Abstract
Regenerated extracellular NH4+in laboratory batch-cultures of the heterotrophic marine microzooplankter Oxyrrhis marina affects the strength and consistency of chemotaxes elicited by synthetic and biogenic chemoattractants. The ecological relevance of experiments with batch-cultured O. marina and limitations of the microcapillary assay for the study of chemosensory behaviours are discussed.
Chemosensory; Chemotaxis; Chemotaxes; Oxyrrhis marina
MEDICAL MICROBIOLOGY
Regenerated extracellular NH4+ affects the motile chemosensory responses of batch-cultured Oxyrrhis marina
Claire M. Martel
Institute of Life Science, School of Medicine, Swansea University, Singleton Park, SA2 8PP, UK
ABSTRACT
Regenerated extracellular NH4+in laboratory batch-cultures of the heterotrophic marine microzooplankter Oxyrrhis marina affects the strength and consistency of chemotaxes elicited by synthetic and biogenic chemoattractants. The ecological relevance of experiments with batch-cultured O. marina and limitations of the microcapillary assay for the study of chemosensory behaviours are discussed.
Key words: Chemosensory, Chemotaxis, Chemotaxes, Oxyrrhis marina
Chemotaxis, the directional movement of cells or organisms in response to chemical stimulation is an ancestral and essential physiological response (18) first described by W. Pfeffer in 1884 following observations of the attraction of fern sperm to the ova. Since then the phenomenon has been observed in cell types ranging from amoeboid slime molds (4) and bacteria (1) to mammalian macrophages (10). In the field of microbial marine ecology, motile chemosensory responses to dissolved chemical stimuli have been documented in marine bacteria (13), autotrophic algae (26) and cryptophytes (17) and are understood to assist in nutrient acquisition and avoidance responses (e.g. to noxious compounds). It is suspected that some symbiotic marine microbes (e.g. the dinoflagellate Symbiodinium microadriaticum) might use chemotaxis to help locate specific hosts (22); another dinoflagellate species - Crypthecodinium cohnii - shows strong positive chemotaxis towards polysaccharides derived from its specialist prey species - the marine microalga Porphyridium (29). Given the specificity of S. microadriaticum and C. cohnii, it is reasonable to infer that the chemosensory apparatus and responses of both could be highly refined. For heterotrophic protozoa that consume a wider variety of prey items -hereafter focusing on the marine microzooplankter Oxyrrhis marina - it has been postulated (9) and demonstrated (19, 21) that chemotaxis can enhance the overall efficiency of prey location. Pertinently, it has long been assumed that O. marina must possess chemosensory apparatus with an affinity for dissolved chemical stimuli (11, 28). However, despite being employed as a model organism for well over a decade (for recent review [20]) the biochemical basis of any such apparatus remains poorly investigated. Instead, studies designed to explore chemotaxis have tended to record high variability in the sensitivity and consistency of chemotaxes observed in O. marina. For example, in 1985 it was reported that O. marina only showed motile chemoresponses towards a shrimp extract (that elicited positive chemotaxis in other heterotrophic protozoa) 72 hours after being transferred from batch-culture with the diatom Phaeodactylum, to culture with the microalgae Dunaliella salina (27). At this time, it was proposed that the chemosensory apparatus of O. marina might only be activated when cells are maintained using certain prey species (27); however no explanation as to why this may be is given. Similarly, a recent study found variation in threshold detection limits (the lowest concentration of a stimulant required to elicit chemotaxis) and inconsistencies between the compounds that evoked the strongest and weakest chemosensory responses in batch-cultured O. marina (19).
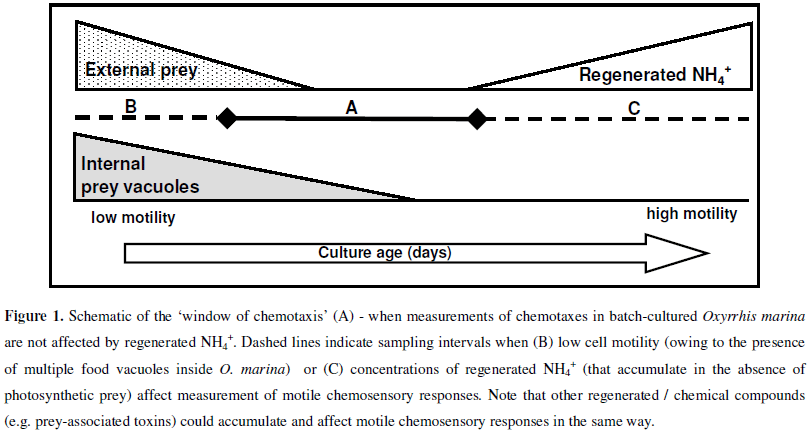
The aim of experimental work reported here was to simply explore the importance of batch-culture conditions - specifically the effect/s of regenerated extracellular NH4+-on the motile chemosensory responses of O. marina. It should first be noted that Oxyrrhis marina is a heterotrophic (non-photosynthetic) species in which losses of cell-carbon during respiration are coupled with the regeneration of nitrogen - as extracellular NH4+(5, 14). Secondly, when used in laboratory studies, O. marina is frequently maintained in batch-culture on autotrophic prey (e.g. the microalgae Dunaliella primolecta) that assimilate NH4+during photosynthesis (12). Thirdly, previous work has shown that synthetic and biogenic sources of NH4+elicit positive chemotaxis in O. marina (19). On the basis of the latter consideration, it was reasoned that accumulation of regenerated extracellular NH4+in prey-deplete batch-cultures of O. marina (Fig. 1) could affect the strength of motile chemosensory responses elicited by alternative stimuli (particularly if they are assayed at lower concentrations than ambient NH4+). To investigate this possibility, a standard microcapillary assay (2) was used to measure the motile chemosensory responses elicited by known chemoattractants (19) in O. marina that were removed from a batch-culture (Batch-culture study, Table 1a) as it progressed from a preysaturated, low NH4+condition (Fig. 1b) to a prey-depleted, high NH4+condition (Fig. 1c). A second experiment was then undertaken to determine whether chemotaxis in O. marina could be suppressed and recovered; first by incubating cells in seawater-based NH4Cl, and then by transferral of the same cells back into NH4+-free seawater (NH4Cl addition and removal experiment, Table 1b).
In all experimental work, monoxenic batch-cultures of Oxyrrhis marina Dujardin (CCAP 1133/5) were maintained using exponential phase Dunaliella primolecta Butcher (CCAP 11/34) grown at 18ºC (±1ºC) in an 18:6 hour light:dark cycle (180fmol photons m-2 s-1) using modified (440fM nitrate) f/2 media (15). Measurements of chemotaxis were made using a modification of Adler's (1973) micropipette technique (2, 19). Briefly, disposable 2fL microcapillary tubes (CAMAG Biosciences, UK) were filled via capillary action with a test or control (NH4+-free seawater) solution and placed in Petri dishes containing O. marina (4×104 cells mL-1) that had been washed and resuspended (for 3 hours) in NH4+-free seawater. After 2 minutes counts of the number of O. marina observed inside experimental (containing a test solution) or control tubes (5 tubes per assay) were made using light microscopy. These counts were used to derive a (t2min) response index as: mean number O. marina experimental tube-1 ÷ mean number O. marina control tube-1. All response indices were rounded (up or down) to the nearest integer such that, in this report: a response index of 1.0 reflects no chemotaxis (the motile response elicited by a test chemoattractant was no different from that elicited by the seawater control solution); a response index of 2 (or higher) is indicative of positive, measureable chemotaxis (Table 1).
For the batch-culture study (Table 1a), a prey-saturated (high prey, low NH4+; Fig. 1) culture was established by mixing O. marina with D. primolecta to achieve starting (day 1) cell densities of 3.0 × 104 cells mL-1 and 3.5 × 105 cells mL-1 respectively. The culture flask was then wrapped in aluminium foil to prevent photosynthesis (NH4+-uptake) by uneaten D. primolecta and to allow accumulation of regenerated extracellular NH4+. Cell numbers and biovolumes for O. marina and D. primolecta were monitored for 8 days using an Elzone 282 PC particle analyser (Particle Data Inc); this enabled estimation of the size (biovolume) of individual O. marina as they progressed from a vacuole-replete (containing prey multiple vacuoles) to a vacuole-deplete (no prey vacuoles) status. At each sampling interval, the concentration of regenerated extracellular NH4+ in the batch-culture was estimated according to the method of Holmes et al., (16). The motile chemosensory responses elicited in O. marina (3, 6, 12, 24 and 48 hours after transfer of cells from the experimental batch-culture to NH4+-free seawater) by i) cellfree filtrate from the original culture flask, ii) 100µM arginine and iii) 100µM seawater-based NH4Cl (Table 1a) were then assayed. It should be noted that the maximum (48 hour) transfer time was chosen largely because after this time, levels of NH4+in the NH4+-free seawater began to increase (owing to continuing respiration and NH4+- regeneration from transferred O. marina). For the second NH4Cl addition and removal experiment (Table 1b), chemotactic O. marina (showing positive chemotaxis towards 100µM arginine) were incubated (for 3 hours) in 0µM, 5µM, 10µM, 20µM or 50µM (final concentration) seawater-based NH4Cl. The motile chemosensory responses of O. marina to arginine were assayed with the microcapillary technique i) 'in situ' (using a 100µM arginine test solution) and ii) 3 hours after washing and transfer of cells back into NH4+-free seawater (using 1µM, 10µM, 100µM, 500µM or 1mM arginine test solutions, Table 1b).
In the batch-culture study, measurements of chemotaxis were affected by the satiation status of O. marina and - as hypothesised - concentrations of regenerated extracellular NH4+(Table 1 and Fig. 1). O. marina removed from the batch culture on day 1 were visibly vacuole-replete (containing >5 Dunaliella prey vacuoles; mean O. marina biovolume 4.96 pL cell-1) and displayed very low motility. Measurable motile chemosensory responses (response indices > 2.0) to i) cell-free culture filtrate, ii) 100µM arginine and iii) 100µM NH4Cl solutions were only observed 12, 24 and 48 hours after transfer of these (day 1) O. marina to NH4+-free seawater - after which time many divided, yielding smaller (2.0 pL cell-1) motile daughter-cells. O. marina removed from the batch-culture on day 2 contained fewer (1-5) D. primolecta prey vacuoles, were small (≈ 2.6 pL cell-1) and visibly more motile. However - despite their increased motility - they only responded to arginine and NH4Cl chemoattractants 12 hours after transfer to NH4+-free seawater (Table 1a). Interestingly, positive chemotaxis was elicited by cell-free culture filtrate (= 8µM NH4+) just 3 hours after transfer. As shown previously (19) these results demonstrate that regenerated NH4+(and other non-quantified biogenic stimuli [e.g. amino acids leaked from Regenerated extracellular NH4+of batch-cultured O. marina conspecifics]) elicit strong, positive chemotaxis in O. marina. Furthermore, they illustrate that, following transfer between different culture conditions, a recovery or adjustment period is required before measurable chemotaxes are inducible in O. marina. Importantly, the length of this adjustment period appears to be shorter for biogenic chemoattractants - possibly because they comprise a mixture of stimulatory compounds that act synergistically on any chemosensory apparatus involved in the propagation of chemotaxis in O. marina. It is significant that - even after a 48 hour recovery period in NH4+free seawater (Table 1a) -no measurable motile chemosensory responses were observed in O. marina removed from the batchculture on days 4, 6 or 8 (extracellular NH4+concentrations of 26µM, 66µM and 123µM respectively). It might be that chronic exposure of O. marina to regenerated NH4+(and other biogenic compounds that accumulate in the batch-culture system) results in long-term acclimatisation to ambient chemical conditions (8) such that cells no longer respond to NH4+. The sensitivity of motile chemosensory responses may be downregulated (e.g. 24) or even the repressed at the genetic level in order to maintain intracellular homeostasis. Alternatively it might be that chronic exposure of O. marina to NH4+and/or additional biogenic chemical stressors has adverse effects on aspects of cell physiology (e.g. causing damage to cellular organelles) that regulate cell motility and/or the propagation of chemosensory responses.
In the second NH4Cl addition and removal experiment (Table 1b), the motile responses of chemotactic O. marina (showing positive chemotaxis towards 100µM arginine) were clearly affected by ambient (5µM-1000µM) concentrations of NH4Cl. Despite being between 2 and 20-fold more concentrated than 'in situ' concentrations of NH4Cl, the 100µM arginine test solution still failed to elicit positive chemotaxis in O. marina (Table 1b). Even after washing and transfer of O. marina back into NH4+-free seawater, measureable motile chemosensory responses towards a greater range of arginine test solutions (10-1000µM) were only recovered in cells that had not been incubated in NH4Cl solutions greater than 5µM (Table 1b). It appears that brief (i.e. 3 hour) exposure to low (<5µM) concentrations of NH4+has reversible effects on the motile chemosensory responses of O. marina. Conversely, incubation in higher (>5µM) concentrations and/or chronic exposure to NH4+(e.g. regenerated extracellular NH4+in batch-cultures) can result in irreversible impairment of chemotaxis in O. marina.
The sensory apparatus (chemoreceptors, signal transduction pathways etc) that regulates chemotaxis in O. marina still requires fuller investigation. However, a consideration of the mechanisms that control chemotaxis in the much-studied freshwater ciliate Paramecium tetraurelia provides some potential insights into results from experimental work with O. marina. In Paramecium, motile chemosensory responses are mediated by membrane electrical changes; chemoattractants elicit membrane hyperpolarisation and positive chemotaxis while repellents cause depolarisation and negative chemotaxis (30). Some chemoattractants (e.g. glutamate) elicit chemotaxis after binding with specific cellsurface chemoreceptors (31); other non-specific mechanisms of activation are under also being investigated. Pertinently, ammonium chloride (NH4Cl) elicits positive chemotaxis in Paramecium although cell-surface receptors specific for NH4+have not yet been elucidated (6). Instead it is suspected that alteration of intracellular pH is the likely mechanism by which NH4Cl induces chemotaxis in Paramecium (6). Briefly, in solution, NH4Cl is in equilibrium with the membrane permeant molecule ammonia (NH3) which (on passing into the cell) triggers hyperpolarisation. It is entirely possible that the chemosensory responses elicited by NH4+ in O. marina (like those in Paramecium) are not receptor based. Indeed, diffusion of NH4+across the membrane of O. marina might result in activation of any cellular apparatus implicated in the control of chemotaxis. Regardless of the mechanism through which NH4+elicits chemotaxis, it is clear that its excitatory effect can render O. marina unable to detect alternative stimuli that may elicit their effects via similar (or more specific, receptor based) modes of action; results from the NH4Cl addition and removal experiment underscore this observation. Here, the stimulatory effect elicited by 'in situ' NH4+concentrations of 5µM (or higher) completely masked responses to 100µM arginine (Table 1b).
That regenerated NH4+appears to have such a marked effect on the strength and consistency of chemotaxis in O. marina raises some important considerations relating to the maintenance and use of batch-cultured cells for research. Firstly, because relatively low (e.g. 5µM) concentrations of NH4+(note that concentrations of regenerated NH4+in preydeplete batch-cultures frequently exceed 100µM) affect cells, thorough investigations of chemotaxes and characterisation of any chemosensory apparatus that might exist in O. marina are likely to be very difficult to conduct using batch-cultured cells. In some batch-culture studies, there may be a 'window of chemotaxis' in which ambient culture conditions (low-prey, low-NH4+) are more conducive to quantifying and observing chemotaxis in O. marina (Fig. 1a). However, the temporal existence of this window will vary from culture to culture and will be dependent on i) prey abundance and type (photosynthetic versus non-photosynthetic prey), ii) predator abundance (rapid grazing versus slow grazing activity) and iii) prey quality (high C:N versus low C:N prey) all of which affect levels of NH4+-regeneration by O. marina (5). Moreover, while this study has focused on regenerated NH4+, it is important to note that other regenerated compounds (e.g. amino acids leaked from conspecifics) and/or biogenic exudates (including toxins) from prey species will also affect the physiology and chemotaxis of batch-cultured O. marina. Clearly, to minimise (or at least regulate) the physiological effects of extracellular chemical stressors, O. marina from chemostat cultures should be used if at all possible; standard recovery periods (following washing and transfer of O. marina between different culture environments) must also be employed.
It should be highlighted that the 'window of chemotaxis' is so named, primarily because it represents a window of opportunity in which an experimenter is most likely to be able to measure motile chemosensory responses in laboratory-cultured O. marina. However, the conditions necessary for this 'window' also occur in the natural marine environment. As depicted (Fig. 1a) the 'window of chemotaxis' corresponds to prey-depleted, low-NH4+conditions, where motile, vacuoledeplete (containing no or very few prey vacuoles) O. marina are present. These conditions would - for example -prevail i) at the tail-end of grazing activity on an algal bloom, and ii) when toxic, graze-resistant or non-palatable algal prey are present. In both instances, the presence of residual algal prey would result in the uptake (for photosynthesis) of regenerated NH4+and thus low levels of extracellular NH4+. Pertinently, in such circumstances, O. marina would be vacuole-depleted, motile and perhaps more reliant on chemosensory mechanisms to help locate new (or more suitable) sources of prey. It is noteworthy that conditions outside of the 'window of chemotaxis' (Fig. 1b and 1c) might also occur naturally. For example, large, prey-saturated, O. marina containing multiple internal prey vacuoles (Fig. 1b) would be present during the early stages of grazing activity on a dense algal bloom. For prey-saturated O. marina, motile chemosensory responses (for the location of new prey) are perhaps unnecessary and (owing to decreased cell-motility) ineffective. Conversely, small, preydeplete, highly motile O. marina (Fig. 1c) would predominate in prey-starved conditions (e.g. at the end of an algal bloom or in the oligotrophic open ocean). It is reasonable to suspect that localised high-NH4+, low-prey conditions (Fig. 1c.) might occur over short-term time frames in certain marine habitats (e.g. within rock pools or microscale aggregations of O. marina). However, in the oligotrophic open ocean, physical dispersion and biological (e.g. bacterial) NH4+-uptake processes are likely to dictate that the high concentrations of regenerated NH4+seen in laboratory batch-cultures (Table 1a) would not persist in the longer-term.
Clearly, the results reported here underscore the need to treat any ecological assumptions based studies with batch-cultured O. marina with caution. As previously highlighted (19), the positive chemotaxis elicited by NH4+in O. marina could facilitate population cohesion in prey depleted, low Reynolds number environments (e.g. the typical rock pool habitat of O. marina [7]) where NH4+regenerated by conspecifics would accumulate in the absence of photosynthetic prey. It follows that in prey depleted environments O. marina populations would remain compacted as cells sense and respond to NH4+and additional biogenic cues leaked from conspecifics. Conversely, under low -NH4+conditions and/or when strong 'non-self' stimuli (e.g. exudates from a phytoplankton bloom) are encountered, O. marina populations may engage in more active foraging. Because cohesion maintained by regenerated compounds is likely to be strongest at the centre of a microbial population, the directional responses of cells at its periphery (those exposed to non-self signals) could guide others in a follow-the-leader manner towards new foraging areas. However, it should be noted that in the oligotrophic ocean, concentrations of NH4+may not reach (or persist at) those observed in laboratory studies due to biological uptake processes and nutrient cycling within marine 'microbial loop' communities (3, 23).
Finally, a few points on the suitability of the microcapillary assay for studying motile chemosensory responses in O. marina should be noted. Indeed, the microcapillary assay can be useful for the investigation of motile chemosensory responses in general. For example, as predatory cells approach prey items, they pass through gradients of excreted metabolites (a zone termed the phycosphere - [25]). The microcapillary technique simulates 'phycosphere' conditions to some degree; however it cannot be used to recreate more discrete chemical gradients or near-field biochemical changes (e.g. chemical pulses) that are suspected to be associated with the presence of prey items (20, 32). Importantly, because (in this and other studies [19, 27]) the microcapillary assay is used to quantify chemoresponses to chemical gradients after relatively long (e.g. 2 minute) integration periods, it is not possible to infer the effects of test stimuli on chemosensory behaviour over more immediate (micro/millisecond) timescales. On this point, it is perhaps noteworthy that in the NH4Cl addition and removal study reported here (Table 1b), no O. marina were observed (at t2min) inside control tubes (containing NH4+-free seawater) when 'in situ' concentrations of NH4+exceeded 5µM. Conversely, when ambient NH4+was non-detectable (typically following washing and transfer of O. marina to NH4+- free seawater), the number of O. marina observed inside control tubes at t2min increased to between 8 and 15. This observation suggests that O. marina does indeed respond to discrete chemical gradients, and that (once adjusted to ambient conditions) will orientate towards similar (or away from dissimilar) conditions - perhaps to help maintain intracellular homeostasis. Given these considerations, use of the microcapillary assay to investigate stimuli (e.g. algal toxins) that might be expected to elicit negative chemotaxis in the natural marine environment is fundamentally flawed. The failure of O. marina to enter microcapillary tubes may not be indicative of an avoidance response or true negative chemotaxis but rather the affinity of cells for dissolved stimuli (e.g. regenerated NH4+) present in the extracellular batchculture environment. Novel methodologies, particularly those designed for the study of cellular responses to near-field chemical signals, are now required.
ACKNOWLEDGEMENTS
The author wishes to thank Professor Kevin J Flynn (Swansea University, UK) and the Natural Environmental Research Council (NERC, UK).
Submitted: June 01, 2009; Returned to authors for corrections: June 30, 2009; Approved: October 06, 2009.
* Corresponding Author. Mailing address: Institute of Life Science, School of Medicine, Swansea University, Singleton Park, SA2 8PP, UK.; E-mail: c.m.martel@swansea.ac.uk
- 1. Adler, J. (1966). Chemotaxis in Bacteria. Science., 153, 708-716.
- 2. Adler, J. (1973). A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J. Gen. Microbiol, 74, 77-91.
- 3. Azam, F.; Fenchel, T.; Field, J.G.; Gray, J.S.; Meyerreil, L.A.; Thingstad, F. (1983). The ecological role of water-column microbes in the sea. Mar. Ecol. Prog. Ser., 10, 257-263.
- 4. Bonner, J.T. (1947). Evidence for the formation of aggregates by chemotaxis in the development of the slime mold Dictyostelium discoideum. J. Exp. Zool, 106, 1-26.
- 5. Davidson, K.; Cunningham, A.; Flynn, K.J. (1995). Predator-prey interactions between Isochrysis galbana and Oxyrrhis marina 3. Mathematical modelling of predation and nutrient regeneration. J. Plankton Res, 17, 465-492.
- 6. Davis, D.P.; Fiekers, J.F.; Van Houten, J.L. (1998). Intracellular pH and chemoresponse to NH4+in Paramecium. Cell. Motil. Cytoskeleton., 40, 107-118.
- 7. Droop, M.R. (1959). A note on some physical conditions for culturing Oxyrrhis marina. J. Mar. Biol. Assoc. U. K, 38, 599-604.
- 8. Dryl, S. (1952). The dependence of chemotropism in Paramecium caudatum on the chemical changes in the medium. Acta. Biol. Exp, 16, 23-53.
- 9. Fenchel, T.; Blackburn, N. (1999). Motile chemosensory behaviour of phagotrophic protists: Mechanisms for and efficiency in congregating at food patches. Protist, 150, 325-336.
- 10. Fischer, T.J.; Klein, R.B.; Borut, T.C.; Gard, S.E.; Rachelefsky, G.S.; Stiehm, E.R. (1977). Monocyte chemotaxis in health and disease. Pediatr Res, 11, 486-486.
- 11. Flynn, K.J.; Fielder, J. (1989). Changes in intracellular and extracellular amino-acids during the predation of the chlorophyte Dunaliella primolecta by the heterotrophic dinoflagellate Oxyrrhis marina and the use of the glutamine glutamate ratio as an indicator of nutrient status in mixed populations. Mar. Ecol. Prog. Ser., 53, 117-127.
- 12. Flynn, K.J.; Garrido, J.L.; Zapata, M.; Opik, H.; Hipkin, C.R. (1992). Changes in fatty-acids, amino-acids and carbon nitrogen biomass during nitrogen starvation of ammonium-grown and nitrate-grown Isochrysis galbana. J. App. Phycol., 4, 95-104.
- 13. Goldman, J.C.; Dennett, M.R. (2001). Rapid nitrogen uptake by marine bacteria. Limnol. Oceanogr, 46, 1195-1198.
- 14. Goldman, J.C.; Dennett, M.R.; Gordin, H. (1989). Dynamics of herbivorous grazing by the heterotrophic dinoflagellate Oxyrrhis marina. J. Plankton Res, 11, 391-407.
- 15. Guillard, R.R.L. (1975). Culture of phytoplankton for feeding marine invertebrates. In: Smith, W.L; Chanely, M.H. (eds). Culture of marine invertebrates Plenum Press: New York, p. 26-60.
- 16. Holmes, R.M.; Aminot, A.; Kerouel, R.; Hooker, B.A.; Peterson, B.J. (1999). A simple and precise method for measuring ammonium in marine and freshwater ecosystems. Can. J. Fish. Aquat. Sci., 56, 1801-1808.
- 17. Lee, E.S.; Lewitus, A.J.; Zimmer, R.K. (1999). Chemoreception in a marine cryptophyte: Behavioral plasticity in response to amino acids and nitrate. Limnol. Oceanogr, 44, 1571-1574.
- 18. Lenhoff, H.M. (1974). On the mechanism of action of feeding and the evolution of receptors associated with feeding and digestion. In: Muscatine, L.; Lenhoff, H.M. (eds). Coelenterate Biology Academic Press: New York, p. 238-239.
- 19. Martel, C.M. (2006). Prey location, recognition and ingestion by the phagotrophic marine dinoflagellate Oxyrrhis marina. J. Exp. Mar. Biol. Ecol, 335, 210-220.
- 20. Martel, C.M. (2009). Conceptual bases for prey biorecognition and feeding selectivity in the microplanktonic marine phagotroph Oxyrrhis marina. Microb. Ecol., 57 (4), 589-597.
- 21. Menden-Deuer, S. ; Grünbaum, D. (2006). Individual foraging behaviors and population distributions of a planktonic predator aggregating to phytoplankton thin layers. Limnol. Oceanogr, 51, 109-116.
- 22. Pasternak, Z.; Bachar, A.; Abelson, A.; Achituv, Y. (2004). Initiation of symbiosis between the soft coral Heteroxenia fuscescens and its zooxanthellae. Mar. Ecol. Prog. Ser., 279, 113-116.
- 23. Pomeroy, L.R. (1974). The oceans food web, a changing paradigm. Bioscience, 24, 499-504.
- 24. Preston, R.R.; Hammond, J.A. (1998). Long-term adaptation of Ca2+dependent behaviour in Paramecium tetraurelia. J. Exp. Biol., 201, 1835-1846.
- 25. Sieburth, J.M. (1968). The influence of algal antibiosis on the ecology of marine microorganisms. In: Droop, M.R.; Wood, E.J.F. (eds). Advances in microbiology of the sea Academic Press: London, p. 63-94.
- 26. Sjoblad, R.D.; Chet, J.; Mitchell, R. (1978). Chemoreception in the green alga Dunaliella tertiolecta. Curr. Microbiol, 1, 305-307.
- 27. Spero, H.J. (1985). Chemosensory capabilities in the phagotrophic dinoflagellate Gymnodinium fungiforme. J. Phycol., 21, 181-184.
- 28. Tarran, G.A. (1991). Aspects of the grazing behaviour of the marine dinoflagellate Oxyrrhis marina Dujardin. PhD thesis: Southampton University, Southampton.
- 29. Ucko, M.; Geresh, S.; Simonberkovitch, B.; Arad, S.M. (1994). Predation by a dinoflagellate on a red microalga with a cell-wall modified by sulfate and nitrate starvation. Mar. Ecol. Prog. Ser., 104, 293-298.
- 30. Van Houten, J. (1990). Chemosensory Transduction in Paramecium. In: Armitage, J.; Lackie, J.M. (eds). Biology of the chemotactic response Cambridge University Press, p. 297-321.
- 31. Van Houten, J.L.; Yang, W.Q.; Bergeron, A. (2000). Glutamate chemosensory signal transduction in Paramecium. J. Nutr, 130, 946S-949S.
- 32. Wolfe, G.V. (2000). The chemical defence ecology of marine unicellular plankton: Constraints, mechanisms, and impacts. Biol. Bull., 198, 225-244.
Publication Dates
-
Publication in this collection
16 Apr 2010 -
Date of issue
June 2010
History
-
Reviewed
30 June 2009 -
Received
01 June 2009 -
Accepted
06 Oct 2009