al. (18), and by Manachini et al. (10).
ACKNOWLEDGMENTS
The authors thank the FAPERJ (Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro) for financial support and the CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for providing a scholarship to posgraduate student W.C.A.Nascimento.
Submitted: February 03, 2003; Returned to authors: June 03, 2003; Approved: March 04, 2003.
- 1. Adams, M.W.W.; Kelly, R.M. Finding and using thermophilic enzymes. Trends Biotechnol, 16: 329-332, 1998.
- 2. Aleksieva, P.; Djerova, A.; Tchorbanov, B.; Girarov, J. Submerged cultivation of a strain of Humicola lutea 72 producing acid protease. Eur. J. App. Microbiol. Biotech, 13: 165-169, 1981.
- 3. Banerjee, U.C.; Sani, R.K.; Azmi, W.; Soni, R. Thermostable alkaline protease from Bacillus brevis and its characterization as a laundry detergent additive. Proc. Biochem., 35: 213-219, 1999.
- 4. Beg, Q.K.; Gupta, R. Purification and characterization of an oxidation-stable, thiol-dependent serine alkaline protease from Bacillus mojavensis Enz. and Microbial Techn., 32: 294-304, 2003.
- 5. Ferrero, M.A.; Castro, G.R.; Abate, C.M.; Baigori, M.D.; Singeriz, F. Thermostable alkaline proteases of Bacillus licheniformis MIR 29: isolation, production and characterization. Appl. Microbiol. Biotechnol., 45: 327-332, 1996.
- 6. Horikoshi, K. Enzymes of alkalophilies. In: Microbial Enzyme and Biotechnology, 2nd, 275-94, 1990.
- 7. Janssen, P.H.; Peek, K.; Morgan, H.W. Effect of culture conditions on the production of a extracellular proteinase by Thermus sp. Rt41A. Appl. Microbiol. Biotechnol, 41: 400-406, 1994.
- 8. Johnvesly, B.; Naik, G.R. Studies on production of thermostable alkaline protease from thermophilic and alkaliphilic Bacillus sp. JB-99 in a chemically defined medium. Proc. Biochem., 37: 139-144, 2001.
- 9. Kumar, C.G.; Tiwari, M.P.; Jany, K.D. Novel alkaline serine proteases from alkalophilic Bacillus spp.: Purification and some properties. Proc. Biochem., 34: 441-449, 1999.
- 10. Manachini, P.L.; Fortina, M.G.; Parini, C. Thermostable alkaline protease produced by Bacillus thermoruber a new species of Bacillus Appl. Microbiol, 28: 409-413, 1988.
- 11. Nunes, A.S. Influência da temperatura sobre os requerimentos nutricionais de um Bacillus sp. termofílico. Tese (Mestrado em Produção Vegetal) - Campos dos Goytacazes - RJ, Universidade Estadual do Norte Fluminense - UENF, 63p. 2000.
- 12. Nunes, A.S.; Martins, M.L.L. Isolation, properties and kinetics of growth of a thermophilic Bacillus Braz. J. Microbiol, 32: 271-275, 2001.
- 13. Pastor, M.D.; Lorda, G.S.; Balatti, A. Protease obtention using Bacillus subtilis 3411 and amaranth seed meal medium at different aeration rates. Braz. J. Microbiol., 32: 1-8, 2001.
- 14. Peterson, G.L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Analytical Biochem, 83: 346-356, 1977.
- 15. Phadatare, S.U.; Deshpande, V.V.; Srinivasan, M.C. High activity alkaline protease from Conidiobolus coronatus (NCL 86.8.20): Enzyme production and compatability with commercial detergents. Enz. Microbiol. Technol, 15: 72-76, 1993.
- 16. Ponsare, A.C.; Venugopal, V.; Lewis, N.F. A note on nutritional influence on extracellular protease synthesis in Aeromonas hydrophila J. Appl. Bacteriol., 58: 101-104.
- 17. Priest, F.G. Extracellular enzyme synthesis in the genus Bacillus Bacteriol. Rev, 41: 711-753, 1977.
- 18. Rahman, R.N.Z.A.; Razak, C.N.; Ampom, K.; Basri, M.; Yunus, W.M.Z.W.; Salleh, A.B. Purification and characterization of a heat-stable alkaline protease from Bacillus stearothermophilus F1. Appl. Microbiol. Biotechnol, 40: 822-827, 1994.
- 19. Singh, J.; Batra, N.; Sobti, C.R. Serine alkaline protease from a newly isolated Bacillus sp. SSR1. Proc. Biochem., 36: 781-785, 2001.
- 20. Sinha, N.; Satyanarayana, T. Alkaline protease by thermophilic Bacillus licheniformis Indian J. Microbiol., 31: 425-430, 1991.
- 21. Sonnleitner, B.; Fiechter, A. Advantages of using thermophiles in biotechnological processes: expectations and reality. Trends Biotechnol., 1: 74-80, 1983.
- 22. Sookkheo, B.; Sinchaikul, S.; Phutrakul, S.; Chen, S.T. Purification and characterization of the highly thermostable proteases from Bacillus stearothermophilus TLS33. Prot. Exp. Pur., 20: 142-151, 2000.
- 23. Zeikus, J.G.; Vieille, C.; Savchenko, A. Thermozymes: Biotechnology and structure-function relationship. Extremophiles, 1: 2-13, 1998.
- 24. Ward, O.P. Proteolytic enzymes. In: M. Moo-Young Editor, Comprehensive Biotechnol, 3: 789-818, 1985.
Publication Dates
-
Publication in this collection
16 Nov 2004 -
Date of issue
June 2004
History
-
Accepted
04 Mar 2003 -
Reviewed
03 June 2003 -
Received
03 Feb 2003