Abstract
Urogenital infections affect millions of people every year worldwide. The treatment of these diseases usually requires the use of antimicrobial agents, and more recently, the use of probiotic lactic acid bacteria (LAB) cultures for the management of vaginal infections has been extensively studied. In this work, 11 vaginal lactobacilli isolates, previously obtained from healthy patients, were studied to screen microorganisms with probiotic properties against Candida spp. The LAB were tested for their ability of auto-aggregation, coaggregation with C. albicans, C. glabrata, C. krusei, and C. tropicalis, adhesion to Caco-2 epithelial cells and production of lactic acid and hydrogen peroxide (H2O2). All lactobacilli isolates tested were able to auto-aggregate (ranging from 25.3% to 75.4% assessed at 4 hours of incubation) and to co-aggregate with the four Candida species into different degrees; among them L. crispatus showed the highest scores of coaggregation. The highest amount of lactic acid was produced by L. salivarius (13.9 g/l), followed by L. johnsonii (6.5 g/l), L. acidophilus (5.5 g/l), and L. jensenii (5.4 g/l). All isolates produced H2O2 , but the highest levels (3 -10 mg/l) were observed for L. acidophilus, L. crispatus, L. gasseri, L. johnsonii, and L. vaginalis. Only L. agilis, L. jensenii, L. johnsonii and L. ruminus were able to adhere to epithelial Caco-2 cells. Among the isolates evaluated, L agilis, L. jensenii, L. johnsonii, and L. ruminus exhibited simultaneously several desirable properties as potential probiotic strains justifying future studies to evaluate their technological properties in different pharmaceutical preparations for human use.
Lactobacillus spp.; probiotic; Candida spp
MEDICAL
Vaginal lactobacilli as potential probiotics against Candida spp.
Natalia F. Gil; Rafael C.R. Martinez; Bruna C. Gomes; Auro Nomizo; Elaine C. P. De Martinis* * Corresponding Author. Mailing address: Departamento de Análises Clínicas, Toxicológicas e Bromatológicas, Faculdade de Ciências Farmacêuticas de Ribeirão Preto - Universidade de São Paulo, Av. do Café, s/n, 14040-903 - Ribeirão Preto - SP - Brazil.; Tel. +55 16 36024267 Fax +55 16 36024725.; E-mail: edemarti@usp.br
Faculdade de Ciências Farmacêuticas de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, SP, Brasil
ABSTRACT
Urogenital infections affect millions of people every year worldwide. The treatment of these diseases usually requires the use of antimicrobial agents, and more recently, the use of probiotic lactic acid bacteria (LAB) cultures for the management of vaginal infections has been extensively studied. In this work, 11 vaginal lactobacilli isolates, previously obtained from healthy patients, were studied to screen microorganisms with probiotic properties against Candida spp. The LAB were tested for their ability of auto-aggregation, coaggregation with C. albicans, C. glabrata, C. krusei, and C. tropicalis, adhesion to Caco-2 epithelial cells and production of lactic acid and hydrogen peroxide (H2O2). All lactobacilli isolates tested were able to auto-aggregate (ranging from 25.3% to 75.4% assessed at 4 hours of incubation) and to co-aggregate with the four Candida species into different degrees; among them L. crispatus showed the highest scores of coaggregation. The highest amount of lactic acid was produced by L. salivarius (13.9 g/l), followed by L. johnsonii (6.5 g/l), L. acidophilus (5.5 g/l), and L. jensenii (5.4 g/l). All isolates produced H2O2 , but the highest levels (3 -10 mg/l) were observed for L. acidophilus, L. crispatus, L. gasseri, L. johnsonii, and L. vaginalis. Only L. agilis, L. jensenii, L. johnsonii and L. ruminus were able to adhere to epithelial Caco-2 cells. Among the isolates evaluated, L agilis, L. jensenii, L. johnsonii, and L. ruminus exhibited simultaneously several desirable properties as potential probiotic strains justifying future studies to evaluate their technological properties in different pharmaceutical preparations for human use.
Key words: Lactobacillus spp., probiotic, Candida spp.
INTRODUCTION
In women of childbearing age, the vaginal ecosystem is dominated by Lactobacillus spp. (41). These microorganisms can prevent the colonization of the urogenital tract by several pathogens and they are important for women's reproductive and general healthy (17, 24, 40, 42).
Lactobacilli modulate the vaginal microbiota by different mechanisms such as: (i) auto-aggregation, (ii) production of lactic acid, hydrogen peroxide, bacteriocins, and biosurfactants, (iii) co-aggregation with pathogenic microorganisms, and (iv) adhesion to epithelial cells (17, 29, 37).
Bacterial vaginosis (BV) and vulvovaginal candidiasis (VVC) are the most prevalent vaginal infections worldwide (30). BV is responsible for up to 50% of all the cases of vaginal infections and it is characterized by a significant reduction in lactobacilli population, and increase in facultative aerobic and anaerobic pathogens (10, 16).
VVC affects up to 75% of women at least once in their lives and despite pruritus and vaginal discharge are usual complaints associated with this disease neither is specific to the infection (34). The majority of cases of VVC (ca. 90%) caused by Candida albicans are treated with oral or topical antifungal agents, with increasing reports on episodes of VVC due to non-albicans species (27, 28). There is an overgrowing concern about the spread use of over-the-counter preparations (such as topical azole agents) which may contribute for the selection of non-albicans resistant strains that are normally more difficult to be eradicated (23, 35).
Probiotics are defined as live microorganisms which when administered in adequate quantity confer health benefits to the host and lactobacilli of human origin are potential probiotics against urogenital tract infections (11, 25). Some clinical studies showed positive results for the use of L. fermentum RC-14 and L. rhamnosus GR-1 to treat patients with BV by oral intake and intravaginal administration (1, 2). Also, a recent clinical trial showed that oral administration of capsules containing L. fermentum RC-14 and L. rhamnosus GR-1 was effective as adjuvant in the treatment of patients diagnosed with VVC (20).
Probiotics do not show collateral effects usually seen for traditional antibacterial and antifungal agents because they act by several mechanisms, which minimize punctual mutations involved in the emergence of antimicrobial resistant pathogens. The technology necessary to produce probiotic agents does not appear to be complex, and this can stimulate their production at reasonable costs. This scenario certainly encourages more researches to be undertaken to select and test new strains with probiotic properties.
The aim of the present work was to evaluate the ability of Lactobacillus spp., previously isolated from the vaginal microbiota of healthy Brazilian patients, as potential probiotics against Candida species.
MATERIALS AND METHODS
Strains
A total of 11 vaginal Lactobacillus spp. were previously isolated from a group of 64 healthy Brazilian women (21) and the use of the strains for this study was approved by local Ethic Review Board (250/CEP-CSE-FMRP-USP). The isolates studied were L. acidophilus, L. agilis, L. coleohominis, L. crispatus, L. fermentum, L. gasseri, L. jensenii, L. johnsonii, L. salivarius, L. ruminus and L. vaginalis. Additionally, for the study of adhesion to epithelial cells, L. bulgaricus and L. rhamnosus GG were employed as negative and positive controls, respectively. The bacterial strains were kept at -70ºC in MRS broth (de Man, Rogosa and Sharpe - Oxoid, UK) added of 20 % (v/v) of glycerol.
A total of four Candida spp. strains were used in this study, to know: C. albicans ATCC 18804, C. tropicalis ATCC 750, C. krusei ATCC 20298 and C. glabrata ATCC 2001. The yeast strains were kept in SDA (Sabouraud-dextrose agar - Oxoid, UK) at room temperature.
Auto-aggregation studies
Lactobacillus spp. was grown overnight at 37ºC in MRS broth (1.0%, v/v), centrifuged at 6,000g for 15min (Fanem, mod. 208 N, Brazil) and cell pellets were resuspended in phosphate buffered saline (PBS) to obtain an optical density (O.D.) of 0.6 at 600nm (UVmini-1240, Shimadzu, Japan). Auto-aggregation inversely correlated with O.D. and it was monitored every 1h for up to 4h of incubation (13, 25). Gram staining was used to visualize the aggregates under oil immersion microscopy with 100 times magnification (CX-31 - Olympus, Japan).
Co-aggregation studies
Culture plates of 24 wells containing round glass slides were added of: i) 500µL of an overnight culture of Lactobacillus spp. grown at 37ºC in MRS broth and ii) 500µL of an overnight culture of Candida spp. grown at 37ºC in BHI broth (Brain-Heart Infusion - Oxoid, UK). Plates were incubated at 37ºC for 4h in an orbital shaker at 100 rpm (CT-712, Cientec, Brazil) and co-aggregation was determined by Gram staining of the round glass slides and observation under oil immersion microscopy (CX-31 - Olympus, Japan). Scoring was done according to Reid et al. (31).
Production of lactic acid
Homofermentative metabolism was verified by absence of production of gas from glucose (33) and lactic acid production was quantified in grams per liter, by acid-base titration, according to Edema and Sanni (9).
Production of hydrogen peroxide
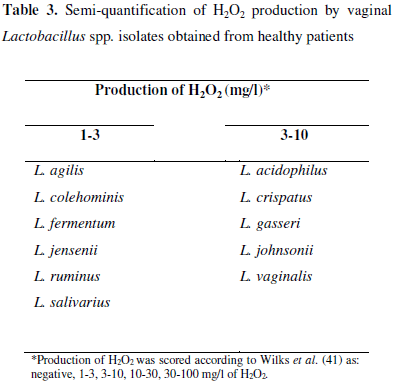
Determination of hydrogen peroxide (H2O2) production by Lactobacillus isolates was performed according to Wilks et al. (41) with modifications. Briefly, lactobacilli were grown in MRS broth (Oxoid, UK) for 24h at 37ºC and 100µl-aliquots of the broths were seeded on MRS agar plates (Oxoid, UK) and incubated for 48h at 37ºC, under anaerobic atmosphere. Selected colonies were put in contact with strips containing peroxidase (Merckoquant Peroxide Test -Merck, Germany). Different tones of blue products were visually compared, with a scale provided by the manufacturer. Results were expressed in ranges of H2O2 production according to Wilks et al. (41).
Adhesion to the epithelial cells
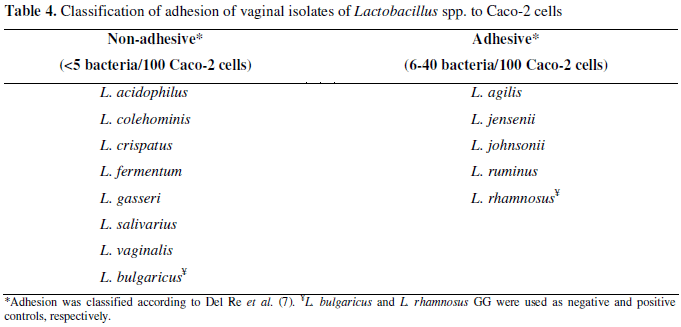
Adhesion to intestinal epithelial Caco-2 cells (ATCC 7348406) was evaluated according to Duprè et al. (8). Briefly, Caco-2 cells were cultivated at 37ºC under 5% CO2 in RPMI medium (Gibco, USA) supplemented with 10% of fetal bovine serum and 100U/ml of streptomycin and penicillin (Sigma, USA). When confluent growth was achieved, adhered cells were trypsinized, transferred to 24-well plates containing round glass slides and re-incubated. After 24 hours, the RPMI medium with antibiotics was removed and replaced by RPMI supplemented with 2% of fetal bovine serum. Bacterial cultures were previously grown overnight at 37ºC in MRS broth, diluted in RPMI containing 2% of fetal bovine serum and added to each well (ca. 106 bacteria) containing the Caco-2 cells and incubation was done at 37ºC for 3 hours. After that, slides were washed, fixed, stained with May-Grunwald- Giemsa and analyzed under oil immersion microscopy with 100 times magnification (CX-31 - Olympus, Japan).
The number of bacteria adhered to Caco-2 cells was obtained by scoring adhesion to 100 random eukaryotic cells, using criteria proposed by Del Re et al. (7) as non-adhesive (<5 bacteria/100 cells), adhesive (6-40 bacteria/100 cells) and strongly adhesive (>40 bacteria/100 cells). L. bulgaricus and L. rhamnosus GG were used as negative and positive controls, respectively.
RESULTS AND DISCUSSION
All lactobacilli isolates tested in the present study exhibited some degree of auto-aggregation at all time-points tested, but the highest degree of auto-aggregation was observed after 4h of incubation at 37ºC for L. jensenii, followed by L. ruminus and L. acidophilus (Figure 1). Figure 1 also illustrates auto-aggregation observed for L. acidophilus in two time-points. The ability of auto-aggregation of vaginal lactobacilli is an intrinsic characteristic and may substantially increase the colonization of environments with short residence times (25). According to Juarez-Tomás et al. (13) the ability of auto-aggregation is higher in acid environments where probiotic lactobacilli are more adapted to survive and represents the first step towards the formation of biofilms by lactobacilli strains, which helps to inhibit the overgrowth and proliferation of pathogenic microorganisms (14, 37).
The co-aggregation scores obtained after 4h of incubation at 37ºC for Lactobacillus spp. and Candida spp. are shown in Table 1 and illustrated in Figure 2. L. crispatus showed macroscopically visible clumps when evaluated with all four Candida strains. The co-aggregation can create a microenvironment around the pathogen with a higher concentration of inhibitory substances and it can also block the dissemination of pathogens to tissue receptors (22, 29).
The results for quantification of lactic acid produced by the lactobacilli isolates are summarized in Table 2. The highest amount of lactic acid was produced by L. salivarius, followed by L. johnsonii, L. acidophilus and L. jensenii. The production of organic acids helps to keep the vaginal pH below 4.5 and creates a hostile environment for the growth and survival of pathogenic microorganisms (3). In a study conducted by Valore et al. (40) with vaginal exudates samples obtained from healthy patients, a higher antimicrobial activity was verified for samples with the highest levels of lactic acid.
Hydrogen peroxide is another antagonistic compound produced by lactobacilli and its production is normally assessed by using qualitative methods, such as incorporation of the peroxide in agar medium and revelation by addition of tetramethylbenzidine (19, 24). However, quantitative results may help to better understand the role of H2O2 in healthy and infected vaginal environments (38, 41). In this study, despite H2O2 production by all isolates, the highest levels were observed for L. acidophilus, L. crispatus, L. gasseri, L. johnsonii, and L. vaginalis (Table 3).
In vaginal exudates, H2O2 is converted to reactive oxygen species (ROS) such as superoxide anions, hydrogen peroxide and hidroxyl free radicals that are highly toxic against several microorganisms (15). Besides that, lactobacilli keep a high oxireduction potential in the vaginal environment, which inhibits multiplication of strictly anaerobic bacteria (3). The absence hydrogen peroxide-producing lactobacilli has been related to a higher risk of BV, recurrent urinary tract infection by E. coli and increased susceptibility to the infection by Human Immunodeficiency Virus (HIV-1) (31, 38).
Atassi et al. (4) affirmed that adhesion to epithelial tissue is the first step towards the formation of a barrier by lactobacilli that will prevent colonization by pathogenic microorganisms. In our study lactobacilli isolates were tested for adhesion to epithelial tissue, using Caco-2 cell, which presents a confluent growth when cultivated in vitro and shows characteristics of mature enterocytes, making it an excellent model for studying adherence of microorganisms (6, 12). Among all lactobacilli isolates tested, only L. agilis, L. jensenii, L. johnsonii and L. ruminus were able to adhere to epithelial Caco-2 cells (Table 4 and Figure 3) indicating adhesion is specific for each bacterial strain, as verified also by Chauvière et al. (6).
Some vaginal Lactobacillus species are capable of synthesizing antimicrobial peptides known as bacteriocins (5). In our study, we have also evaluated bacteriocin production of all lactobacilli isolates against Candida strains by agar antagonism method, but no inhibitory activity was detected (data not shown). Osset et al. (26) studied the production of bacteriocin by several LAB isolates against C. albicans and C. glabrata and they did not observe inhibition zones when agar plate method was used. However, in broth cultures those authors verified that some lactobacilli were inhibitory against C. albicans to some extent. In our experiments, highest inhibitory activity against C. albicans assessed in BHI broth was obtained for both L. jensenii and L. johnsonii isolates (data not shown).
Ström et al. (36) observed that several antifungal compounds, such as cyclic dipeptides, pyroglutamic acid and lactones were produced by Lactobacillus isolates and played an important role against Candida spp. Thus further investigation is required to clarify the nature of the inhibitory substance produced by lactobacilli isolates against the yeast in our study.
In conclusion, our results indicated that among the 11 vaginal lactobacilli isolates tested, L agilis, L. jensenii, L. johnsonii, and L. ruminus exhibited desirable properties as potential probiotic strains including the ability to adhere to epithelial mucosa, to auto-aggregate, co-aggregate with Candida species, and to produce both lactic acid and H2O2. Also, future studies are encouraged to assess technological properties of those microorganisms for clinical use, including determination of their viability and stability in pharmaceutical preparations such as capsules resistant to gastrointestinal tract for oral intake and ovules/capsules for intravaginal administration.
ACKNOWLEDGMENTS
Natalia F. Gil is grateful to The State of São Paulo Research Foundation (FAPESP, Process # 06/60105-7) for granting an undergraduate fellowship to do this work. The authors are also grateful to Prof. Gregor Reid (University of Western Ontario - Canada), who kindly donated L. rhamnosus GG strain.
Submitted: July 16, 2008; Returned to authors for corrections: April 09, 2009; Approved: September 28, 2009.
- 1. Anukam, K.; Osazuwa, E.; Ahonkhai, I.; Ngwu, M.; Osemene, G.; Bruce, A.W.; Reid, G. (2006 a). Augmentation of antimicrobial metronidazole therapy of bacterial vaginosis with oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14: randomized, double-blind, placebo controlled trial. Microbes Infect., 8, 1450-1454.
- 2. Anukam, K.C.; Osazuwa, E.; Osemene, G.I.; Ehigiagbe, F.; Bruce, A.W.; Reid, G. (2006 b). Clinical study comparing probiotic Lactobacillus GR-1 and RC-14 with metronidazole vaginal gel to treat symptomatic bacterial vaginosis. Microbes Infect., 8, 2772-2776.
- 3. Aroutcheva, A.; Gariti, D.; Simon, M.; Shott, S.; Faro, J.; Simoes, JA; Gurguis, A; Faro, S. (2001). Defense factors of vaginal lactobacilli. Am. J. Obstet. Gynecol., 185, 375-379.
- 4. Atassi, F.; Brassart, D.; Grob, P.; Graf, F.; Servin, A.L. (2006). Lactobacillus strains isolated from the vaginal microbiota of healthy women inhibit Prevotella bivia and Gardnerella vaginalis in coculture and cell culture. FEMS Immunol. Med. Microbiol., 48, 424-432.
- 5. Boris, S.; Barbès, C. (2000). Role played by Lactobacillus in controlling the population of vaginal pathogens. Microbes Infect., 2, 543-546. 21.
- 6. Chauvière, G.; Coconnier, M.H.; Kernéis, S.; Fourniat, J.; Servin, A.L. (1992). Adhesion of human Lactobacillus acidophilus strain LB to human enterocyte-like Caco-2 cells. J. Gen. Microbiol., 138, 1689-1696.
- 7. Del Re, B.; Sgorbati, B.; Miglioli, M.; Palenzona, D. (2000). Adhesion, autoaggregation and hydrophobicity of 13 strains of Bifidobacterium longum. Lett. Appl. Microbiol. 31, 438-442.
- 8. Duprè, I.; Zanetti, S.; Schito, A.M.; Fadda, G.; Sechi, L.A. (2003). Incidence of virulence determinants in clinical Enterococcus faecium and Enterococcus faecalis isolates collected in Sardinia (Italy). J. Med. Microbiol., 52, 491-498.
- 9. Edema, M.O.; Sanni, A.I. (2008). Functional properties of selected starter cultures for sour maize bread. Food Microbiol., 25, 616-625.
- 10. Eschenbach, D.A.; Hillier, S.; Critchlow, C.; Stevens, C.; DeRouen, T.; Holmes, K.K. (1988). Diagnosis and clinical manifestations of bacterial vaginosis. Am. J. Obstet. Gynecol., 158, 819-828.
- 11. FAO-WHO (2001). Food and agriculture organization of the United Nations, World Health Organization. Report of a joint FAO-WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Cordoba, Argentina, 2001. Available in: ftp://ftp.fao.org./docrep/fao/009/a0512e/a0512e00.pdf
- 12. Hauri, H.P.; Sander, B.; Naim, H. (1994). Induction of lactase biosynthesis in the human intestinal epithelial cell line Caco-2. Eur. J. Biochem., 219, 539-546. 28.
- 13. Juárez Tomás., M.S.; Wiese, B.; Nader-Macías, M.E. (2005). Effects of culture conditions on the growth and auto-aggregation ability of vaginal Lactobacillus johnsonii CRL 1294. J. Appl. Microbiol., 99, 1383-1391.
- 14. Kos, B.; Susković, J.; Vuković, S.; Simpraga, M.; Frece, J.; Matosić, S. (2003). Adhesion and aggregation ability of probiotic strain Lactobacillus acidophilus M92. J. Appl. Microbiol., 94, 981-987. 30.
- 15. Kulisaar, T.; Zilmer, M.; Mikelsaar, M.; Vihalemm, T.; Annuk, H.; Kairane, C.; Kilk, A. (2002). Two antioxidative lactobacilli strains as promising probiotics. Int. J. Food Microbiol., 72, 215-224.
- 16. Larsson, P.G.; Forsum, U. (2005). Bacterial vaginosis - a disturbed bacterial flora and treatment enigma. APMIS, 113, 305-316.
- 17. Lepargneur, J.P.; Rousseau, V. (2002). Protective role of the Doderlein flora. J. Gynecol. Obstet. Biol. Reprod., 31, 485-494.
- 18. Lewus, C.B.; Montville, T.J. (1991). Detection of bacteriocins produced by lactic acid bacteria. J. Microbiol. Methods, 13, 145-150.
- 19. Martin, H.L.; Richardson, B.A.; Nyange, P.M.; Lavreys, L.; Hillier, S.L.; Chohan, B.; Mandaliya, K.; Ndinya-Achola, J.O.; Bwayo, J.; Kreiss, J. (1999). Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J. Infect. Dis., 180, 1863-1868.
- 20. Martinez, R.C.R.; Franceschini, S.A.; Patta, M.C.; Quintana, S.M.; Candido, R.C.; Ferreira, J.C.; De Martinis, E.C.P.; Reid, G. (2009). Improved treatment of vulvovaginal candidiasis with fluconazole plus probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14. Lett. Appl. Microbiol., 48, 269-274.
- 21. Martinez, R.C.R.; Franceschini, A.S.; Patta, M.C.; Quintana, S.M.; Nunes, A.C.; Moreira, J.L.S.; Anukam, K.C.; Reid, G.; De Martinis, E.C.P. (2008). Analysis of vaginal lactobacilli from healthy and infected Brazilian women. Appl. Environ. Microbiol., 74, 4539-4542.
- 22. Mastromarino, P.; Brigidi, P.; Macchia, S.; Maggi, L.; Pirovano, F.; Trinchieri, V.; Conte, U.; Matteuzzi, D. (2002). Characterization and selection of vaginal Lactobacillus strains for the preparation of vaginal tablets. J. Appl. Microbiol., 93, 884-893.
- 23. Mathema, B.; Cross, E.; Dun, E.; Park, S.; Bedell, J.; Slade, B.; Willians, M.; Riley, L.; Chaturvedi, V.; Perlin, D.S. (2001). Prevalence of vaginal colonization by drug-resistant Candida species in college-age women with previous exposure to over-the-counter azole antifungals. Clin. Infect. Dis., 33, 23-27.
- 24. Mijać, V.D.; Dukić, S.V.; Opavski, N.Z.; Dukić, M.K.; Ranin, L.T. (2006). Hydrogen peroxide producing lactobacilli in women with vaginal infections. Eur. J. Obst. Gynecol. Reprod. Biol., 129, 69-76.
- 25. Ocaña, V.S.; Nader-Macías, M.E. (2002). Vaginal lactobacilli: self-and co-aggregation ability. Br. J. Biomed. Sci., 59, 183-190.
- 26. Osset, J.; García, E.; Bartolomé, R.M.; Andreu, A. (2001). Role of Lactobacillus as protector against vaginal candidiasis. Med. Clin., 22, 285-288.
- 27. Owen, M.K.; Clenney, T.L. (2004). Management of vaginits. Am. Fam. Physician., 70, 2125-2132.
- 28. Paulitsch, A.; Weger, W.; Ginter-Hanselmayer, G.; Marth, E.; Buzina, W. (2006). A 5-year (2000-2004) epidemiological survey of Candida and non-Candida yeast species causing vulvovaginal candidiasis in Graz, Austria. Mycoses 49, 471-475.
- 29. Reid, G. (2001). Probiotic agents to protect the urogenital tract against infection. Am. J. Clin. Nutr., 73, 437-443.
- 30. Reid, G.; Bruce, A.W.; Fraser, N.; Heinemann, C.; Owen, J.; Henning, B. (2001). Oral probiotics can resolve urogenital infections. FEMS Immunol. Med. Microbiol., 30, 49-52.
- 31. Reid, G.; MacGroarty, J.A.; Domingue, P.A.G.; Chow, A.W.; Bruce, A.W.; Eisen, A.; Costerton, J.W. (1990). Coaggregation of urogenital bacteria in vitro and in vivo. Current Microbiol., 20, 47-52.
- 32. Reid, G.; Kim, S.O.; Köhler, G.A. (2006). Selecting, testing and understanding probiotic microorganisms. FEMS Immunol. Med. Microbiol., 46, 149-157.
- 33. Schillinger, U.; Lücke, F.K. (1987). Identification of lactobacilli from meat and meat products. Food Microbiol., 4, 199-208.
- 34. Sobel, J.D. (2007). Vulvovaginal candidiasis. Lancet 369, 1961-1971.
- 35. Sobel, J.D. (1998). Vulvovaginitis due to Candida glabrata An emergin problem. Mycoses 41, 18-22.
- 36. Ström, K.; Sjögren, J.; Broberg, A.; Schnürer, J. (2002). Lactobacillus plantarum MiLAB 393 produces the antifungal cyclic dipeptides cyclo (L-Phe-L-Pro) and cyclo (L-Phe-trans-4-OH-L-Pro) and 3-phenyllactic acid. Appl. Environ. Microbiol., 68, 4322-4327.
- 37. Strus, M.; Kucharska, A.; Kukla, G.; Brzychczy-Włoch, M.; Maresz, K.; Heczko, P.B. (2005). The in vitro activity of vaginal Lactobacillus with probiotic properties against Candida. Infect. Dis. Obstet. Gynecol., 13, 69-75.
- 38. Tomas, M.S.; Bru, E.; Nader-Macías, M.E. (2003). Comparison of the growth and hydrogen peroxide production by vaginal lactobacilli under different culture conditions. Am. J. Obstet. Gynecol., 188, 35-44.
- 39. Vallor, A.C.; Antonio, M.A.; Hawes, S.E.; Hillier, S.L. (2001). Factors associated with acquisition of, or persistent colonization by, vaginal lactobacilli: role of hydrogen peroxide production. J. Infect. Dis., 184, 1431-1436.
- 40. Valore, E.V.; Park, C.H.; Igreti, S.L.; Ganz, T. (2002). Antimicrobial components of vaginal fluid. Am. J. Obstet. Gynecol., 187, 561-568.
- 41. Wilks, M.; Wiggins, R.; Whiley, A.; Hennessy, E.; Warwick, S.; Porter, H.; Corfield, A.; Millar, M. (2004). Identification and H2O2 production of vaginal lactobacilli from pregnant womem at high risk o preterm birth and relation with outcome. J. Clin. Microbiol., 42, 713-717.
- 42. Zárate, G.; Nader-Macías, M.E. (2006). Influence of probiotic vaginal lactobacilli on in vitro adhesion of urogenital pathogens to vaginal epithelial cells. Lett. Appl. Microbiol., 43, 174-180.
Publication Dates
-
Publication in this collection
23 Nov 2009 -
Date of issue
Mar 2010
History
-
Reviewed
09 Apr 2009 -
Received
16 July 2008 -
Accepted
28 Sept 2009