Abstract
Due to the fact the incubation conditions may influence the microbiological evaluation of water for dialysis, the objective of the present study was the comparison of the efficiency of R2A and PCA media in the enumeration of heterotrophic bacteria in 193 samples of water collected in dialysis clinics from 12 cities in São Paulo, between October and December 2007. Results showed counts significantly greater in R2A, suggesting that enumeration should be carried out in R2A, suggesting that enumeration should be carried out in R2A agar associated with longer incubation times, because of the greater sensitivity.
Water for dialysis; heterotrophic bacteria; pour plate; Reasoner'2 Agar; Plate Count Agar
MEDICAL
Enumeration of heterotrophic bacteria in water for dialysis: comparison of the efficiency of Reasoner'2 agar and plate count agar
Adriana Bugno* * Corresponding Author. Mailing address: Instituto Adolfo Lutz, Diretoria de Medicamentos, Av. Dr. Arnaldo, 355, Cerqueira Cesar, CEP 01246902, São Paulo, SP, Brasil.; Tel.: 11 30682963.; Email: adrbugno@ial.sp.gov.br ; Adriana Aparecida Buzzo Almodóvar; Tatiana Caldas Pereira
Instituto Adolfo Lutz, Serviço de Medicamentos, São Paulo, SP, Brasil
ABSTRACT
Due to the fact the incubation conditions may influence the microbiological evaluation of water for dialysis, the objective of the present study was the comparison of the efficiency of R2A and PCA media in the enumeration of heterotrophic bacteria in 193 samples of water collected in dialysis clinics from 12 cities in São Paulo, between October and December 2007. Results showed counts significantly greater in R2A, suggesting that enumeration should be carried out in R2A, suggesting that enumeration should be carried out in R2A agar associated with longer incubation times, because of the greater sensitivity.
Key words: Water for dialysis, heterotrophic bacteria, pour plate, Reasoner'2 Agar, Plate Count Agar.
Hemodialysis is a therapeutic procedure for chronic renal insufficiency patients, used to remove waste products, excess water and mineral salts accumulated in the organism as a consequence of renal failure.
The fluid used in dialysis to facilitate the regulation of the electrolytic balance and the removal of toxic substances in the plasma is mainly made up of treated water mixed with a concentrated electrolyte solution, a buffer and glucose (11, 16, 18, 23).
Chronic renal disease patients undergoing regular hemodialysis treatment are submitted to three 3 to 4-hour weekly sessions. They are exposed to approximately 120L of treated water in each session (4, 12, 16, 18, 22, 23). Water is separated from the blood of the patient by a semipermeable membrane, which may enable direct access of contaminants that might be present in the water (6, 9, 10, 11, 22). Monitoring and maintenance of water treatment systems are important to assure its chemical and microbiological quality and to prevent additional risks to the patients (4, 6, 9, 10, 11, 12, 14, 15, 16, 18, 22, 23).
Recognition of the potential risk that the quality of water for dialysis may pose led to the development of criteria and standards for this water, determined by several organs and commissions, such as the European Pharmacopoeia (9, 10, 15, 16, 23), the Association for the Advancement of Medical Instrumentation (AAMI) (4, 10, 13, 16, 21, 23) and, in Brazil, by Resolução RDC # 154/2004 (5).
Microbiological parameters for the quality of treated water are defined in order to prevent the occurrence of bacteremia and pyrogenic reactions. Microorganisms may grow very quickly in water and dialysis fluids, and the development of biofilms enables their persistence in the system, increasing the risk of contamination (3, 4, 6, 9, 10, 12, 13, 15, 16, 18, 22). The intact membrane of the dialysis machine should prevent the contamination of the blood with bacteria from the dialysis fluid. However, infections may still occur when membrane integrity is compromised, when microbial contamination of the water is high or when contamination occurs during the reutilization of the dialysis machines (3, 13, 14, 16, 22).
Classical approaches for the enumeration of microorganisms in water include plate counts, membrane filtration and the most probable number technique (17). As occurs in any microbiological technique, results of microorganism enumeration are influenced by culture media used, as well as by the incubation conditions (1, 13, 16, 17, 20, 21, 23).
There are two basic culture media used in microbiological analyses: complex media with high nutritional content, such as trypticase soy agar (TSA) and plate count agar (PCA). These media are indicated for the isolation and enumeration of heterotrophic bacteria isolated from animals and humans. There are also simple media with few nutrients, such as Reasoner's 2 Agar (R2A), used in the detection of slow-growing, oligotrophic bacteria, and in the enumeration of heterotrophic bacteria adapted to aquatic environments and that require low concentration of nutrients (1, 17, 20). Techniques that use simple culture media, associated with longer incubation periods (5 to 7 days) at lower temperatures (20 to 28ºC) have been shown to be more sensitive in determining microbial contamination of water for human consumption and of water for dialysis (2, 7, 13, 15, 16, 17, 19, 20, 21). However, the use of more complex culture media may improve the recovery of microorganisms when longer incubation times and lower temperatures are used (20). The last editions of the United States Pharmacopeia (20) and the European Pharmacopoeia (8) recommend the use of R2A medium for the enumeration of heterotrophic bacteria in treated water, incubated between 20 and 25ºC for 4 to 7 days, or between 30 and 35ºC from 3 to 4 days.
Considering the importance and the need to evaluate microbiological quality of water used in dialysis centers, the objective of the present study was to compare the efficiency of R2A and PCA agars in the enumeration of heterotrophic bacteria in treated water collected from different dialysis clinics in the city of São Paulo and other cities in the Grande São Paulo region, from October and December 2007. The study was carried out to compare the efficiency of R2A and PCA in the enumeration of heterotrophic bacteria. PCA was used as the standard for the evaluation.
A total of 193 samples of treated water were collected in dialysis clinics in the city of São Paulo and in other cities in the Grande São Paulo region, during the Programa Estadual de Monitoramento da Qualidade da Água Tratada em Serviços de Diálise [State Program for the Monitoring of Water for Dialysis]. Samples were aseptically collected after allowing the water to flow for two or three minutes, according to the recommendations of the American Public Health Association (APHA) (2). Samples were transported to the laboratory in isothermal containers, and the interval between collection of the samples and the beginning of the analyses was no more than six hours. Analyses started upon arrival of the samples.
The pour plate technique was carried out according to the recommendations of official compendia (2, 20) for enumerating of heterotrophic bacteria. Aliquots of 1.0 mL of the samples were transferred in quadruplicates to the center of sterile Petri dishes, and 20.0 mL of R2A, molten and cooled to 45ºC, were placed in two of the dishes; 20.0 mL of PCA, also molten and cooled to 45ºC, were poured in the two other plates. The same procedure was carried out using aliquots of 0.1 mL of the samples. Plates were homogenized and, after medium was solidified, they were incubated in an inverted position at 34 ± 2ºC for 96 hours. After incubation, colonies were counted.
Means of the counts of heterotrophic bacteria/mL obtained with each of the culture media were calculated for the 193 samples. The statistical comparison between the two media used to count heterotrophic bacteria in water for dialysis did not consider 17.10% and 6.22% of the samples analyzed using PCA and R2A, respectively, because these plates did not show any growth. All other valid counts were transformed into a log scale to produce a normal distribution, and statistical analyses were carried out using the software SPSS 15.0 for Windows, with a 95% confidence interval.
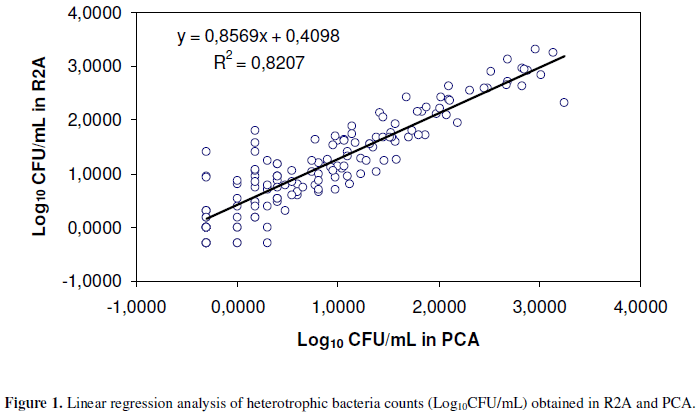
Means obtained in the enumeration of heterotrophic bacteria using R2A and PCA and expressed in Log10 CFU/mL, were respectively, 1.0421 (±0.8894) and 0.8349 (±0.9384), with significant differences between the means (Student's t test < 1.649; p = 0.018). Linear regression analysis was used in order to compare the efficiency in the enumeration of bacteria between the two culture media analyzed. The graph for the regression analysis is presented in Figure 1.
Microorganisms that grow in extreme environments show better results in laboratory culture when they are incubated in conditions that simulate these environments. Because of this, bacteria associated with water for dialysis grow better in low-nutrient culture media, such as R2A, when incubated for more than 48 hours and at temperatures around 25ºC (1, 7, 9, 16, 17, 19-21). Results of this study showed high correlation between log counts in the media evaluated (r = 0.9056, p < 0.01), and counts significantly greater in R2A than in PCA, even using higher temperatures for incubation. Counts in R2A were greater than those in PCA in 81.22% of the samples, whereas 21.88% of the samples showed greater counts in PCA than in R2A.
Besides comparing the efficiency in the enumeration, this study also evaluated the impact of the culture media in the evaluation of the quality of the samples, considering the maximum threshold of 200 CFU/mL determined by Resolução RDC # 154/2004 (5). In the 193 samples, counts over 200 CFU/mL were obtained in 7.77% of them using PCA, and in 10.36% of them using R2A. In PCA 6 samples (3.11%), counts were lower than 200 CFU/mL. However, they were greater than this threshold when R2A was used. Only one sample in R2A (0.52%) showed counts lower than 200 CFU/mL, whereas in PCA, counts were greater than the threshold. These data suggest that PCA underestimated bacterial contamination of the samples and may erroneously indicate that these samples complied with the microbiological standards determined by the official regulations.
R2A, a low-nutrient culture medium, showed better results than PCA in the evaluation of bacterial contamination in water for dialysis, when incubated at around 34ºC for 96 hours. Enumeration of heterotrophic bacteria in water for dialysis should be carried out in R2A associated with longer incubation times, in order to minimize the risks to the patient under dialysis, because of the greater sensitivity of this culture medium.
Submitted: August 28, 2008; Returned to authors for corrections: November 06, 2008; Approved: July 24, 2009.
- 1. Allen, M.J.; Edberg, S.C.; Reasoner, D.J. (2004). Heterotrophic plate count bacteria - what is their significance in drinking water? Int. J. Food Microbiol. 92, 265-274.
- 2. American Public Health Association. (1998). Standard Methods for the examination of Water and Wasterwater 20Ş ed. United Book Press, Inc, Maryland, Baltimore, p. 9-19-9-21, 9-33-9-38, 9-53-9-54.
- 3. Archibald, L.K.; Khoi, N.N.; Jarvis, W.R.; Reller, L.B.; Cam, P.D.; Thu, T.A.; Hung, N.V. (2006). Pyrogenic reactions in hemodialysis patients, Hanoi, Vietnam. Infect. Control Hosp. Epidemiol. 27 (4), 424-426.
- 4. Arvanitidou, M.; Spaia, S.; Katsinas, C.; Pangidis, P.; Constantinidis, T.; Katsouyannopoulos, V.; Vayonas, G. (1998). Microbiological quality of water and dialysate in all haemodialysis centres of Greece. Nephrol. Dial. Transplant. 13, 949-954.
-
5Brasil, Leis e Decretos. (2004). Resolução RDC nº154 de 15 de junho de 2004. Estabelece o Regulamento Técnico para funcionamento dos Serviços de Diálise. Disponível em: http://e-legis.anvisa.gov.br/leisref/public/showAct.php?id=22875&word Acesso em 17 de maio de 2008.
- 6. Brunet, P.; Berland, Y. (2000). Water quality and complications of haemodialysis. Nephrol. Dial. Transplant 15, 578-580.
- 7. Carter, J.T.; Rice, E.W.; Buchberger, S.G.; Lee, Y. (2000). Relationships between levels of heterotrophic bacteria and water quality parameters in a drinking water distribution system. Wat. Res 34 (5), 1495-1502.
- 8. European Pharmacopoeia Comission (1997). European Pharmacopoeia. 3Ş. Ed. Council of Europe, Strasburg, 1799 p.
- 9. Gomila, M.; Gascó, J.; Busquets, A.; Gil, J.; Bernabeu, R.; Buades, J.M.; Lalucat, J. (2005). Identification of culturable bacteria present in haemodialysis water anf fluid. FEMS Microbiol. Ecology 52, 101-114.
- 10. Hoenick, N.A.; Levin. R. (2003). The implications of water quality in hemodialysis. Semin. Dial. 16 (6), 492-497.
- 11. Hoenick, N.A.; Ronco, C.; Levin, R. (2006). The importance of water quality and haemodialysis fluid composition. Blood Purif. 24, 11-18.
- 12. Lima, J.R.O.; Marques, S.G.; Gonçalves, A.G.; Filho, N.S.; Nunes, P.C.; Silva, H.S.; Monteiro, S.G.; Costa, J.M.L. (2005). Microbiological analyses of water from hemodialysis services in São Luís, Maranhão, Brazil. Braz. J. Microbiol. 36, 103-108.
- 13. Lonnemann, G. (1998). Assessment of the quality of dialysate. Nephrol. Dial. Transplant 13 (suppl 5), 17-20.
- 14. Oie, S.; Kamiya, A.; Yoneda, I.; Uchiyama, K.; Tsuchida, M.; Takai, K.; Naito, K. (2003). Microbial contamination of dialysate and its prevention in haemodialysis units. J. Hosp. Infect. 54, 115-119.
- 15. Pérez-Garcia, R.; Rodríguez-Benitez, P.O.C. (2000). Why and how to monitor bacterial contamination of dialysate? Nephrol. Dial. Transplant 15, 760-764.
- 16. Pontoriero, G.; Pozzoni, P.; Andrulli, S.; Locatelli, F. (2003). The quality of dialysis water. Nephrol. Dial. Transplant 18 (suppl 7), vii21-vii25.
- 17. Reasoner, D.J. (2004). Heterotrophic plate count methodology in the United States. Int. J. Food Microbiol 92, 307-315.
- 18. Silva, A.M.M.; Martins, C.T.B.; Ferraboli, R.; Jorgetti, V.; Romão Junior, J.E. (1996). Revisão/Atualização em Diálise: Água para hemodiálise. J. Bras. Nefrol 18 (2), 180-188.
- 19. Uhl, W.; Schaule, G. (2004). Establishment of HPC(R2A) for regrowth control in non-chlorinated distribution systems. Int. J. Food Microbiol. 92: 317-325.
- 20. United States Pharmacopeial Convention (2008). The United States Pharmacopeia 31Ş ed. Port City Press, Baltimore, p. 105-109, 760-782.
- 21. van der Linde, K.; Lim, B.T.; Rondeel, J.M.M.; Antonissen, L.P.M.T, de Jong, G.M.T. (1999). Improved bacteriological surveillance of haemodialysis fluids: a comparison between Tryptic soy agar and Reasoner's 2A media. Nephrol. Dial. Transplant 14, 2433-2437.
- 22. Varo, S.D.; Martins, C.H.G.; Cardoso, M.J.O.; Sartori, F.G.; Montanari, L.B.; Pires-Gonçalves, R.H. (2007). Isolamentos e fungos filamentosos em água utilizada em uma unidade de hemodiálise. Rev. Soc. Bras. Med. Tropical 40, 326-331.
- 23. Vorbeck-Meister, I.; Sommer, R.; Vorbeck, F.; Hörl, W.H. (1999). Quality of water used for haemodialysis: bacteriological and chemical parameters. Nephrol. Dial. Transplant. 14, 666-675.
Publication Dates
-
Publication in this collection
23 Nov 2009 -
Date of issue
Mar 2010
History
-
Accepted
24 July 2009 -
Reviewed
06 Nov 2008 -
Received
28 Aug 2008