Abstract
Group B Streptococcus (GBS) is still not routinely screened during pregnancy in Brazil, being prophylaxis and empirical treatment based on identification of risk groups. This study aimed to investigate GBS prevalence in Brazilian pregnant women by culture or polymerase chain reaction (PCR) associated to the enrichment culture, and to determine the antimicrobial susceptibility patterns of isolated bacteria, so as to support public health policies and empirical prophylaxis. After an epidemiological survey, vaginal and anorectal specimens were collected from 221 consenting laboring women. Each sample was submitted to enrichment culture and sheep blood agar was used to isolate suggestive GBS. Alternatively, specific PCR was performed from enrichment cultures. Antimicrobial susceptibility patterns were determined for isolated bacteria by agar diffusion method. No risk groups were identified. Considering the culture-based methodology, GBS was detected in 9.5% of the donors. Twenty five bacterial strains were isolated and identified. Through the culture-PCR methodology, GBS was detected in 32.6% specimens. Bacterial resistance was not detected against ampicillin, cephazolin, vancomycin and ciprofloxacin, whereas 22.7% were resistant to erythromycin and 50% were resistant to clindamycin. GBS detection may be improved by the association of PCR and enrichment culture. Considering that colony selection in agar plates may be laboring and technician-dependent, it may not reflect the real prevalence of streptococci. As in Brazil prevention strategies to reduce the GBS associated diseases have not been adopted, prospective studies are needed to anchor public health policies especially considering the regional GBS antimicrobial susceptibility patterns.
Perinatal disease; group B streptococci; antimicrobial drug susceptibility
MEDICAL MICROBIOLOGY
Detection of Group B Streptococcus in Brazilian pregnant women and antimicrobial susceptibility patterns
Didier Silveira Castellano-FilhoI; Vânia Lúcia da SilvaI; Thiago César NascimentoI; Marcel de Toledo VieiraII; Cláudio Galuppo DinizI, * * Corresponding Author. Mailing address: Laboratory of Bacterial Physiology and Molecular Genetics, Department of Parasitology, Microbiology and Immunology, Institute of Biological Sciences, Federal University of Juiz de Fora, 36.036-900, Juiz de Fora, MG, Brazil.; Tel/Fax.: + 55 32 2102-3213.; E-mail: claudio.diniz@ufjf.edu.br
IDepartamento de Parasitologia, Microbiologia e Imunologia, Instituto de Ciências Biológicas, Universidade Federal de Juiz de Fora, Juiz de Fora, MG, Brasil
IIDepartamento de Estatística, Instituto de Ciências Exatas, Universidade Federal de Juiz de Fora, Juiz de Fora, MG, Brasil
ABSTRACT
Group B Streptococcus (GBS) is still not routinely screened during pregnancy in Brazil, being prophylaxis and empirical treatment based on identification of risk groups. This study aimed to investigate GBS prevalence in Brazilian pregnant women by culture or polymerase chain reaction (PCR) associated to the enrichment culture, and to determine the antimicrobial susceptibility patterns of isolated bacteria, so as to support public health policies and empirical prophylaxis. After an epidemiological survey, vaginal and anorectal specimens were collected from 221 consenting laboring women. Each sample was submitted to enrichment culture and sheep blood agar was used to isolate suggestive GBS. Alternatively, specific PCR was performed from enrichment cultures. Antimicrobial susceptibility patterns were determined for isolated bacteria by agar diffusion method. No risk groups were identified. Considering the culture-based methodology, GBS was detected in 9.5% of the donors. Twenty five bacterial strains were isolated and identified. Through the culture-PCR methodology, GBS was detected in 32.6% specimens. Bacterial resistance was not detected against ampicillin, cephazolin, vancomycin and ciprofloxacin, whereas 22.7% were resistant to erythromycin and 50% were resistant to clindamycin. GBS detection may be improved by the association of PCR and enrichment culture. Considering that colony selection in agar plates may be laboring and technician-dependent, it may not reflect the real prevalence of streptococci. As in Brazil prevention strategies to reduce the GBS associated diseases have not been adopted, prospective studies are needed to anchor public health policies especially considering the regional GBS antimicrobial susceptibility patterns.
Key words: Perinatal disease, group B streptococci, antimicrobial drug susceptibility
INTRODUCTION
Lancefield Group B Streptococcus (GBS), or Streptococcus agalactiae, are catalase-negative gram positive cocci, which characteristically occurs in pairs or small chains. Nine distinct serotypes are recognized as part of the human microbiota colonizing mucous membranes, especially the gastrointestinal and genitourinary tracts (7,27).
In the 70's GBS was recognized as the main etiology of early-onset neonatal sepsis, with evidence pointing to vertical transmission (mother-to-infant), chiefly by contact with and aspiration of vaginal secretions from the colonized birth canal during labor (21,28). Among GBS-related neonatal infections, sepsis and pneumonia are the most important, followed, less frequently, by meningitis, celullitis, osteomyelitis and septic arthritis (28).
The rates of GBS-colonized pregnant women range worldwide from 3% to 41% (13,26,32,33,35). Brazilian authors have found colonization rates from 5% to 25% in regional studies (3,22,23,26,29,30). GBS colonization may be transient, chronic or intermittent (28). Regardless of the kind of delivery (vaginal or cesarean section), 50% of neonates from colonized mothers become also colonized. Among colonized neonates, 2% may develop GBS infection. Signs of severe infection appear within the first 72 hours of life, already being present in the first 24 hours in 85% of the cases (25).
Due to the incidence and severity of neonatal GBS infection, the Centers for Disease Control and Prevention (CDC), the American College of Obstetricians and Gynecologists (ACOG) and the American Academy of Pediatrics (AAP) issued the first guidelines for prevention of early neonatal streptococcal disease in 1996 (7). The recommendations were revised in 2002, when guidelines for prevention of vertical transmission, through routine screening (culture of vaginal and anorectal secretions between the 35th and 37th gestational week) and intrapartum antibiotic prophylaxis of the colonized women were definitely established (28).
Until today there are no public health policies or strategies in Brazil aimed at the reduction of GBS neonatal infection, the topic is conspicuously absent from the Prenatal and Puerperium - Qualified Care Technical Manual issued by the Ministry of Health. In this regard, prophylaxis and empirical treatment are based on identification of risk groups (31).
This paper describes the GBS prevalence in a population of pregnant women followed-up at a maternity facility belonging to the Brazilian Health Unified System, as well as the antimicrobial drug susceptibility patterns of the isolated bacteria, in order to produce regional knowledge to minimize the risks of irrational use of antimicrobials during empirical prophylaxis and to support public health policies. The data were explored through three sets of analyses. First, isolation of GBS samples presumptively identified by phenotypic characteristics and confirmed by amplification of specific 16S ribosomal RNA encoding DNA. Secondly, direct detection of GBS by polymerase chain reaction after enrichment culture of clinical specimen. Finally, antimicrobial drugs susceptibility patterns were investigated to all isolated bacteria.
MATERIAL AND METHODS
Specimen collection and microbiological culture
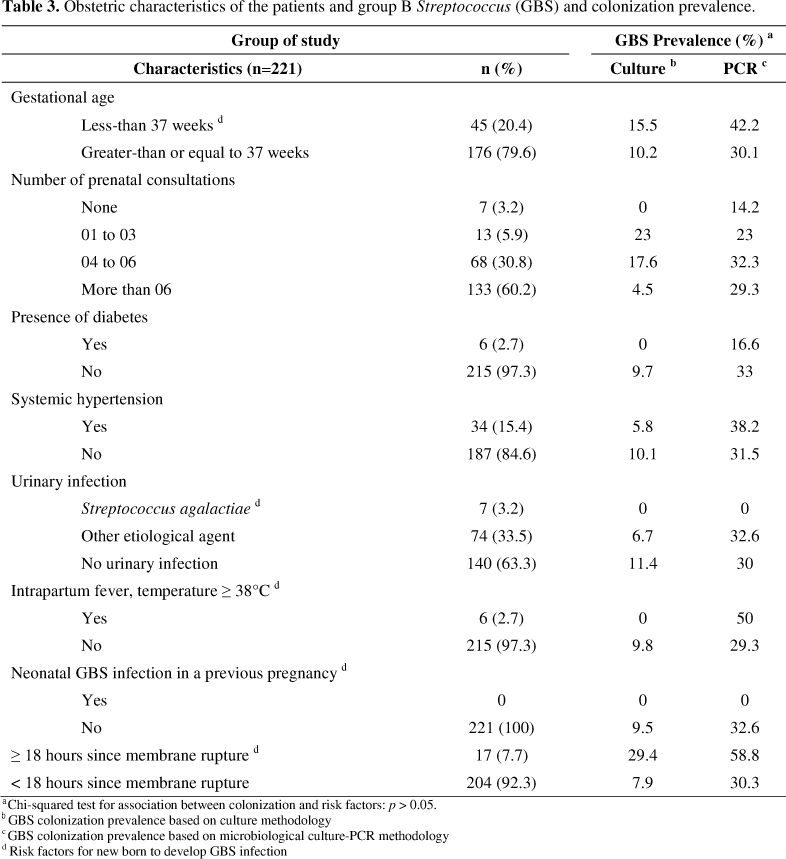
The study was undertaken in the Therezinha de Jesus Maternity Hospital, in the city of Juiz de Fora, Minas Gerais, Brazil, from October 2007 through March 2008. Vaginal and anorectal specimens were collected from 221 pregnant women admitted in labor, which were randomly selected. Besides sociodemographic variables (age, marital status, race, schooling, occupation, place of origin), the following clinical obstetric variables were analyzed: gestational age, number of prenatal consultations, number of pregnancies, parity, presence of diabetes, urinary infection, systemic hypertension, 18 hours or more since membrane rupture, axillary temperature equal to or greater than 38°C, premature labor and neonatal GBS infection in a previous pregnancy.
Admission for labor assistance, regardless of gestational age, was the inclusion criterion. Use of antimicrobial drugs in the 30 days prior to hospital admission and advanced labor with imminent delivery were the exclusion criteria. All the women included in the study signed their informed consent form, in compliance with resolution 196/96 of the Brazilian Health Council. The study was approved by the Committee of Ethics on Research of the Federal University of Juiz de Fora. Sample collection and processing followed the CDC recommendations (7) and were performed by previously trained medical and nursing staff.
Each swab used for sampling was immediately inoculated in Todd-Hewitt broth (Acumedia Manufacturers, Inc. Lansing, MI, USA) supplemented with gentamicin 8μg/ml (Schering-Plough, RJ, Brazil), nalidixic acid 15μg/ml (Homeopatia Santos, MG, Brazil) and sodium azide 0.02% (Sigma-Aldrich, Inc. MO, USA), for enrichment and selective isolation of GBS. The inoculated tubes were incubated at 35.5°C for 18 to 24 hours, at the Laboratory of Bacterial Physiology and Molecular Genetics (Department of Parasitology, Microbiology and Immunology, Institute of Biological Sciences, UFJF).
After enrichment, for isolation of representative GBS colonies, the cultures were streaked onto Petri dishes containing Tryptic Soy Agar (Acumedia Manufacturers, USA) supplemented with 5% defibrinated sheep blood, and incubated at the same condition. Bacterial cultures, beta-hemolytic or not, Gram-positive, with typical morphology and catalase-negative, obtained from isolated suggestive colonies were submitted to the bile-esculin test. The isolates presumably identified as GBS were cryopreserved for further specific identification and assessment of antimicrobial drugs susceptibility.
All studied patients were routinely assessed by the attending obstetricians regarding their risk of GBS colonization, and the antimicrobial prophylaxis was used in all patients who had at least one of the classical risk factors for GBS colonization, according with CDC recommendations.
Molecular identification of bacterial samples
Specific identification of isolated strains or direct detection of GBS after enrichment culture was performed by DNA amplification of a sequence coding for surface immunogenic protein designated as Sip Specific Sequence (SSS) unique for GBS and DNA amplification of a sequence coding for specific 16S RNA region, through the polymerase-chain reaction (PCR), according to the method described by Chotár et al. (8). Genomic DNA from the isolated bacterial samples and the total DNA present in the enrichment culture were extracted by chemical digestion with phenol-chlorophorm, according to the well-established method for obtaining highly purified bacterial DNA (11).
The specific primers pairs SAGA 1 and SAGA 2 or SIP-f and SIP-r (Table 1) were used in two distinct 25μL reactions containing 35μM of each primer, 2μL of the template DNA and 12.5μL of a ready-made commercial solution containing Taq DNA polymerase, dNTPs, MgCl2 and buffers, at an optimum concentration for efficient DNA amplification (PCR Master Mix®, Promega Corporation, Madison, WI, USA). The following amplification conditions were used in both reactions: initial denaturation - 96°C, 5 min, followed by 30 cycles at 96°C, I min; -55°C, 1 min; -72°C, 2 min, followed by final extension of 72°C, 2 min. PCR reactions were made in duplicate and performed in a thermocycler (Techne TC-412 Thermal Cycler, Southam Warwickshire, UK). The amplicons obtained in each reaction were visualized in 1.5% agarose gel in TBE 0.5X buffer, after electrophoresis at constant voltage (120V), for 2 hours. The gels were analyzed in an ultraviolet transilluminator (GE Healthcare, United Kingdom), after treatment with ethidium bromide (Promega Corporation), and recorded by an image photodocumentation system (GE Healthcare, United Kingdom). The amplicon size was estimated with 100 bp Ladder Standard DNA (Promega Corporation) as molecular weight marker. The reference strain Streptococcus agalactiae ATCC 13813 was used as positive control. The negative control was performed in amplification reactions without the DNA template.
Antimicrobial drugs susceptibility assays
Antimicrobial drugs susceptibility patterns were determined through the disk-diffusion method, according to recommendations of the Clinical and Laboratory Standards Institute (9). The following antimicrobials were tested, according to their regional clinical-microbiological relevance: penicillin, ampicillin, clindamycin, erythromycin, cephazolin, ciprofloxacin and vancomycin (Laborclin Laboratory Products, Paraná, Brazil).
Statistical analyses were performed using the SPSS software version 10.0 (SPSS Inc., Chicago, IL, USA). The chi-square test, with the level of significance at the statistical tests at 5%, was used to evaluate the association of GBS colonization and sociodemographics or clinical obstetric variables.
RESULTS
A total of 221 vaginal and 221 anorectal swabs taken from pregnant women admitted in labor were examined. Demographic and clinical obstetric variables are shown in Table 2 and 3. Almost all patients (96.8%) had regularly attended prenatal care consultations in the public municipal health network. Overall the mean number of pregnancies was 2.47 ± 1.89 and the median parity was 1.37 ± 1.87.
GBS colonization was evaluated by using two approaches. The first approach consisted of classical microbiological method (enrichment culture and colony selection in blood agar) followed by specific identification through molecular biology. The second approach consisted of a genetic detection of GBS directly from the enrichment culture. Once two specific GBS genetic markers were assessed, bacterial identification or detection were considered only when the two PCR reactions were positive.
Twenty five bacterial samples were obtained from 21 of the 221 patients (11 from vaginal swabs and 14 from anorectal swabs). Of the positive cultures, microorganisms were simultaneously detected in both sites (vaginal and anorectal) in 4 patients. With this classical microbiological method, GBS colonization prevalence was then 9.5%. With the genetic approach, the test was considered positive for those samples which amplified both segments in a confirmatory way, being GBS identified in 96 of the enrichment cultures (56 vaginal swabs and 40 anorectal swabs) from 72 patients, of the 221 in the study. Of these 96 positive tests, 32 were related only to vaginal and 16 only to anorectal specimens, whereas 24 positives tests were related to both anatomical sites. According to the enrichment culture associated to PCR-based methodology GBS colonization was ascertained in 32.6% of our sample.
Regarding the obstetric characteristics of the patients and GBS colonization prevalence, no statistical difference was observed for detection rates even considering the risk factor for the new born to develop neonatal disease (Table 3). Indeed considering GBS detection in patients with at least one of the risk factors that would indicate the use of antimicrobial prophylaxis (28.8%) and the other patients (34.8%), no statistical difference was observed (p > 0.05).
The occurrence data and relative frequencies considering the two approaches are summarized in Table 4.
All GBS isolates were susceptible to penicillin, ampicillin, cephazolin, ciprofloxacin and vancomycin. However bacterial resistance was observed against erythromycin (22.7%) and clindamycin (50%).
DISCUSSION
There are still no technical recommendations or consensus guidelines on prophylaxis of perinatal streptococcal disease in Brazil (14,31). This was the motivation for this study involving women of any gestational age admitted in labor into a maternity hospital. On the chi-squared test, no significant differences in the GBS colonization rates were detected when the sociodemographic and clinical obstetric variables including the risk factors for the new born to develop infection were considered. Our findings are in agreement with those from other authors (15,23,26) and confirms the poor performance of the risk-based strategy to identify women that should receive antimicrobial prophylaxis. The data support the CDC recommendation that to all pregnant women in the third trimester of gestation should systematically be offered vaginal and anorectal cultures (28).
Even considering the different rates of GBS infection according to the methodological approach used in this study, 9.5% and 32.6%, both values of prevalence are in agreement with literature data, which point to a prevalence range of 3-41% (13,26,27,32,33,35). This worldwide variability is related to different sociocultural, geographic, climatic, biological and methodological determinants. Brazilian studies have found rates ranging from 5 to 25% (3,5,22,23,26,29,30). Our finding of a 32.6% rate of GBS prevalence using the genetic detection directly from the enrichment culture is worrisome and the highest reported in the country to our knowledge so far. On further comparison of the two methodologies, the PCR associated detection directly from enrichment culture was observed to be more sensitive for GBS detection than classical microbiological culture methodology. As the culture-PCR based method, in our study, showed higher GBS detection rates, and two genetic markers were targeted, we believe that such PCR protocol with two distinct reactions might minimize the possibility of false-positive GBS detection based on unspecific DNA amplification. Actually, the literature highlights classical microbiological culture methodology as the gold standard for the epidemiological investigation of GBS prevalence although might be considered a laboring and technician-dependent methodology, especially regarding the colony selection in blood agar plates (1,17,18,19). In a country like Brazil, where routine screening for GBS is not mandatory, staff training at clinical microbiology laboratories is still considered expensive and this lack of professional specific bench expertise would not reflect the real prevalence of streptococci in our region.
Taken together, when the results found considering the both anatomical sites were compared with those from the vaginal site only, there was a 92.3% increase in the GBS detection rate with classical microbiological culture methodology and a 28.2% increase with genetic detection of in the enrichment culture. These findings are supported by the CDC recommendation that samples should be obtained from both sites (28). It is noteworthy that, because anal samples have not been obtained in several studies, the true GBS rates are significantly underestimated.
According to the patients' medical records, they were routinely assessed by the attending obstetricians regarding their risk of GBS colonization and antimicrobial prophylaxis was used based on the clinical evaluation of risk. However, the GBS prevalence rate determined by the culture-PCR based methodology showed that 71.2% of the patients who received intrapartum antibiotic prophylaxis were found to be not colonized. By the other hand, 33.3% of the patients who did not receive intrapartum antibiotic prophylaxis were colonized by GBS. While the first information might indicate that clinical judgment leads to unnecessary use of antibiotics for about seven in each ten patients receiving prophylaxis, the second information might indicate the frequency in which the clinical judgment fails in identifying those who would benefit from prophylaxis.
Considering the antimicrobial susceptibility patterns observed for the isolated bacteria, the results are in accordance with literature data pointing to stable GBS susceptibility to penicillin and ampicillin in the last decades (16). Although the CLSI manual does not indicate the break-point for sensitivity to cephazolin, every isolate susceptible to penicillin must be considered susceptible to cephazolin as well. Our findings (no isolate resistant to cephazolin) confirm literature data (28). In contrast with the situation regarding penicillin, ampicillin and cephazolin, resistance rates to erythromycin and clindamycin, drugs which are considered first-line prophylaxis for those with allergy to penicillin, have progressively increased since 1996 (28). In our study, 22.7% of the isolates were resistant to erythromycin and 50% were resistant to clindamycin. In relation to erythromycin, our data are in agreement with literature data (2,5,10).
As for clindamycin, our rates are higher than those from the literature. This may be accounted for by the small sample number (n=25/221) or still by the increasing use of clindamycin for treatment and prophylaxis of other infectious diseases, such as anaerobic infections in medicine and dentistry. Likewise, anaerobic microorganisms have been growing resistant to clindamycin, something generally explained by their indiscriminate use (6,12).
Although vancomycin is not a first line drug for prophylaxis of perinatal streptococcal disease, some enterococci and Staphylococcus aureus (28) isolates have been proving resistant to this antibiotic. This trend notwithstanding, no vancomycin-resistant GBS isolates have been identified (2,5,10,24,28). In the light of the findings, vancomycin may be considered a better option for prophylaxis of patients allergic to penicillin. As for ciprofloxacin, in our study resistant strains were not detected despite of this drug is not usually mentioned in the guidelines for treatment of GBS in penicillin-allergic patients (7,9). Most authors recommend that quinolones be avoided in pregnancy because of the potential for injury to fetal cartilage. In this regard ciprofloxacin susceptibility was considered due to its microbiological relevance. In fact, some studies have found GBS isolates resistant to fluoroquinolones such as norfloxacin, ciprofloxacin, levofloxacin and gatifloxacin, among others (4,20,34).
Because of the high GBS colonization rates and the antimicrobial susceptibility patterns we found, the role of the obstetrician in the control of this preventable condition becomes easily highlighted. Add to that the need of actualization and systematization of laboratory protocols, as well as financial support on clinical laboratory staff traininship in the detection of GBS. Perinatal streptococcal disease is both an expensive and serious condition that can be effectively prevented by relatively low-cost and fast screening strategies during gestation or at the delivery. We expect our study may contribute to the development of effective public health strategies towards prevention and treatment of this important perinatal threat.
ACKNOWLEDGMENTS
This study was supported by grants from Programa de Pós-graduação em Saúde da Universidade Federal de Juiz de Fora (PPGS/UFJF), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG).
Submitted: August 13, 2009; Returned to authors for corrections: April 19, 2010; Approved: June 21, 2010.
- 1. Atkins, K.L.; Atkinson, R.M.; Shanks, A.; Parvin, A.C.; Dunne, W.M.; Gross, G. (2006). Evaluation of polymerase chain reaction for Group B Streptococcus detection using an improved culture method. Obstet Gynecol, 108 (3), 488-491.
- 2. Barcaite, E.; Bartusevici, A.; Tameliene, R.; Kliucinskas, M.; Maleckiene, L.; Nadisauskiene, R. (2008). Prevalence of maternal group B streptococcal colonisation in European countries. Acta Obstet. Gynecol. Scand., 87 (3), 260-271.
- 3. Beraldo, C.; Brito, A.S.J.; Saridakis, H.O.; Matsuo, T. (2004). Prevalência da colonização vaginal e anorretal por estreptococo do grupo B em gestantes do terceiro trimestre. Rev. Bras. Ginecol. Obstet, 26 (7), 543-549.
- 4. Borchardt, S.M.; Debusscher, J.H.; Tallman, P.A.; Manning, S.D.; Marrs, C.F.; Kurzynski, T.A.; Foxman, B. (2006). Frequency of antimicrobial resistance among invasive and colonizing Group B streptococcal isolates. BMC Infect. Dis., 6 (1), pp.57.
- 5. Borger, I.L.; D'Oliveira, R.E.C.; Castro, A.C.D.; Mondino, S.S.B. (2005). Streptococcus agalactiae em gestantes: prevalência de colonização e avaliação da suscetibilidade aos antimicrobianos. Rev. Bras. Ginecol. Obstet, 27 (10), 575-579.
- 6. Branco, F.P.; Volpato, M.C.; Andrade, E.D. (2007). Profilaxia da endocardite bacteriana na clínica odontológica - o que mudou nos últimos anos? Periodontia, 17 (3), 23-29.
- 7. Centers for Disease Control and Prevention (CDC). (1996). Prevention of Perinatal Group B Streptococcal Disease: A Public Health Perspective. MMWR Recomm. Rep. 45 (RR-7), 1-24.
- 8. Chótar, M.; Vidová, B.; Godány, A. (2006). Development of specific and rapid detection of bacterial pathogens in dairy products by PCR. Folia Microbiol., 51 (6), 639-646.
-
9Clinical and Laboratory Standards Institute (2007). M100-S17. Performance Standards for Antimicrobial Susceptibility Testing. CLSI, Wayne, PA.
- 10. Decoster, L.; Frans, J.; Blankaert, H.; Lagrou, K.; Verhaegen, J. (2005). Antimicrobial susceptibility of group B Streptococci collected in two Belgian hospitals. Acta Clin. Belg., 60 (4), 180-184.
- 11. Diniz, C.G.; Farias, L.M.; Carvalho, M.A.R.; Rocha, E.R.; Smith, C.J. (2004). Differential gene expression in a Bacteroides fragilis metronidazole-resistant mutant. J. Antimicrob. Chemother., 54 (1), 100-108.
- 12. DiPersio, L.P.; DiPersio, J.R. (2006). High rates of erythromycin and clindamycin resistance among OBGYN isolates of group B Streptococcus Diagn. Microbiol. Infect. Dis, 54 (1), 79-82.
- 13. Eisenberg, V.H.; Raveh, D.; Meislish, Y.; Rudensky, B.; Ezra, Y.; Samueloff, A.; Eidelman, A.I.; Schimmel, M.S. (2006). Prevention of early-onset neonatal Group B Streptococcal infection: is universal screening by culture universally applicable? Isr. Med. Assoc. J., 8, 698-702.
-
14Federação Brasileira das Associações de Ginecologia e Obstetrícia (FEBRASGO), 2006. Assistência Pré-natal, 2ed, Febrasgo, São Paulo.
- 15. Fernández, J.; Sánchez, J.; Feris, J.M.; Gómez, E.; Serulle, Y.; Demorizi, J.; Rivera, L.; Rivera-Almodóvar, E.; Mercedes, H.; Pérez-Then, E. (2006). Prevalencia de estreptococo grupo B (EGB) en embarazadas dominicanas. Rev. Panam. Infectol, 8 (1), 26-32.
- 16. Fracalanzza, S.E.L.; Benchetrit, J.R.L.C. (1986). Susceptibilidade de estreptococos do grupo B isolados no período perinatal aos antimicrobianos. Rev. Bras. Med, 43 (8), 221-224.
- 17. Gavino, M.; Wang, E. (2007). A comparison of a new rapid real-time polymerase chain reaction system to traditional culture in determining group B Streptococcus colonization. Am. J. Obstet. Gynecol, 197 (4), 388.e1-388.e4.
- 18. Goodrich, J.S.; Miller, M.B. (2007). Comparison of culture and 2 real-time polymerase chain reaction assays to detect group B Streptococcus during antepartum screening. Diagn. Microbiol. Infect. Dis, 59 (1), 17-22.
- 19. Honest, H.; Sharma, S.; Khan, K.S. (2006). Rapid tests for Group B Streptococcus colonization in laboring women: a systematic review. Pediatrics, 117 (4), 1055-1066.
- 20. Miró, E.; Rebollo, M.; Rivera, A.; Álvarez, M.T.; Navarro, F.; Mirelis, B.; Coll, P. (2007). Streptococcus agalactiae altamente resistente a fluoroquinolonas. Enferm. Infect. Microbiol. Clin, 24 (9), 562-563.
- 21. Moyo, S.R.; Hägerstrand, I.; Nyström, L.; Tswana, S.A.; Blomberg, J.; Bergström, S.; Ljung, A. (1996). Stillbirth and intrauterine infection, histological chorioamnionitis and microbiological findings. Int. J. Gynaecol. Obstet., 54 (2), 115-123.
- 22. Oliveira, A.I.F.; Jácomo, A.J.D.; Filho, L.C.; Cordeiro, D.; Carvalho, N.N. (1985). Colonização em gestantes por estreptococo do grupo B. J. Pediatr, 58 (6), 381-382.
- 23. Pellegrini, R. (1999). Freqüência de colonização por Streptococcus agalactiae em gestantes da cidade de Salvador, Bahia. Rev. Soc. Bras. Med. Trop., 32 (4), 451-452.
- 24. Persson, E.; Berg, S.; Bergseng, H.; Bergh, K.; Valsö-Lyng, R.; Trollfors, B. (2008). Antimicrobial susceptibility of invasive group B Streptococcal isolates from south-west Sweden 1988-2001. Scand. J. Infect. Dis, 40 (4), 308-313.
- 25. Pettersson, K. (2007). Perinatal infection with Group B streptococci. Semin. Fetal Neonatal Med, 12, 193-197.
- 26. Pogere, A.; Zoccoli, C.M.; Tobouti, N.R.; Freitas, P.F.; D'Acampora, A.J.; Zunino, J.N. (2005). Prevalência da colonização pelo estreptococo do grupo B em gestantes atendidas em ambulatório de pré-natal. Rev. Bras. Ginecol. Obstet., 27 (4), 174-180.
- 27. Savoia, D.; Gottimer, C.; Crocilla, C.; Zucca, M. (2008). Streptococcus agalactiae in pregnant women: phenotypic and genotypic characters. J. Infect., 56 (2), 120-125.
- 28. Schrag, S.; Gorwitz, R.; Fultz-Butts, K.; Schuchat, A. (2002). Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm. Rep., 51(RR- 11), 1-22.
- 29. Simões, J.A.; Alves, V.M.N.; Fracalanzza, E.L.; Camargo, R.P.S.; Mathias, L.; Milanez, H.M.B.P.; Brolazo, E. M. (2007). Phenotypical characteristics of group B Streptococcus in parturients. Braz. J. Infect. Dis, 11 (2), 261-266.
- 30. Smânia Júnior, A.; Benchetrit, L.C.; Smânia, E.F.A.; Fracalanzza, S.E.L. (1986). Isolamento de estreptococos do grupo B, de gestantes e neonatos, em Florianópolis, Santa Catarina. Rev. Bras. Anal. Clin., 18 (4), 103-108.
- 31. Soares Filho, A.M.; Serra, A.S.L.; Rattner, D.; Cruz, D.R.N.; Cezimbra, G.S.S.; Pires, H.M.B. (2007). Pré-natal e Puerpério - Atenção Qualificada e Humanizada - Manual Técnico. Editora MS, Brasília.
- 32. Thibaudon, B.C.; Stroebel, N.A.; Boulard, M.I.; Djavadzadeh-Amini, M.; Kacet, N.; Truffert, P; Subtil, D.; Dubos, J.P. (2008). Prevention of early-onset group B Streptococcus neonatal diseases. The 2005 experience of the Lille University Health Center. J. Gynecol. Obstet. Biol. Reprod, 37 (4), 392-399.
- 33. Vaciloto, E.; Richmann, R.; Costa, H.P.F.; Kusano, E.J.U.; Almeida, M.F.B.; Amro, E.R. (2002). A Survey of the incidence of neonatal sepsis by Group B Streptococcus during a decade in a Brazilian maternity hospital. Braz. J. Infect. Dis., 6 (2), 55-62.
- 34. Wehbeh, W.; Rojas-Diaz, R.; Li, X.; Mariano, M.; Grenner, L.; Segal-Maurer, S.; Tommasulo, B.; Drlica, K.; Urban, C.; Rahal, J.J. (2005). Fluoroquinolone-resistant Streptococcus agalactiae: epidemiology and mechanism of resistance. Antimicrob. Agents Chemother., 49 (6), 2495-2497.
- 35. Winn, H.N. (2007). Group B Streptococcus in pregnancy. Clin. Perinatol, 34 (3), 387-392.
Publication Dates
-
Publication in this collection
09 Aug 2010 -
Date of issue
Dec 2010
History
-
Reviewed
19 Apr 2010 -
Received
13 Aug 2009 -
Accepted
21 June 2010