Abstract
Amylases are one of the main enzymes used in industry. Such enzymes hydrolyze the starch molecules into polymers composed of glucose units. Amylases have potential application in a wide number of industrial processes such as food, fermentation and pharmaceutical industries. α-Amylases can be obtained from plants, animals and microorganisms. However, enzymes from fungal and bacterial sources have dominated applications in industrial sectors. The production of α-amylase is essential for conversion of starches into oligosaccharides. Starch is an important constituent of the human diet and is a major storage product of many economically important crops such as wheat, rice, maize, tapioca, and potato. Starch-converting enzymes are used in the production of maltodextrin, modified starches, or glucose and fructose syrups. A large number of microbial α-amylases has applications in different industrial sectors such as food, textile, paper and detergent industries. The production of α-amylases has generally been carried out using submerged fermentation, but solid state fermentation systems appear as a promising technology. The properties of each α-amylase such as thermostability, pH profile, pH stability, and Ca-independency are important in the development of fermentation process. This review focuses on the production of bacterial and fungal α-amylases, their distribution, structural-functional aspects, physical and chemical parameters, and the use of these enzymes in industrial applications.
α-Amylases; enzyme production; bacterial and fungal amylase; starch
REVIEW
Application of microbial α-amylase in industry - A review
Paula Monteiro de Souza; Pérola de Oliveira e Magalhães
Departamento de Ciências Farmacêuticas, Faculdade de Ciências da Saúde, Universidade de Brasília, Brasília, DF, Brasil
ABSTRACT
Amylases are one of the main enzymes used in industry. Such enzymes hydrolyze the starch molecules into polymers composed of glucose units. Amylases have potential application in a wide number of industrial processes such as food, fermentation and pharmaceutical industries. α-Amylases can be obtained from plants, animals and microorganisms. However, enzymes from fungal and bacterial sources have dominated applications in industrial sectors. The production of α-amylase is essential for conversion of starches into oligosaccharides. Starch is an important constituent of the human diet and is a major storage product of many economically important crops such as wheat, rice, maize, tapioca, and potato. Starch-converting enzymes are used in the production of maltodextrin, modified starches, or glucose and fructose syrups. A large number of microbial α-amylases has applications in different industrial sectors such as food, textile, paper and detergent industries. The production of α-amylases has generally been carried out using submerged fermentation, but solid state fermentation systems appear as a promising technology. The properties of each α-amylase such as thermostability, pH profile, pH stability, and Ca-independency are important in the development of fermentation process. This review focuses on the production of bacterial and fungal α-amylases, their distribution, structural-functional aspects, physical and chemical parameters, and the use of these enzymes in industrial applications.
Key words: α-Amylases; enzyme production; bacterial and fungal amylase; starch
INTRODUCTION
α-Amylases (E.C.3.2.1.1) are enzymes that catalyses the hydrolysis of internal α-1,4-glycosidic linkages in starch in low molecular weight products, such glucose, maltose and maltotriose units (29, 42, 66). Amylases are among the most important enzymes and are of great significance for biotechnology, constituting a class of industrial enzymes having approximately 25% of the world enzyme market (66, 68). They can be obtained from several sources, such as plants, animals and microorganisms. Today a large number of microbial amylases are available commercially and they have almost completely replaced chemical hydrolysis of starch in starch processing industry. The amylases of microorganisms have a broad spectrum of industrial applications as they are more stable than when prepared with plant and animal α-amylases (81). The major advantage of using microorganisms for the production of amylases is the economical bulk production capacity and the fact that microbes are easy to manipulate to obtain enzymes of desired characteristics. α-Amylase has been derived from several fungi, yeasts and bacteria. However, enzymes from fungal and bacterial sources have dominated applications in industrial sectors (29).
α-Amylases have potential application in a wide number of industrial processes such as food, fermentation, textile, paper, detergent, and pharmaceutical industries. Fungal and bacterial amylases could be potentially useful in the pharmaceutical and fine-chemical industries. However, with the advances in biotechnology, the amylase application has expanded in many fields such as clinical, medicinal and analytical chemistry, as well as their widespread application in starch saccharification and in the textile, food, brewing and distilling industries (29, 42, 61).
α-Amylases are one of the most popular and important form of industrial amylases and the present review point out the microorganisms that produce this enzymes.
STRUCTURAL AND FUNCTIONAL CHARACTERISTICS OF α-AMYLASE
The α-amylase (α-1,4-glucan-4-glucanohydrolase) can be found in microorganisms, plants and higher organisms (42). The α-amylase belongs to a family of endo-amylases that catalyses the initial hydrolysis of starch into shorter oligosaccharides through the cleavage of α-D-(1-4) glycosidic bonds (9, 36, 42, 80). Neither terminal glucose residues nor α-1,6-linkages can be cleaved by α-amylase (88). The end products of α-amylase action are oligosaccharides with varying length with an α-configuration and α-limit dextrins (86), which constitute a mixture of maltose, maltotriose, and branched oligosaccharides of 6-8 glucose units that contain both α-1,4 and α-1,6 linkages (88). Others amylolytic enzymes participate in the process of starch breakdown, but the contribution of α-amylase is the mos important for the initiation of this process (80).
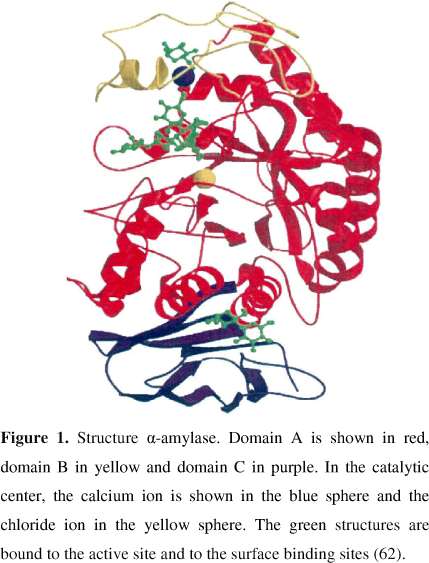
The amylase has a three-dimensional structure capable of binding to substrate and, by the action of highly specific catalytic groups, promote the breakage of the glycoside links (36). The human α-amylase is a classical calcium-containing enzyme composed of 512 amino acids in a single oligosaccharide chain with a molecular weight of 57.6 kDa (88). The protein contains 3 domains: A, B, and C (Figure 1). The A domain is the largest, presenting a typical barrel shaped (β/α)8 super structure. The B domain is inserted between the A and C domains and is attached to the A domain by disulphide bond. The C domain has a β sheet structure linked to the A domain by a simple polypeptide chain and seems to be an independent domain with unknown function. The active site (substrate-binding) of the α-amylase is situated in a long cleft located between the carboxyl end of the A and B domains. The calcium (Ca2+) is situated between the A and B domains and may act in the stabilization of the three-dimensinoal structure and as allosteric activator. Binding of substrate analogs suggest that Asp206, Glu230 and Asp297 participate in catalysis (56). The substrate-binding site contains 5 subsites with the catalytic site positioned at subsite 3. Substrate can bind to the first glucose residue in subsite 1 or 2, allowing cleavage to occur between the first and second or second and third glucose residues (88).
STARCH
Starch is an important constituent of the human diet and, for this purpose, is used chemically and enzymatically processed into a variety of different products such as starch hydrolysates, glucose syrups, fructose, maltodextrin derivatives or cyclodextrins, used in food industry. In addition to that, the sugars produced can be fermented to produce ethanol. In spite of the large number of plants able to produce starch, only a few plants are important for industrial starch processing. The major industrial sources are maize, tapioca, potato, and wheat, but limitations such as low shear resistance, thermal resistance, thermal decomposition and high tendency towards retrogradation limit its use in some industrial food applications (1, 28, 86). Among carbohydrate polymers, starch is currently enjoying increased attention due to its usefulness in different food products. Starch contributes greatly to the textural properties of many foods and is widely used in food and industrial applications as a thickener, colloidal stabilizer, gelling agent, bulking agent and water retention agent (37).
Starch is a polymer of glucose linked to another one through the glycosidic bond. Two types of glucose polymers are present in starch: amylose and amylopectin (Figura 2a and 2b). Amylose and amylopectin have different structures and properties. Amylose is a linear polymer consisting of up to 6000 glucose units with α-1,4 glycosidic bonds. Amylopectin consists of short α-1,4 linked to linear chains of 10-60 glucose units and α-1,6 linked to side chains with 15-45 glucose units. Granule bound starch synthase can elongate malto-oligosaccharides to form amylose and is considered to be responsible for the synthesis of this polymer. Soluble starch synthase is considered to be responsible for the synthesis of unit chains of amylopectin. α-Amylase is able to cleave α-1,4 glycosidic bonds present in the inner part of the amylose or amylopectin chain (56, 78, 84, 86).
Starch is hydrolyzed into smaller oligossaccharides by α-amylase, wich is one of the most important commercial enzyme processes. Amylases find application in all the industrial processes such as in food, detergents, textiles and in paper industry, for the hydrolysis of starch (29, 47, 81). Saccharide composition obtained after hydrolyze of starch is highly dependent on the effect of temperature, the conditions of hydrolysis and the origin of enzyme. Specificity, thermostability and pH response of the enzymes are critical properties for industrial use (42).
α-AMYLASE PRODUCTION
The production of α-amylase by submerged fermentation (SmF) and solid state fermentation (SSF) has been investigated and depend on a variety of physicochemical factors. SmF has been traditionally used for the production of industrially important enzymes because of the ease of control of different parameters such as pH, temperature, aeration and oxygen transfer and moisture (16, 22).
SSF systems appear promising due to the natural potential and advantages they offer. SSF resembles the natural habitat of microorganism and is, therefore, the preferred choice for microorganisms to grow and produce useful value added products. SmF can be considered as a violation of their natural habitat, especially of fungi (74). Fungi and yeast were termed as suitable microorganisms for SSF according to the theoretical concept of water activity, whereas bacteria have been considered unsuitable. However, experience has shown that bacterial cultures can be well managed and manipulated for SSF processes (60). There are others advantages of SSF over SmF, including superior productivity, simpler technique, lower capital investment, lower energy requirement and less water output, better product recovery and lack of foam build up, besides it is reported to be the most appropriate process for developing countries. Recently, researches evaluated whether SSF is the best system for producing enzymes. They found that SSF is appropriate for the production of enzymes and other thermolabile products, especially when higher yields can be obtained when compared to SmF (16, 82).
The optimization of fermentation conditions, particularly physical and chemical parameters, are important in the development of fermentation processes due to their impact on the economy and practicability of the process (21). The role of various factors, including pH, temperature, metal ions, carbon and nitrogen source, surface acting agents, phosphate and agitation have been studied for α-amylase production. The properties of each α-amylase such as thermostability, pH profile, pH stability, and Ca-independency must be matched to its application. For example, α-amylases used in starch industry must be active and stable at low pH, but at high pH values in the detergent industry. Most notable among these are the composition of the growth medium, pH of the medium, phosphate concentration, inoculum age, temperature, aeration, carbon source and nitrogen source (16, 69). The physical and chemical parameters of α-amylases from bacteria and fungi have been widely studied and described (29). Table 1 shows properties of some amylases from microorganisms.
BACTERIAL AMYLASES
α-Amylase can be produced by different species of microorganisms, but for commercial applications α-amylase is mainly derived from the genus Bacillus. α-Amylases produced from Bacillus licheniformis, Bacillus stearothermophilus, and Bacillus amyloliquefaciens find potential application in a number of industrial processes such as in food, fermentation, textiles and paper industries (46, 61).
Thermostability is a desired characteristic of most of the industrial enzymes. Thermostable enzymes isolated from thermophilic organisms have found a number of commercial applications because of their stability. As enzymatic liquefaction and saccharification of starch are performed at high temperatures (100-110ºC), thermostable amylolytic enzymes have been currently investigated to improve industrial processes of starch degradation and are of great interest for the production of valuable products like glucose, crystalline dextrose, dextrose syrup, maltose and maltodextrins (6, 26, 79). Bacillus subtilis, Bacillus stearothermophilus, Bacillus licheniformis, and Bacillus amyloliquefaciens are known to be good producers of thermostable α-amylase, and these have been widely used for commercial production of the enzyme for various applications (64). Thermostable α-amylases have been reported from several bacterial strains and have been produced using SmF as well as SSF (83). However, the use of SSF has been found to be more advantageous than SmF and allows a cheaper production of enzymes (76). The production of α-amylase by SSF is limited to the genus Bacillus, and B. subtilis , B. polymyxia , B.mesentericus, B. vulgarus, B. megaterium and B.licheniformis have been used for α-amylase production in SSF (8). Currently, thermostable amylases of Bacillus stearothermophilus or Bacillus licheniformis are being used in starch processing industries (26).
Enzymes produced by some halophilic microorganisms have optimal activity at high salinities and could therefore be used in many harsh industrial processes where the concentrated salt solutions used would otherwise inhibit many enzymatic conversions (4, 63). In addition, most halobacterial enzymes are considerably thermotolerant and remain stable at room temperature over long periods (51). Halophilic amylases have been characterized from halophilic bacteria such as Chromohalobacter sp. (63), Halobacillus sp. (4), Haloarcula hispanica (34), Halomonas meridiana (15), and Bacillus dipsosauri (18).
FUNGAL AMYLASES
Most reports about fungi that produce α-amylase have been limited to a few species of mesophilic fungi, and attempts have been made to specify the cultural conditions and to select superior strains of the fungus to produce on a commercial scale (29). Fungal sources are confined to terrestrial isolates, mostly to Aspergillus and Penicillium (43).
The Aspergillus species produce a large variety of extracellular enzymes, and amylases are the ones with most significant industrial importance (32). Filamentous fungi, such as Aspergillus oryzae and Aspergillus niger, produce considerable quantities of enzymes that are used extensively in the industry. A. oryzae has received increased attention as a favourable host for the production of heterologous proteins because of its ability to secrete a vast amount of high value proteins and industrial enzymes, e.g. α-amylase (39). Aspergillus oryzae has been largely used in the production of food such as soy sauce, organic acid such as citric and acetic acids and commercial enzymes including α-amylase (41). Aspergillus niger has important hydrolytic capacities in the α-amylase production and, due to its tolerance of acidity (pH < 3), it allows the avoidance of bacterial contamination (19).
Filamentous fungi are suitable microorganisms for solidstate fermentation (SSF), especially because their morphology allows them to colonize and penetrate the solid substrate (65). The fungal α-amylases are preferred over other microbial sources due to their more accepted GRAS (Generally Recognized As Safe) status (29).
The thermophilic fungus Thermomyces lanuginosus is an excellent producer of amylase. Jensen (38) and Kunamneni (48) purified the α-amylase, proving its thermostability.
PURIFICATION OF α-AMYLASE
Industrial enzymes produced in bulk generally require little downstream processing and hence are relatively crude preparations. The commercial use of α-amylase generally does not require purification of the enzyme, but enzyme applications in pharmaceutical and clinical sectors require high purity amylases. The enzyme in the purified form is also a prerequisite in studies of structure-function relationships and biochemical properties (29). Different strategies for purification of enzymes have been investigated, exploiting specific characteristics of the target biomolecule. Laboratory scale purification for α-amylase includes various combinations of ion exchange, gel filtration, hydrophobicity interactions and reverse phase chromatography. Alternatively, α-amylase extraction protocols using organic solvents such as ethanol, acetone and ammonium sulfate precipitation (25, 31, 44) and ultrafiltration have been proposed (53). These conventional multi-step methods requires expensive equipments at each step, making them laborious, time consuming, barely reproducible and may result in increasing loss of the desired product (5). However, liquid-liquid extractions consist of an interesting purification alternative since several features of the early processing steps can be combined into a single operation. Liquid-liquid extraction is the transfer of certain components from one phase to another when immiscible or partially soluble liquid phases are brought into contact with each other. This process is widely employed in the chemical industry due to its simplicity, low costs, and ease of scale up. Purification of biomolecules using liquid-liquid extraction has been successfully carried out on a large scale for more than a decade. Advantages of using this system are lower viscosity, lower cost of chemicals and shorter phase separation time. The dynamic behavior of these systems has to be investigated and understood to enhance plant-wide control of continuous liquid-liquid extraction and to assess safety and environmental risks at the earliest possible design stage (49).
INDUSTRIAL APPLICATION OF α-AMYLASE
Starch conversion
The most widespread applications of α-amylases are in the starch industry, wich are used for starch hydrolysis in the starch liquefaction process that converts starch into fructose and glucose syrups (57). The enzymatic conversion of all starch includes: gelatinization, which involves the dissolution of starch granules, thereby forming a viscous suspension; liquefaction, which involves partial hydrolysis and loss in viscosity; and saccharification, involving the production of glucose and maltose via further hydrolysis (29, 64). Initially, the α-amylase of Bacillus amyloliquefaciens was used but it has been replaced by the α-amylase of Bacillus stearothermophilus or Bacillus licheniformis (86). The enzymes from the Bacillus species are of special interest for large-scale biotechnological processes due to their remarkable thermostability and because efficient expression systems are available for these enzymes (64).
Detergent industry
Detergent industries are the primary consumers of enzymes, in terms of both volume and value. The use of enzymes in detergents formulations enhances the detergents ability to remove tough stains and making the detergent environmentally safe. Amylases are the second type of enzymes used in the formulation of enzymatic detergent, and 90% of all liquid detergents contain these enzymes (29, 33, 50). These enzymes are used in detergents for laundry and automatic dishwashing to degrade the residues of starchy foods such as potatoes, gravies, custard, chocolate, etc. to dextrins and other smaller oligosaccharides (54, 58). Amylases have activity at lower temperatures and alkaline pH, maintaining the necessary stability under detergent conditions and the oxidative stability of amylases is one of the most important criteria for their use in detergents where the washing environment is very oxidizing (13, 45). Removal of starch from surfaces is also important in providing a whiteness benefit, since starch can be an attractant for many types of particulate soils. Examples of amylases used in the detergent industry are derived from Bacillus or Aspergillus (50).
Fuel alcohol production
Ethanol is the most utilized liquid biofuel. For the ethanol production, starch is the most used substrate due to its low price and easily available raw material in most regions of the world (14). In this production, starch has to be solubilized and then submitted to two enzymatic steps in order to obtain fermentable sugars. The bioconversion of starch into ethanol involves liquefaction and saccharification, where starch is converted into sugar using an amylolytic microorganism or enzymes such as α-amylase, followed by fermentation, where sugar is converted into ethanol using an ethanol fermenting microorganism such as yeast Saccharomyces cerevisiae (53, 59). The production of ethanol by yeast fermentation plays an important role in the economy of Brazil (17). In order to obtain a new yeast strain that can directly produce ethanol from starch without the need for a separate saccharifying process, protoplast fusion was performed between the amylolytic yeast Saccharomyces fibuligera and S. cerevisiae (14). Among bacteria, α-amylase obtained from thermoresistant bacteria like Bacillus licheniformis or from engineered strains of Escherichia coli or Bacillus subtilis is used during the first step of hydrolysis of starch suspensions (70).
Food industry
Amylases are extensively employed in processed-food industry such as baking, brewing, preparation of digestive aids, production of cakes, fruit juices and starch syrups (16). The α-amylases have been widely used in the baking industry. These enzymes can be added to the dough of bread to degrade the starch in the flour into smaller dextrins, which are subsequently fermented by the yeast. The addition of α-amylase to the dough results in enhancing the rate of fermentation and the reduction of the viscosity of dough, resulting in improvements in the volume and texture of the product. Moreover, it generates additional sugar in the dough, which improves the taste, crust colour and toasting qualities of the bread. Besides generating fermentable compounds, α-amylases also have an anti-staling effect in bread baking, and they improve the softness retention of baked goods, increasing the shelf life of these products (29, 86). Currently, a thermostable maltogenic amylase of Bacillus stearothermophilus is used commercially in the bakery industry (86). Amylases are also used for the clarification of beer or fruit juices, or for the pretreatment of animal feed to improve the digestibility of fiber (23, 24, 86).
Textile industry
Amylases are used in textile industry for desizing process. Sizing agents like starch are applied to yarn before fabric production to ensure a fast and secure weaving process. Starch is a very attractive size, because it is cheap, easily available in most regions of the world, and it can be removed quite easily. Starch is later removed from the woven fabric in a wet-process in the textile finishing industry. Desizing involves the removal of starch from the fabric which serves as the strengthening agent to prevent breaking of the warp thread during the weaving process. The α-amylases remove selectively the size and do not attack the fibres (3, 20, 29). Amylase from Bacillus stain was employed in textile industries for quite a long time.
Paper industry
The use of α-amylases in the pulp and paper industry is for the modification of starch of coated paper, i.e. for the production of low-viscosity, high molecular weight starch (29, 86). The coating treatment serves to make the surface of paper sufficiently smooth and strong, to improve the writing quality of the paper. In this application, the viscosity of the natural starch is too high for paper sizing and this can be altered by partially degrading the polymer with α-amylases in a batch or continuous processes. Starch is a good sizing agent for the finishing of paper, improving the quality and erasebility, besides being a good coating for the paper. The size enhances the stiffness and strength in paper (10, 29). Examples of amylases obtained from microorganisms used in paper industry includes Amizyme® (PMP Fermentation Products, Peoria, USA), Termamyl®, Fungamyl, BAN® (Novozymes, Denmark) and α-amylase G9995® (Enzyme Biosystems, USA) (72).
CONCLUSION
The use of α-amylase in starch based industries has been prevalent for many decades and a number of microbial sources exist for the efficient production of this enzyme, but only a few selected strains of fungi and bacteria meet the criteria for commercial production. The search for new microorganisms that can be used for amylase production is a continuous process. More recently, many authors have presented good results in developing α-amylase purification techniques, which enable applications in pharmaceutical and clinical sectors which require high purity amylases.
ACKNOWLEDGEMENTS
This research was supported by grants from the Coordination for Higher Level Graduate Improvements (Capes - Brazil), National Council for Scientific and Technological Development (CNPq - Brazil) and State of Distrito Federal Research Support Foundation (FAPDF - Brazil).
Submitted: March 23, 2010; Returned to authors for corrections: March 30, 2010; Approved: May 24, 2010.
* Corresponding Author. Mailing address: Departamento de Ciências Farmacêuticas, Faculdade de Ciências da Saúde, Universidade de Brasília, Campus Darcy Ribeiro, Asa Norte, Brasília, DF, Brasil.; E-mail: paulasouza@unb.br
- 1. Agrawal, M.; Pradeep, S.; Chandraraj, K.; Gummadi, S.N. (2005). Hydrolysis of starch by amylase from Bacillus sp. KCA102: a statistical approach. Process Biochemistry 40, 2499 - 2507.
- 2. Aguilar, G.; Morlon-Guyot, J.; Trejo-Aguilar, B.; Guyot, J.P. (2000). Purification and characterization of an extracellular alpha-amylase produced by Lactobacillus manihotivorans LMG 18010(T), an amylolytic lactic acid bacterium. Enzyme Microb Technol 27, 406-413.
- 3. Ahlawat, S.; Dhiman, S.S.; Battan, B.; Mandhan, R.P.; Sharma, J. (2009). Pectinase production by Bacillus subtilis and its potential application in biopreparation of cotton and micropoly fabric. Process Biochemistry 44, 521-526.
- 4. Amoozegar, M.A.; Malekzadeh, F.; Malik, K.A. (2003). Production of amylase by newly isolated moderate halophile, Halobacillus sp. strain MA-2. J Microbiol Methods 52, 353-359.
- 5. Arauza, L.J.; Jozalaa, A.F.; Mazzolab, P.G.; Penna, T.C.V. (2009). Nisin biotechnological production and application: a review. Trends Food Sci Technol 20, 146-154.
- 6. Asgher, M.; Asad, M.J.; Rahman, S.U.; Legge, R.L. (2007). A thermostable α-amylase from a moderately thermophilic Bacillus subtilis strain for starch processing. J Food Process Eng 79, 950 - 955.
- 7. Asoodeh, A.; Chamani, J.; Lagzian, M. (2010). A novel thermostable, acidophilic alpha-amylase from a new thermophilic "Bacillus sp. Ferdowsicous" isolated from Ferdows hot mineral spring in Iran: Purification and biochemical characterization. Int J Biol Macromol 46, 289-297.
- 8. Baysal, Z.; Uyar, F.; Aytekin, C. (2003). Solid state fermentation for production of α-amylase by a thermotolerant Bacillus subtilis from hot-spring water. Process Biochemistry 38, 1665-1668.
- 9. Brayer, G.D.; Luo, Y.; Withers, S.G. (1995). The structure of human pancreatic alpha-amylase at 1.8 A resolution and comparisons with related enzymes. Protein Sci 4, 1730-1742.
- 10. Bruinenberg, P.M.; Hulst, A.C.; Faber, A.; Voogd, R.H. (1996) A process for surface sizing or coating of paper. In: European Patent Application.
- 11. Calderon, M.; Loiseau, G.; Guyot, J.P. (2003). Fermentation by Lactobacillus fermentum Ogi E1 of different combinations of carbohydrates occurring naturally in cereals: consequences on growth energetics and alpha-amylase production. Int J Food Microbiol 80, 161-169.
- 12. Chen, W.M.; Chang, J.S.; Chiu, C.H.; Chang, S.C.; Chen, W.C.; Jiang, C.M. (2005). Caldimonas taiwanensis sp. nov., α-amylase producing bacterium isolated from a hot spring. Syst Appl Microbiol 28, 415-420.
- 13. Chi, M.; Chen, Y.; Wu, T.; Lo, H.; Lin, L. (2009). Engineering of a truncated α-amylase of Bacillus sp. strain TS-23 for the simultaneous improvement of thermal and oxidative stabilities. J. Biosci. Bioeng. xx, xxx-xxx.
- 14. Chi, Z.; Chi, Z.; Liu, G.; Wang, F.; Ju, L.; Zhang, T. (2009). Saccharomycopsis fibuligera and its applications in biotechnology. Biotechnol Adv 27, 423-431.
- 15. Coronado, M.; Vargas, C.; Hofemeister, J.; Ventosa, A.; Nieto, J.J. (2000). Production and biochemical characterization of an alpha-amylase from the moderate halophile Halomonas meridiana FEMS Microbiol Lett 183, 67-71.
- 16. Couto, S.R.; Sanromán, M.A. (2006). Application of solid-state fermentation to food industry- A review. Journal of Food Engineering 76, 291-302.
- 17. de Moraes, L.M.; Astolfi-Filho, S.; Oliver, S.G. (1995). Development of yeast strains for the efficient utilisation of starch: evaluation of constructs that express alpha-amylase and glucoamylase separately or as bifunctional fusion proteins. Appl Microbiol Biotechnol 43, 1067-1076.
- 18. Deutch, C.E. (2002). Characterization of a salt-tolerant extracellular α-amylase from Bacillus dipsosauri Lett Appl Microbiol 35, 78-84.
- 19. Djekrif-Dakhmouche, S.; Gheribi-Aoulmi, Z.; Meraihi, Z.; Bennamoun, L. (2006). Application of a statistical design to the optimization of culture medium for á-amylase production by Aspergillus niger ATCC 16404 grown on orange waste powder. J Food Process Eng 73, 190197.
- 20. Feitkenhauer, H. (2003). Anaerobic digestion of desizing wastewater: influence of pretreatment and anionic surfactant on degradation and intermediate accumulation. Enzyme Microb. Technol. 33, 250-258.
- 21. Francis, F.; Sabu, A.; Nampoothiri, K.M.; Ramachandran, S.; Ghosh, S.; Szakacs, G.; Pandey, A. (2003). Use of response surface methodology for optimizing process parameters for the production of α-amylase by Aspergillus oryzae Biochem. Eng. J. 15, 107-115.
- 22. Gangadharan, D.; Sivaramakrishnan, S.; Nampoothiri, K.M.; Sukumaran, R.K.; Pandey, A. (2008). Response surface methodology for the optimization of alpha amylase production by Bacillus amyloliquefaciens Bioresour Technol 99, 4597-4602.
- 23. Gavrilescu, M.; Chisti, Y. (2005). Biotechnology-a sustainable alternative for chemical industry. Biotechnol Adv 23, 471-499.
- 24. Ghorai, S.; Banik, S.P.; Verma, D.; Chowdhury, S.; Mukherjee, S.; Khowala, S. (2009). Fungal biotechnology in food and feed processing. Food Res. Int. 42, 577-587.
- 25. Glymph, J.L.; Stutzenberger, F.J. (1977). Production, purification, and characterization of alpha-amylase from Thermomonospora curvata. Appl Environ Microbiol 34, 391-397.
- 26. Gomes, I.; Gomes, J.; Steiner, W. (2003). Highly thermostable amylase and pullulanase of the extreme thermophilic eubacterium Rhodothermus marinus: production and partial characterization. Bioresour Technol 90, 207-214.
- 27. Goto, C.E.; Barbosa, E.P.; Kistner, L.C.; Moreira, F.G.; Lenartovicz, V.; Peralta, R.M. (1998). Production of amylase by Aspergillus fumigatus utilizing alpha-methyl-D-glycoside, a synthetic analogue of maltose, as substrate. FEMS Microbiol Lett 167, 139-143.
- 28. Goyal, N.; Gupta, J.K.; Soni, S.K. (2005). A novel raw starch digesting thermostable α-amylase from Bacillus sp. I-3 and its use in the direct hydrolysis of raw potato starch. Enzyme Microb. Technol. 37, 723-734.
- 29. Gupta, R.; Gigras, P.; Mohapatra, H.; Goswami, V.K.; Chauhan, B. (2003). Microbial α-amylases: a biotechnological perspective. Process Biochem 38, 1599 - 1616.
- 30. Hamilton, L.M.; Kelly, C.T.; Fogarty, W.M. (1999). Production and properties of the raw starch-digesting α-amylase of Bacillus sp. IMD 435. Process Biochem 35, 27-31.
- 31. Hamilton, L.M.; Kelly, C.T.; Fogarty, W.M. (1999). Purification and properties of the raw starch degrading -amylase of Bacillus sp. IMD434. Biotechnol. Lett. 21, 111-115.
- 32. Hernández, M.S.; Rodríguez, M.R.; Guerra, N.P.; Rosés, R.P. (2006). Amylase production by Aspergillus niger in submerged cultivation on two wastes from food industries. J Food Process Eng 73, 93-100.
- 33. Hmidet, N.; El-Hadj Ali, N.; Haddar, A.; Kanoun, S.; Alya, S.; Nasri, M. (2009). Alkaline proteases and thermostable α-amylase co-produced by Bacillus licheniformis NH1: Characterization and potential application as detergent additive. Biochemical Engineering Journal 47, 71-79.
- 34. Hutcheon, G.W.; Vasisht, N.; Bolhuis, A. (2005). Characterisation of a highly stable alpha-amylase from the halophilic archaeon Haloarcula hispanica Extremophiles 9, 487-495.
- 35. Ikram ul, H.; Ashraf, H.; Iqbal, J.; Qadeer, M.A. (2003). Production of alpha amylase by Bacillus licheniformis using an economical medium. Bioresour Technol 87, 57-61.
- 36. Iulek, J.; Franco, O.L.; Silva, M.; Slivinski, C.T.; Bloch, C., Jr.; Rigden, D.J.; Grossi de Sa, M.F. (2000). Purification, biochemical characterisation and partial primary structure of a new alpha-amylase inhibitor from Secale cereale (rye). Int J Biochem Cell Biol 32, 1195-1204.
- 37. Jaspreet Singha, J.; Kaurb, L.; McCarthy, O.J. (2007). Factors influencing the physico-chemical, morphological, thermal and rheological properties of some chemically modified starches for food applications-A review. Food Hydrocolloids 21, 1-22.
- 38. Jensen, B.; Nebelong, P.; Olsen, J.; Reeslev, M. (2002). Enzyme production in continuous cultivation by the thermophilic fungus, Thermomyces lanuginosus Biotechnology Letters 24, 41-45.
- 39. Jin, B.; van Leeuwen, H.J.; Patel, B.; Yu, Q. (1998). Utilisation of starch processing wastewater for production of microbial biomass protein and fungal α-amylase by Aspergillus oryzae Bioresour. Technol. 66, 201-206.
- 40. Kajiwara, Y.; Takbshima, N.; Ohba, H.; Omori, T.; Shimoda, M.; Wada, H. (1997). Production of Acid-Stable α-Amylase by Aspergillus during Barley Shochu-Koji Production. J. Ferment. Bioeng. 84, 224-227.
- 41. Kammoun, R.; Naili, B.; Bejar, S. (2008). Application of a statistical design to the optimization of parameters and culture medium for alpha-amylase production by Aspergillus oryzae CBS 819.72 grown on gruel (wheat grinding by-product). Bioresour Technol 99, 5602-5609.
- 42. Kandra, L. (2003). α-Amylases of medical and industrial importance. Journal of Molecular Structure (Theochem) 666-667, 487-498.
- 43. Kathiresan, K.; Manivannan, S. (2006). α-Amylase production by Penicillium fellutanum isolated from mangrove rhizosphere soil. Afr. J. Biotechnol. 5, 829-832.
- 44. Khoo, S.L.; Amirul, A.A.; Kamaruzaman, M.; Nazalan, N.; Azizan, M.N. (1994). Purification and characterization of alpha-amylase from Aspergillus flavus. Folia Microbiol (Praha) 39, 392-398.
- 45. Kirk, O.; Borchert, T.V.; Fuglsang, C.C. (2002). Industrial enzyme applications. Curr Opin Biotechnol 13, 345-351.
- 46. Konsoula, Z.; Liakopoulou-Kyriakides, M. (2007). Co-production of alpha-amylase and beta-galactosidase by Bacillus subtilis in complex organic substrates. Bioresour Technol 98, 150-157.
- 47. Konsula, Z.; Liakopoulou-Kyriakides, M. (2004). Hydrolysis of starches by the action of an α-amylase from Bacillus subtilis Process Biochem 39, 1745-1749.
- 48. Kunamneni, A.; Permaul, K.; Singh, S. (2005). Amylase production in solid state fermentation by the thermophilic fungus Thermomyces lanuginosus J Biosci Bioeng 100, 168-171.
- 49. Mazzola, P.G.; Lopes, A.M.; Hasmann, F.A.; Jozala, A.F.; Penna, T.C.V.; Magalhaes, P.O.; Rangel-Yagui, C.O.; Pessoa, A. (2008). Liquid-liquid extraction of biomolecules: an overview and update of the main techniques. J Chem Technol Biotechnol 83, 143-157.
- 50. Mitidieri, S.; Souza Martinelli, A.H.; Schrank, A.; Vainstein, M.H. (2006). Enzymatic detergent formulation containing amylase from Aspergillus niger: a comparative study with commercial detergent formulations. Bioresour Technol 97, 1217-1224.
- 51. Mohapatra, B.R.; Banerjee, U.C.; Bapuji, M. (1998). Characterization of a fungal amylase from Mucor sp. Associated with the marine sponge Spirastrella sp. J. Biotechnol. 60, 113-117.
- 52. Moller, K.; Sharif, M.Z.; Olsson, L. (2004). Production of fungal alpha-amylase by Saccharomyces kluyveri in glucose-limited cultivations. J Biotechnol 111, 311-318.
- 53. Moraes, L.M.P.; Filho, S.A.; Ulhoa, C.J. (1999). Purification and some properties of an α-amylase glucoamylase fusion protein from Saccharomyces cerevisiae World J. Microbiol. Biotechnol. 15, 561-564.
- 54. Mukherjee, A.K.; Borah, M.; Raí, S.K. (2009). To study the influence of different components of fermentable substrates on induction of extracellular α-amylase synthesis by Bacillus subtilis DM-03 in solid-state fermentation and exploration of feasibility for inclusion of α-amylase in laundry detergent formulations. Biochem. Eng. J. 43, 149-156.
- 55. Murado, M.A.; Gonzfilez, M.P.; Torrado, A.; Pastrana, L.M. (1997). Amylase production by solid state culture of Aspergillus oryzae on polyurethane foams. Some mechanistic approaches from an empirical model. Process Biochem 32, 35-42.
- 56. Muralikrishna, G.; Nirmala, M. (2005). Cereal α-amylases - an overview. Carbohydrate Polymers 60, 163-173.
- 57. Nielsen, J.E.; Borchert, T.V. (2000). Protein engineering of bacterial alpha-amylases. Biochim Biophys Acta 1543, 253-274.
- 58. Olsen, H.S.O.; Falholt, P. (1998). The Role of Enzymes in Modern Detergency. Journal of Surfactants and Detergents 1, 555-567.
- 59. Öner, E.T. (2006). Optimization of ethanol production from starch by an amylolytic nuclear petite Saccharomyces cerevisiae strain. Yeast 23, 849-856.
- 60. Pandey, A. (2003). Solid-state fermentation. Biochem. Eng. J. 13, 81-84.
- 61. Pandey, A.; Nigam, P.; Soccol, C.R.; Soccol, V.T.; Singh, D.; Mohan, R. (2000). Advances in microbial amylases. Biotechnol Appl Biochem 31 (Pt 2), 135-152.
- 62. Payan, F. (2004). Structural basis for the inhibition of mammalian and insect alpha-amylases by plant protein inhibitors. Biochim Biophys Acta 1696, 171-180.
- 63. Prakash, B.; Vidyasagar, M.; Madhukumar, M.S.; Muralikrishna, G.; Sreeramulu, K. (2009). Production, purification, and characterization of two extremely halotolerant, thermostable, and alkali-stable α-amylases from Chromohalobacter sp. TVSP 101. Process Biochem 44, 210-215.
- 64. Prakash, O.; Jaiswal, N. (2009). alpha-Amylase: An Ideal Representative of Thermostable Enzymes. Appl Biochem Biotechnol,
- 65. Rahardjo, Y.S.P.; Weber, F.J.; Haemers, S.; Tramper, J.; Rinzema, A. (2005). Aerial mycelia of Aspergillus oryzae accelerate α-amylase production in a model solid-state fermentation system. Enzyme Microb. Technol. 36, 900-902.
- 66. Rajagopalan, G.; Krishnan, C. (2008). Alpha-amylase production from catabolite derepressed Bacillus subtilis KCC103 utilizing sugarcane bagasse hydrolysate. Bioresour Technol 99, 3044-3050.
- 67. Rao, J.L.U.M.; Satyanarayana, T. (2007). Improving production of hyperthermostable and high maltose-forming α-amylase by an extreme thermophile Geobacillus thermoleovorans using response surface methodology and its applications. Bioresour. Technol. 98, 345-352.
- 68. Reddy, N.S.; Nimmagadda, A.; Sambasiva Rao, K.R.S. (2003). An overview of the microbial α-amylase family. Afr. J. Biotechnol. 2, 645-648.
- 69. Sajedi, R.H.; Naderi-Manesh, H.; Khajeh, K.; Ahmadvand, R.; Ranjbar, B.; Asoodeh, A.; Moradian, F. (2005). A Ca-independent α-amylase that is active and stable at low ph from the Bacillus sp. KR-8104. Enzyme Microb. Technol. 36, 666-671.
- 70. Sanchez, O.J.; Cardona, C.A. (2008). Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour Technol 99, 5270-5295.
- 71. Saxena, R.K.; Dutt, K.; Agarwal, L.; Nayyar, P. (2007). A highly thermostable and alkaline amylase from a Bacillus sp. PN5. Bioresour Technol 98, 260-265.
- 72. Saxena, R.K.; Malhotra, B.; Batra, A. (2004). Commercial Importance of Some Fungal Enzymes. In: Arora, D.K. (ed) Handbook of fungal biotechnology. Marcel Dekker, New York, USA, p 287-298.
- 73. Schwab, K.; Bader, J.; Brokamp, C.; Popovic, M.K.; Bajpai, R.; Berovic, M. (2009). Dual feeding strategy for the production of alpha-amylase by Bacillus caldolyticus using complex media. N Biotechnol 26, 68-74.
- 74. Singhania, R.R.; Patel, A.K.; Soccol, C.R.; Pandey, A. (2009). Recent advances in solid-state fermentation. Biochem. Eng. J. 44, 13-18.
- 75. Siqueira, E.M.A.; Mizuta, K.; Giglio, J.R. (1997). Pycnoporus sanguineus: a novel source of α-amylase. Mycol. Res. 101, 188-190.
- 76. Sodhi, H.K.; Sharma, K.; Gupta, J.K.; Soni, S.K. (2005). Production of a thermostable α-amylase from Bacillus sp. PS-7 by solid state fermentation and its synergistic use in the hydrolysis of malt starch for alcohol production. Process Biochem 40, 525-534.
- 77. Soni, S.K.; Kaur, A.; Gupta, J.K. (2003). A solid state fermentation based bacterial α-amylase and fungal glucoamylase system and its suitability for the hydrolysis of wheat starch. Process Biochem 39, 185-192.
- 78. Sorensen, J.F.; Kragh, K.M.; Sibbesen, O.; Delcour, J.; Goesaert, H.; Svensson, B.; Tahir, T.A.; Brufau, J.; Perez-Vendrell, A.M.; Bellincampi, D.; D'Ovidio, R.; Camardella, L.; Giovane, A.; Bonnin, E.; Juge, N. (2004). Potential role of glycosidase inhibitors in industrial biotechnological applications. Biochim Biophys Acta 1696, 275-287.
- 79. Stamford, T.L.; Stamford, N.P.; Coelho, L.C.; Araujo, J.M. (2001). Production and characterization of a thermostable alpha-amylase from Nocardiopsis sp. endophyte of yam bean. Bioresour Technol 76, 137-141.
- 80. Tangphatsornruang, S.; Naconsie, M.; Thammarongtham, C.; Narangajavana, J. (2005). Isolation and characterization of an alpha-amylase gene in cassava (Manihot esculenta). Plant Physiol Biochem 43, 821-827.
- 81. Tanyildizi, M.S.; Ozer, D.; Elibol, M. (2005). Optimization of α-amylase production by Bacillus sp. using response surface methodology Process Biochem 40, 2291-2296.
- 82. Tanyildizi, M.S.; Ozer, D.; Elibol, M. (2007). Production of bacterial α-amylase by B. amyloliquefaciens under solid substrate fermentation. Biochem. Eng. J. 37, 294-297.
- 83. Teodoro, C.E.S.; Martins, M.L.L. (2000). Culture conditions for the production of thermostable amylase by Bacillus sp. Braz. J. Microbiol. 31, 298-302.
- 84. Tester, R.F.; Karkalas, J.; Qi, X. (2004). Starch-composition, fine structure and architecture. J. Cereal Sci. 39, 151-165.
- 85. Uguru, G.C.; Akinyauju, J.A.; Sani, A. (1997). The use of yam peel for growth of locally isolated Aspergillus niger and amylase production. Enzyme Microb. Technol. 21, 46-51.
- 86. van der Maarel, M.J.; van der Veen, B.; Uitdehaag, J.C.; Leemhuis, H.; Dijkhuizen, L. (2002). Properties and applications of starch-converting enzymes of the alpha-amylase family. J Biotechnol 94, 137-155.
- 87. Wanderley, K.J.; Torres, F.A.; Moraes, L.M.; Ulhoa, C.J. (2004). Biochemical characterization of alpha-amylase from the yeast Cryptococcus flavus FEMS Microbiol Lett 231, 165-169.
- 88. Whitcomb, D.C.; Lowe, M.E. (2007). Human pancreatic digestive enzymes. Dig Dis Sci 52, 1-17.
Publication Dates
-
Publication in this collection
09 Aug 2010 -
Date of issue
Dec 2010
History
-
Received
23 Mar 2010 -
Reviewed
30 Mar 2010 -
Accepted
24 May 2010