Abstract
Chronic osteomyelitis of maxilla and mandible is rare in industrialized countries and its occurrence in developing countries is associated with trauma and surgery, and its microbial etiology has not been studied thoroughly. The aim of this investigation was to evaluate the microbiota associated with osteomyelitis of mandible or maxilla from some Brazilian patients. After clinical and radiographic evaluation, samples of bone sequestra, purulent secretion, and biopsies of granulomatous tissues from twenty-two patients with chronic osteomyelitis of mandible and maxilla were cultivated and submitted for pathogen detection by using a PCR method. Each patient harbored a single lesion. Bacterial isolation was performed on fastidious anaerobe agar supplemented with hemin, menadione and horse blood for anaerobes; and on tryptic soy agar supplemented with yeast extract and horse blood for facultative bacteria and aerobes. Plates were incubated in anaerobiosis and aerobiosis, at 37ºC for 14 and 3 days, respectively. Bacteria were cultivated from twelve patient samples; and genera Actinomyces, Fusobacterium, Parvimonas, and Staphylococcus were the most frequent. By PCR, bacterial DNA was detected from sixteen patient samples. The results suggest that cases of chronic osteomyelitis of the jaws are usually mixed anaerobic infections, reinforcing the concept that osteomyelitis of the jaws are mainly related to microorganisms from the oral environment, and periapical and periodontal infections may act as predisposing factors.
osteomyelitis; bacteria; anaerobes; maxilla; mandible
MEDICAL MICROBIOLOGY
Microbiota associated with chronic osteomyelitis of the jaws
Elerson Gaetti-Jardim JúniorI, III, * * Corresponding Author. Mailing address: School of Dentistry of Araçatuba, Department of Pathology, São Paulo State University-UNESP, Araçatuba, SP, Brazil, Rua José Bonifácio 1193, 16015-050, Araçatuba, SP, Brazil.; Tel.: (+55) 18 36362797 Fax: (+55) 18 36364125.; E-mail : egaettij@foa.unesp.br ; Angélica Cristiane FardinI; Ellen Cristina Gaetti-JardimI; Alvimar Lima de CastroI; Christiane Marie SchweitzerII; Mario Julio Avila-CamposIII
IFaculdade de Odontologia de Araçatuba, Departamento de Patologia, Universidade Estadual Paulista, Araçatuba, SP, Brasil
IICentro de Matemática, Computação e Cognição, Universidade Federal do ABC, Santo André, SP, Brasil
IIILaboratório de Anaeróbios, Departamento de Microbiologia, Universidade de São Paulo, São Paulo, Brasil
ABSTRACT
Chronic osteomyelitis of maxilla and mandible is rare in industrialized countries and its occurrence in developing countries is associated with trauma and surgery, and its microbial etiology has not been studied thoroughly. The aim of this investigation was to evaluate the microbiota associated with osteomyelitis of mandible or maxilla from some Brazilian patients. After clinical and radiographic evaluation, samples of bone sequestra, purulent secretion, and biopsies of granulomatous tissues from twenty-two patients with chronic osteomyelitis of mandible and maxilla were cultivated and submitted for pathogen detection by using a PCR method. Each patient harbored a single lesion. Bacterial isolation was performed on fastidious anaerobe agar supplemented with hemin, menadione and horse blood for anaerobes; and on tryptic soy agar supplemented with yeast extract and horse blood for facultative bacteria and aerobes. Plates were incubated in anaerobiosis and aerobiosis, at 37ºC for 14 and 3 days, respectively. Bacteria were cultivated from twelve patient samples; and genera Actinomyces, Fusobacterium, Parvimonas, and Staphylococcus were the most frequent. By PCR, bacterial DNA was detected from sixteen patient samples. The results suggest that cases of chronic osteomyelitis of the jaws are usually mixed anaerobic infections, reinforcing the concept that osteomyelitis of the jaws are mainly related to microorganisms from the oral environment, and periapical and periodontal infections may act as predisposing factors.
Key words: osteomyelitis, bacteria, anaerobes, maxilla, mandible.
INTRODUCTION
Chronic osteomyelitis is a relapsing and persistent infection that evolves over months to years and is characterized by low-grade inflammation, presence of bone sequestra, new bone apposition, and, some times, fistulous tracts. (28) In contrast to acute purulent osteomyelitis, chronic osteomyelitis is characterized by a low-grade clinical presentation, with lymphocyte and plasma cell infiltrates with necrotic areas and some bone condensation (28).
Its occurrence in the craniofacial skeleton is rarely observed in industrialized countries (1). However, this infection is prevalent in developing countries, where it is frequently associated with trauma and surgery, and its incidence, clinical signs and microbial etiology have not been studied thoroughly (22). In the maxillofacial skeleton, chronic osteomyelitis is more often observed in the mandible and maxilla; and it can be limited to a unique anatomic site or spread to other areas, particularly in individuals presenting impairment of the immune response, non-controlled diabetes and hospitalized patients (3).
The pathogenesis of osteomyelitis may be induced either by hematogenous origin or by dissemination of local infections, and its treatment involves removal of bone sequestra, lesion debridement, and bone decortication associated with systemic antibiotic therapy (7, 10). However, in some patients, these infections are refractory to surgery and antibiotic therapy (7), requiring an adequate microbiological diagnosis (10, 16).
The occurrence, type, severity and clinical prognosis of osteomyelitis depend upon several factors, including the characteristics and virulence of the infecting pathogen, host immune response and source of infection (3). In addition, rapid and accurate microbiological osteomyelitis diagnosis is crucial to delineate the appropriate antibiotic therapy.
Thus, the aim of the present study was to evaluate the microbiota associated with chronic osteomyelitis of maxilla and mandible.
MATERIAL AND METHODS
Patients and Sample Collection
A total of twenty-two patients (14 males and 8 females); aged from 13 to 67 years old (mean, 51.43 years) with clinical and radiographic signs of chronic osteomyelitis seen at the School of Dentistry of Araçatuba, São Paulo State University (UNESP) and at School of Dentistry of Santa Fé do Sul (FUNEC), Brazil were selected. Samples were collected between February 2000 and November 2008. All patients gave written informed consent to be included in this study, which was approved by the Institutional Review Boards of the Universities mentioned above (UNESP, 0063/2000 and 136/2007; FUNEC, 03/2008).
All patients underwent clinical interview to obtain information about their identification, age, ethnic aspects, family and past medical history, followed by clinical and radiographic evaluation of the bone lesion, periodontium and teeth. The most frequent clinical findings are presented in Table 2. Patients exhibiting uncontrolled significant cardiovascular, pulmonary, diabetes mellitus, renal or hepatic disease; submitted to radiotherapy or therapy with bisphosphonates, steroid drugs treatment, or self-medication with antibiotics three months prior to the beginning of the treatment were excluded from this study (28). Six months prior to treatment, six patients had made use of self-medication with amoxicillin and two patients had taken ciprofloxacin and cephalexin orally for 7-14 days.
Only bone biopsy, bone sequestra, bone marrow, granulation tissue and aspirated pus specimens were acceptable for microbial diagnosis. In order to avoid cross-contamination from oral microorganisms, the clinical specimens were obtained intra-orally through surgical procedures, avoiding contact with soft tissues, oral environment and sinus tracts. When possible, samples of bone or granulation tissues were also obtained for histopathological analysis (22). When bone biopsies were removed, areas of the margin of the osteolytic lesion were preferred and removed with chisels and osteotome.
Lesion samples were immediately placed into a tube containing VMGA III medium (21) and 250 μl of ultra-pure water (Milli-Q Water Purification System, Millipore, Milford, MA, USA), and stored at -196ºC, until DNA extraction.
Isolation and Identification of Microorganisms
The collected samples were processed within 2 h. Samples in VMGA III were submitted to serial 10-fold dilutions and plated onto fastidious anaerobe agar (FAA) enriched with 5 μg/mL hemin, 1 μg/mL menadione, 0.5% yeast extract and 5% horse blood. Plates were incubated in anaerobiosis (90 % N2 + 10% CO2) at 37ºC, for 10 days, for isolation of anaerobes. Additionally, samples were also incubated onto tryptic soy agar (TSA) supplemented with 5% horse blood, 0.5% yeast extract and incubated at 37ºC for 48 h, for aerobic and facultative bacteria.
Isolates were identified by Gram staining, colony morphology on blood agar plates, and by the use of biochemical identification kits Rapid ID 32 A (BioMérieux SA, Marcy-l'Etoile, France) for strict anaerobic Gram-negative and -positive rods; RapIDANA II System (Innovative Diagnostic Systems Inc., Atlanta, GA, USA) for strict anaerobic Gram-positive cocci; API Staph (BioMérieux) for staphylococci (Gram-positive cocci, catalase-positive); Rapid ID 32Strep (BioMérieux) for streptococci (Gram-positive cocci, catalase-negative); Rapid NH System (Innovative Diagnostic Systems Inc, Atlanta, GA, USA) for genus Eikenella and API 20E (BioMérieux) for facultative enteric rods. In addition, P. intermedia and P. nigrescens isolates were confirmed by DNA amplification through PCR (13).
Detection of Pathogens by PCR
The presence of microorganisms that have frequently been isolated from oral and bone infections was also evaluated by PCR. Presence of Actinomyces israelii, A. naeslundii, A. viscosus (27), Aggregatibacter actinomycetemcomitans, Campylobacter rectus, Dialister pneumosintes, Eikenella corrodens, Fusobacterium nucleatum, Porphyromonas gingivalis, Prevotella intermedia, Tannerella forsythia, Treponema denticola (2), family Enterobacteriaceae (11), genus Enterococcus (13), E. faecalis (12), E. faecium (9), Parvimonas micra, Porphyromonas endodontalis, Prevotella nigrescens (13), Propionibacterium acnes (14), genus Pseudomonas, P. aeruginosa (25), genus Staphylococcus (20), Streptococcus mutans, S. oralis, S. salivarius, S. sanguinis, and S. sobrinus (15) was determined by PCR using specific primer pairs (Table 1). Additionally, a universal primer pair specific for bacterial 16S rDNA was used to detect DNA from all eubacterial species present in the samples (13).
Bacterial DNA from each sample placed in sterile ultra-pure water was obtained by using a QIAamp DNA Mini Kit (Qiagen, Hilden, Germany). Purified genomic DNA from reference strains of tested microorganisms were used as positive controls. DNA concentrations were determined spectrophotometrically (A260nm; Model DU-640, Beckman Instruments, Richmond, Wash, USA).
PCR amplification was performed in volumes of 25 μL containing 1X PCR/Mg++ buffer (Boehring Mannheim, Indianapolis, IN, USA), 0.2 mM of each dNTP (Pharmacia Biotech, Piscataway, NJ, USA), 0.5 U Taq DNA polymerase (Invitrogen do Brasil, São Paulo, SP, Brazil), 0.4 μM of each primer pair (Invitrogen) and 10 ng of DNA template, using a DNA Thermal Cycler (Perkin Elmer, GeneAmp PCR System 9700, Norwalk, CT, USA). The conditions and oligonucleotides used in DNA amplification are presented in Table 1.
Amplification products were submitted to electrophoresis in 1% agarose gel in 1X TBE buffer (1 M Tris, 0.9 M boric acid, 0.01 M EDTA, pH 8.4, Invitrogen, SP, Brazil), stained with 0.5 mg/mL of ethidium bromide and photographed on a UV light transilluminator (Eastman Kodak Co., NY, USA).
Statistical analysis
Differences between clinical parameters and the frequency of pathogen detection for each subject were analyzed by using the Chi-square or Fisher's exact test. Inter-relationships among different microorganisms were evaluated using the Spearman correlation coefficient test.
RESULTS
The data presented in Table 1 evidenced that most patients with chronic osteomyelitis were smokers (59.1%), presented gingivitis (36.4%) or periodontitis (59.1%), and this infection was more frequent in mandible. In contrast, maxilla was affected just in a five cases (22.7%) and the posterior range was the unique anatomical site affected in this bone. In seven patients (31.8%), osteomyelitis showed a possible relationship with previous dental extractions or endodontic treatment.
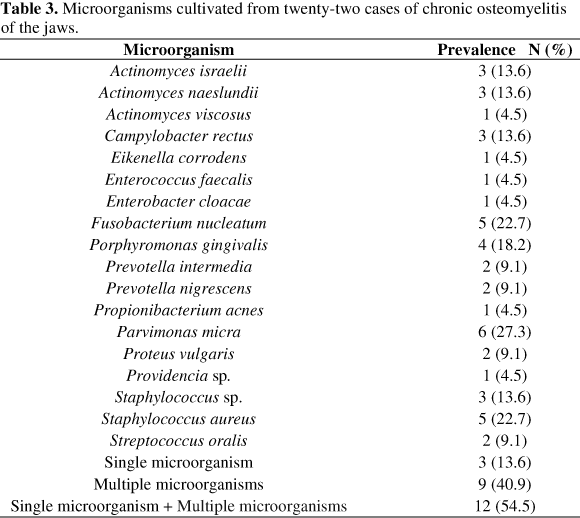
The microbiota associated with maxillary osteomyelitis was similar to that observed in the mandibular cases. By using culture, single or multiple microorganisms were detected from 12 samples (Table 3), while single or multiple microorganisms were detected from 16 samples through PCR (Table 4). All positive samples by culture were also positive by PCR; moreover, two samples of bone biopsy and two specimens of bone sequestra produced positive results only by PCR. Microbial contamination was not detected either by PCR or culture in five osteomyelitis samples; four of the mandible and one of the maxilla.
Cultivated samples evidenced monomicrobial infection by S. aureus in two patients and by Staphylococcus sp. in one patient. Monomicrobial infections caused by strict anaerobes were not observed, but their association with facultative anaerobic cocci and rods was reported in nine cases.
By PCR, mixed infections were detected in fourteen samples, and strict anaerobes were presented in all these positive samples. Monomicrobial infections caused by Staphylococcus sp. were detected in only two samples (Table 4). An average of 3.8 species of strict anaerobes and 1.5 species of aerobes and facultative anaerobes were detected or cultivated per specimen, and this phenomenon was statistically significant (Chi-square test, p= 0.031).
In this study, the most frequently cultivated microorganisms were Parvimonas micra, Staphylococcus aureus, Fusobacterium nucleatum and actinomycetes. Other bacteria, such as Propionibacterium acnes, Porphyromonas gingivalis, Prevotella intermedia, P. nigrescens, Streptococcus oralis, Eikenella corrodens, Enterococcus faecalis, Campylobacter rectus and facultative gram-negative enteric rods were also observed (Table 3). By PCR (Table 4), the detection frequencies of targeted microorganisms were usually higher than those observed by culture.
In the five patients with chronic suppuration and intraoral fistula, high numbers of Fusobacterium nucleatum were observed. Results from PCR suggest a synergistic association between E. corrodens and F. nucleatum (Chi-square test, p= 0.043). Actinomycetes were detected in all lesions with bone sequestra by culture and PCR methods (Fisher's exact test, p = 0.027).
Moreover, A. actinomycetemcomitans, D. pneumosintes, E. faecium, P. endodontalis, S. mutans, S. salivarius, S. sanguinis, S. sobrinus, T. forsythia and pseudomonads were not detected by either culture or PCR methods.
DISCUSSION
Odontogenic infection is the most common cause of osteomyelitis of the jaws, although other causes including injury, malignant tumors, malnutrition, diabetes, chronic systemic diseases and infectious diseases occurring in hypovascularized bone may be associated with this condition (7, 22). In the present study, several fastidious strictly anaerobic bacteria commonly present in the dental biofilm were detected, suggesting that the source of infecting pathogens in osteomyelitis of the jaws is likely to be gingivitis, chronic periodontitis, previous dental extractions or endodontic treatments.
The presence of different oral anaerobic species in mixed infections suggests that ecological associations may be relevant in the development of osteomyelitis of the mandible and maxilla, since most oral anaerobes depend on favorable environmental conditions and complex ecological interactions with different microbial species to develop its capacity to attack and colonize other areas of the host (7, 24). Therefore, it is possible that pathogens different from those of the oral microbiota may reach the bone tissues through transient bacteremia, which are common after surgical procedures or traumas (26).
Mixed anaerobic infections are common in osteomyelitis, especially in skull infections, varying from 33% (4) to 93% (8) of the cases, with a higher prevalence of anaerobes (2.4-3.9 species per sample) than aerobes (0.4 species per sample) (6, 8). In the current study, strict anaerobes were predominant in all mixed infections (3.8 species per sample), while aerobes and facultative anaerobes were less frequent (1.5 species per sample).
The microorganisms detected by culture or PCR from osteolytic lesions or granulation tissues most likely represent the microbiota associated with the lesions and not simply external contaminants. Absence of the most common inhabitants of the oral cavity, including S. mutans, S. salivarius, S. sanguinis, and S. sobrinus, suggests that cross-contamination with oral tissues and dental biofilm was minimized during collection, since samples were obtained through surgical procedures.
Our data showed that the participation of Enterobacteriaceae and other enteric bacteria, such as Enterococcus faecalis, usually associated with immunosuppressed patients, nosocomial and multi-resistant infections (7, 23, 28), is relevant in the microbiota detected in cases of chronic osteomyelitis of the jaws.
Actinomycetes are frequently isolated from oral infections. The results of culture and PCR confirm that A. israelii is the most common member of this genus in chronic osteomyelitis of the jaws. In the current study, all patients exhibited clinical history of periodontal diseases, dental extractions or endodontic treatment, which could facilitate the dissemination of Actinomyces to damaged tissues and the development of chronic infection (18). In this investigation, all samples with Actinomyces species were collected from the mandible, which presents poor vascular supply when compared with the maxilla, as previously reported (5).
Actinomyces is almost invariably isolated as part of polymicrobial microbiota, which commonly includes periodontal bacteria, Staphylococcus, Streptococcus, and Enterococcus spp. (24), as also evidenced in our study. In mixed microbiota, facultative or aerotolerant bacteria, such as actinomycetes and oral streptococci, may facilitate osteolysis, enabling the most active proteolytic Gram-negative anaerobes; for instance P. gingivalis and other black-pigmented Porphyromonas and Prevotella species, to survive in bone lesions or within environments with reduced redox potential inside osteolytic cavities (5, 18, 19). These conditions often result in recurrent disease and inconsistent responses to antimicrobial agents, especially in chronic infections (7).
The prevalence of anaerobic gram-positive cocci, especially Parvimonas micra in osteomyelitis was also previously described (4, 7). These microorganisms are frequently isolated from periodontitis and gingivitis patients, as well as from dental periapical abscesses and other mixed anaerobic infections (17), however their virulence factors have not been fully elucidated (23).
In this study, the presence of F. nucleatum in chronic osteomyelitis was associated with the presence of chronic suppuration and draining sinus tract. This phenomenon may be related to the ability of these anaerobes to stimulate host immune responses and inflammation. In addition, fusobacteria have been implicated in complex ecological interactions, creating conditions for the establishment of black-pigmented Porphyromonas and Prevotella, as well as anaerobic gram-positive cocci, which are commonly associated with osteolytic lesions and other odontogenic infections (7, 17). These Gram-negative anaerobes, particularly P. gingivalis, may also induce tissue damage due to their ability to stimulate cytokine secretion, adhesion and host tissues invasion, and activation of osteoclasts.
This study suggests that cases of chronic osteomyelitis of the jaws are usually due to mixed anaerobic infections caused by oral microorganisms, especially those from the subgingival biofilm, frequently associated to staphylococci and enteric microorganisms.
ACKNOWLEDGMENTS
This study was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Proc. 02/07371-0 and 07/54851-0).
Submitted: August 25, 2009; Returned to authors for corrections: December 11, 2009; Approved: May 25, 2010.
- 1. Antony, B.; Thomas, S.; Chandrashekar, S.C.; Kumar, M.S.; Kumar, V. (2009). Osteomyelitis of the mandible due to Aggregatibacter (Actinobacillus) actinomycetemcomitans Indian J. Pathol. Microbiol 52 (1), 115-116.
- 2. Avila-Campos, M.J.; Velàsquez-Melèndez, G. (2002) Prevalence of putative periodontopathogens from periodontal patients and healthy subjects in São Paulo, SP, Brazil. Rev. Inst. Med. Trop. S. Paulo 44, 1-5.
- 3. Brady, B.A.; Leid, J.G.; Costerton, J.W.; Shirtliff, M.E. (2006). Osteomyelitis: clinical overview and mechanisms of infection persistence. Clin. Microbiol. Newsletter 28, 65-72.
- 4. Brook, I. (1986). Anaerobic osteomyelitis in children. Pediatr. Infect. Dis. 5, 550-556.
- 5. Brook, I. (2008). Actinomycosis: diagnosis and management. Southern Med. J. 101,1019-1023.
- 6. Brook, I. (2008). Microbiology and management of joint and bone infections due to anaerobic bacteria. J. Orthop. Sci. 13, 160-169.
- 7. Brook, I.; Frazier, E.H. (1993). Anaerobic osteomyelitis and arthritis in a military hospital: a 10-year experience. Am. J. Med 94, 2-8.
- 8. Calhoun, K.H.; Shapiro, R.D.; Stiernberg, C.M.; Calhoun, J.H.; Mader, J.T. (1988). Osteomyelitis of the mandible. Arch. Otolaryngol. Head Neck Surg 114, 1157-1162.
- 9. Cheng, S.; McCleskey, F.K.; Gress, M.J.; Petroziello, J.M.; Liu, R.; Namdari, H.; Beninga, K.; Salmen, A.; Del Vecchio, V.G. (1997). A PCR assay for identification of Enterococcus faecium J. Clin. Microbiol 35, 1248-1250.
- 10. Coviello, V.; Stevens, M.R. (2007). Contemporary concepts in the treatment of chronic osteomyelitis. Oral Maxillof. Surg. Clin. N. Am. 19, 523-534.
- 11. Fenollar, F.; Roux, V.; Stein, A.; Drancourt, M.; Raoult, D. (2006) Analysis of 525 samples to determine the usefulness of PCR amplification and sequencing of the 16S rRNA gene for diagnosis of bone and joint infections. J. Clin. Microbiol 44, 1018-28.
- 12. Foschi, F.; Cavrini, F.; Montebugnoli, L.; Stashenko, P.; Sambri, V.; Prati, C. (2005). Detection of bacteria in endodontic samples by polymerase chain reaction assays and association with defined clinical signs in Italian patients. Oral Microbiol. Immunol 20, 289-295.
- 13. Fouad, A.F.; Barry, J.; Caimano, M.; Clawson, M.; Zhu, Q.; Carver, R.; Hazlett, K.; Radolf, J.D. (2002). PCR-based identification of bacteria associated with endodontic infections. J. Clin. Microbiol 40, 3223-3231.
- 14. Harada, K.; Tsuneyama, K.; Sudo, Y.; Masuda, S.; Nakanuma, Y. (2001). Molecular identification of bacterial 16S ribosomal RNA gene in liver tissue of primary biliary cirrhosis: is Propionibacterium acnes involved in granuloma formation? Hepatology 33, 530-536.
- 15. Hoshino, T.; Kawaguchi, M.; Shimizu, N.; Hoshino, N.; Ooshima, T.; Fujiwara, T. (2004). PCR detection and identification of oral streptococci in saliva samples using gtf genes. Diag. Microbiol. Infect. Dis. 48, 195-199.
- 16. Kim, S.G.; Jang, H.S. (2001). Treatment of chronic osteomyelitis in Korea. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 92, 394-398.
- 17. Kuriyama, T.; Karasawa, T.; Nakagawa, K.; Yamamoto, E.; Nakamura, S. (2002). Bacteriology and antimicrobial susceptibility of gram-positive cocci isolated from pus specimens of orofacial odontogenic infections. Oral Microbiol. Immunol. 17, 132-135.
- 18. Long, J.B.; Collins, J.M.; Beauchamp, C.P.; Kho, R.; Cornella, J.L. (2007). Actinomyces meyeri osteomyelitis of the symphysis pubis following pubovaginal sling. Int. Urogynecol. J. 18, 1375-1378.
- 19. Lugassy, G.; Shaham, R.; Nemets, A.; Ben-Dor, D.; Nahlieli, O. (2004). Severe osteomyelitis of the jaw in long-term survivors of multiple myeloma: a new clinical entity. Am. J. Med 117, 440-441.
- 20. Martineau, F.; Picard, F.J.; Ke, D.; Paradis, S.; Roy, P.H.; Ouellette, M.; Bergeron., M.G. (2001). Development of a PCR assay for identification of staphylococci at genus and species levels. J. Clin. Microbiol 39, 2541-2547.
- 21. Möller, A.J. (1966). Microbial examination of root canals and periapical tissues of human teeth: methodological studies. Odontol. Tidskr. 74:1-138.
- 22. Prasad, K.C.; Prasad, S.C.; Mouli, N.; Agarwal, S. (2007). Osteomyelitis in the head and neck. Acta Oto-Laryngol 127, 194-205.
- 23. Scolozzi, P.; Lombardi, T.; Edney, T.; Jaques, B. (2005). Enteric bacteria in mandibular osteomyelitis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 99, 42-46.
- 24. Sharkawy, A.A. (2007). Cervicofacial actinomycosis and mandibular osteomyelitis. Infect. Dis. Clin. N. Am. 21, 543-556.
- 25. Spilker, T.; Coenye, T.; Vandamme, P.; Lipuma, J.J. (2004). PCR-based assay for differentiation of Pseudomonas aeruginosa from other Pseudomonas species recovered from cystic fibrosis patients. J. Clin. Microbiol 42, 2074-2079.
- 26. Takai, S.; Kuriyama, T.; Yanagisawa, M.; Nakagawa, K.; Karasawa, T. (2005). Incidence and bacteriology of bacteremia associated with various oral and maxillofacial surgical procedures. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod 99, 292-298.
- 27. Xia, T.; Baumgartner, J.C. (2003). Occurrence of Actinomyces in infections of endodontic origin. J. Endod 29, 549-552.
- 28. Zuluaga, A.F.; Galvis, W.; Saldarriaga, J.G.; Agudelo, M.; Salazar, B.E.; Vesga, O. (2006). Etiologic diagnosis of chronic osteomyelitis. Arch Intern Med 166, 95-100.
Publication Dates
-
Publication in this collection
09 Aug 2010 -
Date of issue
Dec 2010
History
-
Received
25 Aug 2009 -
Accepted
25 May 2010 -
Reviewed
11 Dec 2009