Abstracts
Clostridium perfringens is a normal inhabitant of the intestinal tract of chickens as well as a potential pathogen that causes necrotic enteritis and colangio hepatitis. The minimum inhibitory concentration (MIC) of seven different compounds used for therapy, growth promotion or prevention of coccidiosis was determined by agar dilution method for 55 C. perfringens strains isolated from the intestines of broiler chickens. All strains showed high susceptibility to penicillin, avilamycin, monensin and narasin. Only 7.3% of the strains showed an intermediated sensitivity to lincomycin, and 49 (89.1%) were considered susceptible. For tetracycline and bacitracin, 41.8% and 47.3% of strains, respectively, were considered resistant.
Necrotic enteritis; growth promoters; anticoccidials; antibiotics
Clostridium perfringens é um habitante normal da microbiota intestinal de frangos, sendo um agente potencialmente patogênico, causador de enterite necrótica e colangio-hepatite. A concentração inibitória mínima (CIM) de sete drogas utilizadas na terapêutica, como agentes promotores de crescimento ou na prevenção de coccidiose foi determinada pelo método de diluição em ágar para 55 estirpes de C. perfringens isoladas do intestino de frangos de corte. Todas as estirpes revelaram alta sensibilidade à penicilina, avilamicina, narasin e monensina, apenas 7,3% demonstraram CIM intermediário para lincomicina e 89.1% foram consideradas sensíveis. Para tetraciclina e bacitracina, 41,8% e 47.3% das amostras, respectivamente, foram consideradas resistentes.
Enterite necrótica; promotores de crescimento; coccidiostáticos; antimicrobianos
VETERINARY MICROBIOLOGY
SHORT COMMUNICATION
Antimicrobial susceptibility of Clostridium perfringens strains isolated from broiler chickens
Sensibilidade antimicrobiana de estirpes de Clostridium perfringens isoladas de aves de corte
Silva, R. O. S.1; Salvarani, F.M.1; Assis, R.A.2; Martins, N.R.S.1; Pires, P.S.1; Lobato, F.C.F.I
IEscola de Veterinária, Universidade Federal de Minas Gerais, Departamento de Medicina Veterinária Preventiva, Belo Horizonte, MG, Brasil
IILaboratório Nacional Agropecuário do Ministério da Agricultura, Pecuária e Abastecimento, Pedro Leopoldo, MG, Brasil
Corresponding Author Corresponding Author Lobato, F.C.F. Escola de Veterinária da Universidade Federal de Minas Gerais, Departamento de Medicina Veterinária Preventiva Avenida Antônio Carlos, 6627. Caixa Postal 567, Bairro São Francisco CEP 30123-970, Belo Horizonte, MG Fone: +55 (31) 3409-2103 E-mail: flobato@vet.ufmg.br
ABSTRACT
Clostridium perfringens is a normal inhabitant of the intestinal tract of chickens as well as a potential pathogen that causes necrotic enteritis and colangio hepatitis. The minimum inhibitory concentration (MIC) of seven different compounds used for therapy, growth promotion or prevention of coccidiosis was determined by agar dilution method for 55 C. perfringens strains isolated from the intestines of broiler chickens. All strains showed high susceptibility to penicillin, avilamycin, monensin and narasin. Only 7.3% of the strains showed an intermediated sensitivity to lincomycin, and 49 (89.1%) were considered susceptible. For tetracycline and bacitracin, 41.8% and 47.3% of strains, respectively, were considered resistant.
Key-words: Necrotic enteritis, growth promoters, anticoccidials, antibiotics.
RESUMO
Clostridium perfringens é um habitante normal da microbiota intestinal de frangos, sendo um agente potencialmente patogênico, causador de enterite necrótica e colangio-hepatite. A concentração inibitória mínima (CIM) de sete drogas utilizadas na terapêutica, como agentes promotores de crescimento ou na prevenção de coccidiose foi determinada pelo método de diluição em ágar para 55 estirpes de C. perfringens isoladas do intestino de frangos de corte. Todas as estirpes revelaram alta sensibilidade à penicilina, avilamicina, narasin e monensina, apenas 7,3% demonstraram CIM intermediário para lincomicina e 89.1% foram consideradas sensíveis. Para tetraciclina e bacitracina, 41,8% e 47.3% das amostras, respectivamente, foram consideradas resistentes.
Palavras-chave: Enterite necrótica, promotores de crescimento, coccidiostáticos, antimicrobianos.
Clostridium perfringens is a common environmental bacterium and is readily isolated from the intestine of birds and mammals (14). In broiler chicken, it is responsible for necrotic enteritis, an acute enteric disease, and for a subclinical disease with focal necrosis in the intestine or C. perfringens-associated hepatitis, with cholangio hepatitis or fibrinoid necrosis in the liver (7). Besides the economic importance of C. perfringens in poultry, it also constitutes a risk to the public health through the food chain (8), being one of the most frequently isolated bacterial pathogens in foodborne disease outbreaks in humans (20). It is thought that antibacterial substances used for growth promotion or anticcocidials may prevent pathogenic effects of C. perfringens (13), but there are few data about this microorganism susceptibility to those substances in Brazil. The objetive of this study was to determine the in vitro susceptibility of C. perfringens to some antimicrobials of relevance for poultry production.
A total of 55 strains were isolated from broiler chicken intestines in a slaughterhouse in Patos de Minas, Minas Gerais, Brazil. Specimens of duodenal contents were subjected to a bacteriologic culture on C. perfringens selective agar (SPS, Difco, USA). Plates were incubated at 37ºC in an anaerobic atmosphere with 10% H2, 10% CO2, 80% N2 and were examined after 48 hours. Presumptive identification of C. perfringens was determined by colonial and microscopical morphology and confirmed by biochemical tests and multiplex-PCR (15). The following antibiotics manufactured for analytical pourpose were tested: lincomycin, penicilin, tetracyclin, bacitracin (Sigma, USA), avilamycin, narasin and monensin (Elanco Animal Health, Brazil).
Minimum inhibitory concentrations (MICs) were determined on Brucella agar (Difco, USA) plates containing doubling diluitions of the antibiotics, from 0,25 to 256,0 mg/L, and suplemmented with 5% of sheep blood (16). Prior to antimicrobial susceptibility testing, isolates were subcultured on a tioglicolate broth (Difco, USA). After incubation in anaerobic atmosphere for 18 hours at 37ºC, the cultures were suspended in a 0,85% NaCl to an optical density equivalent to that of a McFarland 0,5 standart. Strains were inoculated with a Steers inoculum replicator. All strain were tested twice and, for negative control, Bacteroides fragillis (ATCC25285) was included in every test plates.
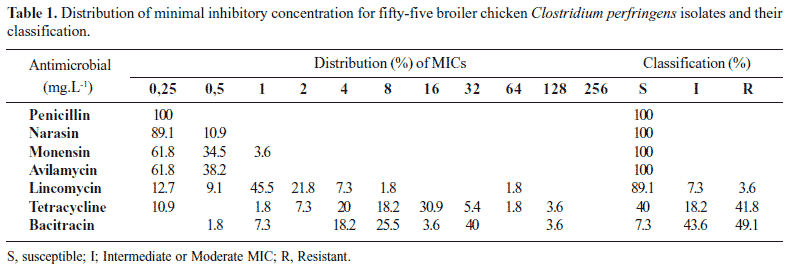
The results are summarize at Table 1. C. perfringens isolates examined was highly susceptible to the two ionophore antibiotics, narasin and monensin, and to penicillin and avilamycin. Lincomycin demonstrated a low MIC for 49 (89.1%) isolates, while 23 (41.8%) and 26 (47.3%) strains were considered resistant to tetracycline and bacitracin, respectivelly.
Despite the decreased sensitivity of C. perfringens isolates from cattle to beta-lactamic antibiotics has been described (17), penicillin caused inhibition of all strains at lowest concentration tested (0,25 mg/L) in this study, in agreement to previous works with C. perfringens poultry isolates (4,9,13,21). Avilamycin showed a good effect too, similar to reported by Martel et al. (13). Higher MICs to avilamycin have also shown by others investigations (6,9,21), but those results were interpreted as inherent lower susceptibility to these drugs, and not as resistance mediated by acquired genes or mutation.
For lincomycin, 49 (89,1%) and four (7.3%) showed a low and a moderate MIC, respectivelly. Only two (3.6%) isolates were considered resistant. Similar results were reported by Martel et al. (13), whereas Watkins et al. (21) described a higher number of lincomycin-resistant strains in broiler chicken and turkey C. perfringens isolates. Resistance genes studies for lincomycin are poorly reported and resistance may be due to as yet unknown genes (13). For bacitracin, 26 (47.3%) strains were considered resistant, while five (9.1%) and 24 (43.6%) strains showed a low and a moderate MIC, respectivelly. Although Watkins et al. (21) and Chalmers et al. (4) reported 88% and 95% resistant C. perfringens strains, other investigations have shown the effectiveness of bacitracin in the control of necrotic enteritis in broiler chicken (2,18). However, Johansson et al. (9) found a low rate of resistance in Swedish and Danish isolates, countries in which bacitracin was no longer in use.
High MICs for tetracycline have already been described for poultry, cattle, dogs and human C. perfringens isolates (4,9,10,12,13,17,21). It was previously been reported that Clostridium species can carry tetracycline resistance genes witch encode a ribosome protecting cytoplasmatic protein (5).
In Brazil, clostridiosis is controlled by a routine combination of antimicrobial growth promoters (AGPs) and ionophorous anticoccidials, both of which have an antibiotic effect on C. perfringens. Increasingly, the use of antibiotics in animal production is linked to the emergence of antibiotic-resistant bacterial strains in humans (3). As a result, the World Health Organization has recommended that AGPs should be replaced by alternative strategies (22). In 2006, the European Union banned all AGPs in animal feed (1). Since then, a growing pressure for AGP withdrawal in Brazil has been put forward by poultry meat importers, recommending the control of necrotic enteritis by anticoccidials, good hygiene management and modified diet. In that case, anticoccidials susceptibility to C. perfringens will be essential. The present study showed that narasin and monensin have a good effect against C. perfringens strains, similar to previously described (4,21). Martel et al. (13) and Johansson et al. (9) reported similar results for Northern Europe farms C. perfringens isolates, where narasin has been used in almost all broiler flocks only up to 1998. These authors suggested that the development of resistance to these ionophore antimicrobial is slow.
Hughes et al. (8), studying the reasons for prescription of antibiotic use in broiler farms, reported that the major indications for therapeutic purposes in the United Kingdom were enteric diseases, particularly necrotic enteritis. Considering the world tendency to ban AGPs from animal production, manufacturers should consider a management that prevents known predisposing factors, including the control of coccidiosis, composition and form of the feed, good hygiene of environment. But, recently the disease re-emerged in countries which banned AGPs (20), confirming that alternatives to prevention and control of C. perfringens infections in poultry needs to be more investigated. Recent studies showed that vaccination of poultry against C. perfringens could be helpful. Lovland et al. (11) reported a vaccine based on C. perfringens type A and C toxoids for the prevention of subclinical lesions, while a live attenuated vaccine was recently reported by Thompson et al. (19). Prebiotics, probiotics and competitive exclusion products are on research too, but more efforts should be done in this complex area (20).
This is the first study of antibotics and anticoccidials suceptibility of Clostridium perfringens isolated from broiler chicken in Brazil.
ACKNOWLEDGEMENTS
This work was supported by funds from Fapemig and CNPq. We thank Elanco Animal Health for the antibiotics and Maria Auxiliadora Roque de Carvalho (ICB/UFMG) for the reference strain.
Submitted: June 06, 2008; Returned to authors for corrections: August 08, 2008; Approved: April 16, 2009
- 1. Bedford, M. (2000). Removal of antibiotic growth promoters from poultry diets: implications and strategies to minimise subsequent problems. World Poult. Sci. J 56, 347-365.
- 2. Brennan, J.; Skinner, J.; Barnum, D.A.; Wilson, J. (2003). The efficacy of bacitracin methylene disalicylate when fed in combination with narasin in the management of necrotic enteritis in broiler chickens. Poult. Sci 82 (3), 360-363.
- 3. Bywater, R.J. (2005). Identification and surveillance of antimicrobial resistance dissemination in animal production. Poult. Sci 84 (4), 644-648.
- 4. Chalmers, G.; Martin, S.W.; Hunter, D.B.; Prescott, J.F.; Weber, L.J.; Boerling, P. (2008). Genetic diversity of Clostridium perfringens isolated from healthy broiler chicken at a commercial farm. Vet. Microbiol 127, 116-127.
- 5 Chopra, I.; Roberts, M. (2001). Tetracycline antibiotics: mode of action, application, molecular biology and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev 65, 232-260.
- 6. Devriese, L.A.; Daube, G.; Hommez, J.; Haesebrouck, F. (1993). In vitro susceptibility of Clostridium perfringens isolated from farm animals to growth-enhancing antibiotics. J. Appl. Microbiol 75 (1), 55-57.
- 7. Engstrom, B.E.; Fermer, C.; Lindberg, A.; Saarinen, E.; Baverud; Gunnarsson, A. (2003). Molecular typing of isolates of Clostridium perfringens from healthy and diseased poultry. Vet. Microbiol 94, 225-235.
- 8. Hughes, L.; Hermans, P.; Morgan, K. (2008). Risk factors for the use of prescription antibiotics on UK broiler farms. J.Antimicrob. Chemother 1-6.
- 9. Johansson, A.; Greko, C.; Engstrom, B.E.; Karlsson, M. (2004). Antimicrobial susceptibility of Swedish, Norwegian and Danish isolates of Clostridium perfringens from poultry, and distribution of tetracycline resistance gentes. Vet. Microbiol 99, 251-257.
- 10. Kather, E.J.; Marks, S.L.; Foley, J.E. (2006). Determination of the prevalence of antimicrobial resistance genes in canine Clostridium perfringens isolates. Vet. Microbiol 113 (1-2), 97-101.
- 11. Lovland, A.; Kaldhusdal, M.; Redhead, K.; Skejerve, E.; Lillehaung, A. (2004). Maternal vaccination against subclinical necrotic enteritis in broilers. Avian Pathol 33 (1), 83-92.
- 12. Marks, S.L.; Kather, E.J. (2003). Antimicrobial susceptibilities of canine Clostridium difficile and Clostridium perfringens isolates to commonly utilized antimicrobial drugs. Vet. Microbiol 94, 39-45.
- 13. Martel, A.; Devriese, L.A.; Cauwerts, K.; De Gussem, K.; Decostere, A.; Haesebrouck, F. (2004). Susceptibility of Clostridium perfringens strains from broiler chicken to anbiotics and anticoccidials. Avian Pathol 33, 3-7.
- 14. Mccourt, M.T.; Finlay, D.A.; Laird, C.; Smyth, J.A.; Bell, C.; Ball, H.J. (2005). Sandwich ELISA detection of Clostridium perfringens cells and a-toxin from field cases of necrotic enteritis of poultry. Vet. Microbiol 106, 259-264.
- 15. Meer, R.R.; Songer, J.G. (1997). Multiplex polimerase chain reaction assay for genotyping of Clostridium perfringens Am. J. Vet. Res 58, 702-705.
-
16NCCLS. (2004). Methods for antimicrobial susceptibility testing of anaerobic bacteria. Fifht edition. Approved Standart M11-A6. National Committee for Clinical Laboratory Standards, Wayne, P.A.
- 17. Sasaki, Y.; Yamamoto, K.; Tamura, Y.; Takahashi, T. (2001). Tetracycline-resistance genes of Clostridium perfringens, Clostridium septicum and Clostridium sordellii isolated from cattle affected with malignant edema. Vet. Microbiol 81 (1), 61-69.
- 18. Si, J.; Gong, J.; Han, Y.; Yu, H.; Brennan, J.; Zhou, H.; Chen, S. (2007). Quantification of cell proliferation and alpha-toxin gene expression of Clostridium perfringens in the development of necrotic enteritis in broiler chickens. Appl. Environ. Microbiol 73 (21), 7110-7113.
- 19. Thompson, D.R.; Parreira, V.R.; Kulkarni, R.R.; Prescott, J.F. (2006). Live attenuated vaccine-based control of necrotic enteritis of broiler chickens. Vet. Microbiol 113, 25-34.
- 20. Van Immerseel, F.; De Buck, J.; Pasmans, F.; Huyghebaert, G.; Haesebrouck, F.; Ducatelle, R. (2004). Clostridium perfringens in poultry: an emerging threat for animal and public health. Avian Pathol 33 (6), 537-549.
- 21. Watkins, K.L.; Shryock, T.R.; Dearth, R.N.; Saif, Y.M. (1997). In-vitro antimicrobial susceptibility of Clostridium perfringens from commercial turkey and broiler chicken origin. Vet. Microbiol 54, 195-200.
-
22World Health Organization. (1997). The medical impact of the use of antimicrobial in food animals. Publication WHO/EMZ/ZOO/97.4. World Health Organisation, Geneva, Switzerland.
Publication Dates
-
Publication in this collection
24 July 2009 -
Date of issue
June 2009
History
-
Reviewed
08 Aug 2008 -
Received
06 June 2008 -
Accepted
16 Apr 2009