Abstract
Colletotrichum lindemuthianum, the causative agent of bean anthracnose, is one of the most common pathogens leading to expressive damage to plants beyond presenting noticeable variability. The knowledge on vegetative compatibility groups (VCGs) is of particular interest in asexual fungi as they subdivide the population in groups that can exchange genetic information via heterokaryosis and the parasexual cycle. Among the techniques used in studies about vegetative compatibility groups, the obtainment of nit mutants is apparent. This paper is aimed at obtaining heterokaryons between different isolates of C. lindemuthianum, grouping them in VCGs and evaluating their genetic variability by using the nit mutants system. Nit mutants were obtained from 20 single spore isolates. The mutants were phenotypically classified and paired for complementation and formation of heterokaryons so as to group them in VCGs. Seventeen mutants from the different phenotypic-rates were recovered: nit1, nit2, nit3 and nitM. At the same time, 10 mutants were selected for pairing and division of the anastomosis groups. Nine heterokaryons were obtained and the isolates were divided into 9 vegetative compatibility groups. In the combinations for the formation of anastomosis, 31 compatible combinations and 24 incompatible combinations were observed. It was concluded that the methodology used to select nit mutants in C. lindemuthianum made it possible to determine the vegetative compatibility groups and that such a technique was adequate to prove genetic variability.
Colletotrichum lindemuthianum; anthracnose; heterokaryosis; nit mutants; VCG
GENETICS AND MOLECULAR MICROBIOLOGY
Vegetative compatibility and heterokaryon formation between different isolates of Colletotrichum lindemuthianum by using the nit mutant system
Camila Rodrigues de Carvalho; Maria Cristina Mendes-Costa* * Corresponding Author. Mailing address: Research Laboratory I (Fungi), Centro Universitário de Lavras, CEP 37200-000, Lavras, MG, Brazil.; E-mail: mcmcosta@unilavras.edu.br
Laboratório de Pesquisa I, Centro Universitário de Lavras, Lavras, MG, Brasil
ABSTRACT
Colletotrichum lindemuthianum, the causative agent of bean anthracnose, is one of the most common pathogens leading to expressive damage to plants beyond presenting noticeable variability. The knowledge on vegetative compatibility groups (VCGs) is of particular interest in asexual fungi as they subdivide the population in groups that can exchange genetic information via heterokaryosis and the parasexual cycle. Among the techniques used in studies about vegetative compatibility groups, the obtainment of nit mutants is apparent. This paper is aimed at obtaining heterokaryons between different isolates of C. lindemuthianum, grouping them in VCGs and evaluating their genetic variability by using the nit mutants system. Nit mutants were obtained from 20 single spore isolates. The mutants were phenotypically classified and paired for complementation and formation of heterokaryons so as to group them in VCGs. Seventeen mutants from the different phenotypic-rates were recovered: nit1, nit2, nit3 and nitM. At the same time, 10 mutants were selected for pairing and division of the anastomosis groups. Nine heterokaryons were obtained and the isolates were divided into 9 vegetative compatibility groups. In the combinations for the formation of anastomosis, 31 compatible combinations and 24 incompatible combinations were observed. It was concluded that the methodology used to select nit mutants in C. lindemuthianum made it possible to determine the vegetative compatibility groups and that such a technique was adequate to prove genetic variability.
Key words: Colletotrichum lindemuthianum; anthracnose; heterokaryosis; nit mutants; VCG.
INTRODUCTION
Colletotrichum lindemuthianum (Sacc. & Magn.) Scrib. (= Glomerella cingulata (Stonem Spaulde & Schrenck) f. sp. phaseoli) is the causal agent of bean anthracnose and stands out as one of the most common pathogens that provokes expressive damage to these plants. The utilization of bean varieties resistant to anthracnose is one form of preventing the disease, but it is made difficult due to the great variability of fungi. However, the genetic mechanisms responsible for the great variability presented by this phytopathogen are not completely known.
The formation of heterokaryons between different strains is an important and common component of the life cycle of many filamentous fungi. Lineages that are capable of fusing (anastomosis) and forming stable and functional heterokaryons are known as sexually or vegetatively compatible, the former being frequently described as members of the same group of vegetative compatibility or vegetative compatibility group - VCG (13). Vegetative incompatibility or heterokaryon incompatibility is a genetic mechanism that restricts the heterokaryosis between individuals who differ in one or more het or vic loci (10, 19).
The knowledge of vegetative compatibility groups between different lineages is of particular interest in asexual fungi such as Colletotrichum spp. since the VCGs subdivide the population into groups that can exchange genetic information via heterokaryosis and the parasexual cycle (4). Studies on VCGs involve obtaining mutants that are incapable of utilizing nitrate as the only source of nitrogen (nit mutants) and are resistant to chlorate, which is a toxic analog of nitrate.
Complementation among different nit mutants is indicated by the development of dense mycelia in the zone of contact between the two mutant colonies (heterokaryon) so that the two isolates belong to the same vegetative compatibility group (13). Thus, mutants can once again present wild-type growth since complementation with another mutant isolate of the same VCG has occurred, and usage of nitrate is now possible (6).
Heterokaryosis can be a probable cause of the increased genetic variation in this species. The present study brought this fact into focus to obtain heterokaryons between different isolates of C. lindemuthianum, group them in VCGs and evaluate their genetic variability by using nit mutants system.
MATERIAL E METHODS
Origin of fungal isolates
The isolates (Table 1) of C. lindemuthianum employed were kindly donated by Elaine A. Souza, PhD (Biology Department of the Universidade Federal de Lavras - MG, Brazil) and twenty single spore isolates were used.
Recovering of nit mutants
After cultures were grown in solid M3 culture medium (11), a mycelial fragment was transferred from the isolates to the center of the Petri dishes containing minimal medium (4) with 1.5% potassium chlorate (MMC), using the technique described by Brooker et al. (4). The Petri dishes were incubated and examined after 14 to 21 days for sector verification. Fragments from these cultures were transferred to Petri dishes containing a minimal medium + NaNO3 (MM) (4). The isolates that presented poor growth colonies in this medium and little mycelial production were considered to be nit mutants, while those presenting dense aerial mycelium growth, or wild-type, were discarded (14).
Phenotypic classification of the nit mutants
For the phenotypic classification of the nit mutants, mycelial fragments from the same Petri dishes containing MM were selected and transferred to the center of dishes containing basal medium (BM) supplemented with sodium nitrite (0,5 g/L), sodium nitrate (2,0 g/L), hypoxanthine (0,5 g/L), ammonium tartrate (1,0 g/L) and uric acid (0,2 g/L) (4). Each nit mutant was transferred to three dishes (100 x 15 mm) with each of the aforementioned media; totaling 15 dishes for each isolate. These dishes were maintained in a BOD incubator between 22/25ºC for a period of 14 to 21 days. Two evaluations were carried out: the former on the 14th and the latter on the 21st day.
The phenotypic classification was done according to the mycelial growth of the mutants in media supplemented with different sources of nitrogen: BM + sodium nitrate (MM), BM + sodium nitrite (NM), BM + hypoxanthine (HM), BM + ammonium tartrate (AM) and BM + uric acid (UAM). Media supplemented with sodium nitrate and ammonium tartrate were used as negative and positive controls, respectively.
Anastomoses formation
A number of 10 mutants were selected in the test for formation of anastomoses and a pairwise confrontation of all the isolates was performed using the methodology described by Rodriguez-Guerra et al. (17). The hyphae were stained with aceto-orcein staining solution (2%). By means of an optical microscope the origin of the anastomosed cells were traced, confirming the anastomose between the isolates - all the confrontations were done thrice. The anastomoses were classified as positive when there was fusion of the hyphae between the confronted isolates and both isolates were considered to belong to the same anastomosis group. In order to confirm whether the isolates formed stable heterokaryons - and therefore belonged to the same vegetative compatibility group -, dishes containing the same pairs were left in culture for 10 consecutive days and later analyzed for the appearance of cell death and hyphal parasitism.
Heterokaryon formation and VCG classification
The heterokaryons were formed when the colonies of different nit mutants were confronted in Petri dishes (100 x 15 mm) at a 1 cm distance in nitrate medium (MM). The dishes were stored in a greenhouse, in the dark, at a temperature between 22/25 ºC. After 14 to 21 days they were analyzed on a weekly basis to verify the existence of heterokaryons.
In order to carry out the confrontations, combinations were done whereby each dish contained five different isolates, and a mycelial fragment was taken from a determined isolate from the center of the dish and in the other four isolates from the margins, i.e., each mutant selected from a determined isolate was paired with all the other mutants from the other isolates so as to determine the number of complementary groups to which the distinct nit mutants belonged. A total of 40 combinations were done and each one was repeated thrice.
Statistical analysis
The results obtained from the formation of anastomoses were analyzed in terms of similarity and the groups were obtained using the program NTSYS-pc 2.1. A matrix was built using the compatibility data. For the estimation of similarity, the Russel and Rao coefficient was employed, and the dendrogram was obtained through analyses of similar groups using the arithmetic mean method (UPGMA).
RESULTS AND DISCUSSION
Recovering of nit mutants
After growth of the colonies, a sector of each colony was isolated according to Leslie (12). However, Brooker et al. (4), isolated 40 sectors per isolate when working with 7 different species of the genus Colletotrichum.
The sectors were observed after 21 to 30 days of growth in culture. Leslie (12) mentions that the frequency of spontaneous sectors in culture varies within the species and within isolates of the same species. The frequency of the colonies is also influenced by environmental factors, like temperature, level of nutrients and selective pressure. For C. lindemuthianum Barcelos (2) obtained sectors after 21 days with a greater frequency after 5 or 6 weeks. For Fusarium solani and F. oxysporum, Oliveira and Costa (16) and Zamani et al. (20), respectively, obtained sectors resistant to potassium chlorate after 7 to 10 days.
From the total of twenty C. lindemuthianum isolates cultivated in MMC, 18 nit mutants were recovered after the transfer to MM. Nit mutants are resistant to the toxic effect attributed to chlorate, but lose their ability to catabolize nitrate in this process (12). They present poor growth colonies with little mycelial production. Two isolates presented growth dense aerial mycelium, wild-type. This isolates were characterized as non-mutants and were discarded later.
Only one of the eighteen nit mutants reversed its phenotype to the wild phenotype a few days after the transfer to the MM; it was also discarded. This reversion rate was also found by Fávaro et al. (8) in studies with C. sublineolum. The authors suggest that the instability of the isolates can be a result of the activity of transposable elements.
Phenotypic classification of nit mutants
After the growth of the nit mutants in basal medium supplemented with different sources of nitrogen took place the phenotyping (Figure 1). Leslie (13) explains the use of different nitrogen sources referring to the fact that nit mutants have altered metabolic pathways.
The nit mutants that presented poor growth colonies in MM and wild-type growth in NM, AM, HM and UAM were classified as nit 1. The colonies with growth dense aerial mycelium only in AM and poor growth in other media were denominated nit 2. Nit 3 mutants are the colonies presenting dense aerial mycelium growth in AM, HM and UAM, and should grow poorly on this MM and NM. Nit M mutants are those colonies with poor growth colonies in MM and HM, and growth dense aerial mycelium in NM, AM and UAM (4).The phenotypic classification of nit mutants is described in Table 1. All the mutants present a peculiar growth in the negative and positive controls: media supplemented with sodium nitrate and ammonium tartrate, respectively.
Anastomoses formation
In the first analyses carried out after five to seven days of incubation, 32 compatible pairs were obtained due to formation of anastomoses between the hyphae, and 23 incompatible ones. The most frequent type among the anastomoses was in H form (Figure 2). The anastomoses are important as they permit communication between the hyphal compartments and in the homeostasis, apart from being the first step in the formation of compatible vegetative groups.
After 10 consecutive days, the pairs were analyzed once again. In this analysis, a pair with cell death between the hyphae of the isolates R65LV29* and R73LV99 was found. Therefore, the compatible pairs reduced to 31 whereas the incompatible ones increased to 24. An indication of cell death can be observed through the intense hyphae staining. In studies with Trichoderma pseudokoningii, Barcellos (3) was able to notice the presence of hyphal segments containing granulous material in some regions of the heterokaryons. Whole nuclei and the intensely stained segments - which could indicate a cell death process derived from a certain level of incompatibility - were not found.
Glass et al. (10) explains that this heterokaryon incompatibility reaction is governed by the vic or het loci, which restrict the formation of heterokaryons between two genetically distinct individuals. If these individuals differ in one or more het loci, the cells involved in the fusion are compartmentalized and undergo a lytic process that leads to cell death.
None of the isolates presented 100% compatibility with other isolates. In terms of proportion, the isolate LV102 presented the greatest level of compatible reactions which was 80%, while the isolates R65LV94, R81LV91 and R73LV99 presented the lower compatibility indices corresponding to 30%. The least compatibility index presented by the isolate R73LV99 is in accordance with the study done by Castro-Prado et al. (5), who tested the isolates R2047, R65, R23, R89 and R73 in terms of formation of anastomoses and heterokaryons. The authors observed that none of the mentioned isolates formed anastomoses with the isolate R73.
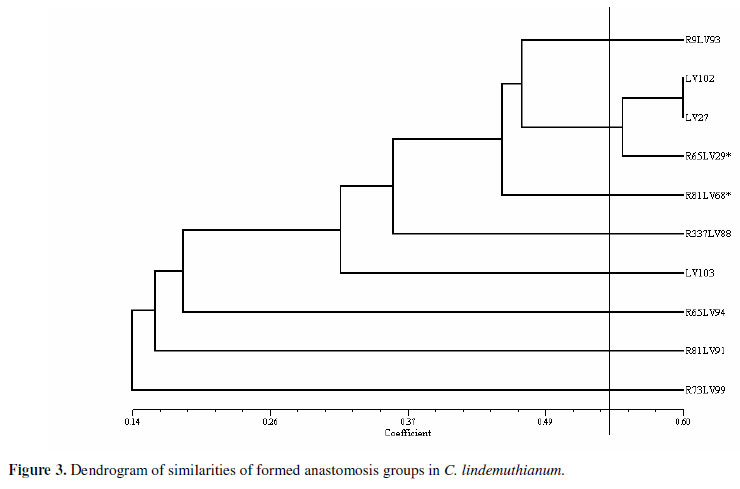
The Russel and Rao coefficient was used to estimate the similarities so as to obtain the groups for anastomoses. The demarcation that represents the maximum similarity value (sgm) (above which the isolates present a 1% probability to be considered similar by the t test) was 0.53. A dendrogram was made through the analysis of the groups of similarities by using the mean arithmetic method (UPGMA). It allowed visualizing the groups of formed anastomoses (Figure 3). Eight different groups were formed, but only the isolates LV102, LV27 and R65LV29* belong to the same group.
Heterokaryon formation and VCG classification
Two evaluations of the pair combinations for heterokaryon formation were made. The first one was carried out on the 14th day and the second on the 21st day. The complementation was more visible in the second evaluation. Complementation can occur between different nit mutants. If two different isolates are incapable of forming a heterokaryon when paired, they are vegetatively incompatible. Two isolates that form a heterokaryon are vegetatively compatible (20).
The formed heterokaryons were characterized by the dense growth of the aerial mycelia in the line of intersection (Figure 4). Nine heterokaryons were obtained from the 40 combinations of five isolates. These heterokaryons occurred between the nit MX nit 2, nit MX nit 1, nit MX nit 3 and nit 1 X nit 3 mutants. Proportionally, the heterokaryons were more frequently formed between nit MX nit 1 mutants with three complementations. This result is also in accordance with the other species of the genus Colletotrichum. In their studies with C. kahawae, Gichuru et al. (9) obtained the best results in the complementation tests between nit 1X nit M and nit 2X nit 3 mutants.
As affirmed by Abang et al. (1), the results of the complementation tests were influenced by the phenotype of the mutants in use. The stronger complementations in the present study occurred between nit 1 and nit 3 mutants with nit M mutant. Várzea et al. (18) explain this point when they assert that the more frequent complementations and those that develop more vigorous heterokaryons occur between the nit M and nit 1 pairs. Between nit 1 and nit 3 mutants, the complementations were weaker, as further observed by Zamani et al. (20) in VCGs studies with F. oxysporum. These authors verified that the complementation was frequently evident for two to three weeks in pairing between nit 1 and nit 3 mutants; Correl et al. (6) added that such complementations could altogether not occur. As expected, no complementation between nit mutants of the same phenotypic class occurred since they bear the same mutation and would not complement one another.
The seventeen isolates used in these pairings formed 9 vegetative compatibility groups (VCG) (Table 1). Vegetatively compatible isolates are probably much more similar than the vegetatively incompatible ones (20). The capability or incapability of two isolates whether to form or not a heterokaryon is genetically controlled by the vegetative incompatibility loci called vic or het. Isolates with the same loci are capable of forming a stable heterokaryon, but if they differ in any of these loci, they are incapable of forming a heterokaryon.
Only one of the eight groups of anastomoses formed in this study is composed of more than one isolate (LV102, LV27, R65LV29*). This fact indicates that these isolates bear the same genes responsible for the pre-fusion and fusion, hsi and vic or het genes, respectively. It was anticipated that these would also constitute the same VCG. However, it was not observed in the present study. Instead, it was verified that these isolates did not belong to the same VCG, thus they should bear the same genes that control the pre-fusion events and allelic differences between the genes that control the vegetative compatibility, in such a manner that they are capable of forming anastomosis, but not heterokaryons.
A relationship between VCG and the locality of the isolates was observed. A VCG composed of more than one isolate has four mutant components of the same locality. Elmer and Stephens (7) and Monteiro (15) also observed this relationship in their studies with F. oxysporum asparagi and Fusarium, respectively. The first authors used 23 isolates originating from Ingham Country, MI and 13 of these were grouped in the same VCG, whereas in the second case only 3 VCGs from a total amount of 16 did not present geographical correlation.
The present study provides evidence of heterokaryosis formation under laboratory conditions, demonstrated that this mechanism could possibly raise genetic variability in this species, and that the technique of determining VCG groups was adequate to evaluate the genetic variability in C.lindemuthianum using nitrate non-utilizing mutants.
ACKNOWLEDGEMENTS
The authors would like to thank the Research Foundation of the State of Minas Gerais (Fundação de Amparo à Pesquisa do Estado de Minas Gerais - FAPEMIG ) for the fellowship granted to the first author.
Submitted: May 23, 2010; Approved: June 21, 2010.
- 1. Abang, M.M.; Hoffmann, P.; Winter, S.; Green, K.R.; Wolf, G.A. (2004). Vegetative Compatibility Among Isolates of Colletotrichum gloeosporides from Yam (Dioscorea spp.) in Nigeria. J.Phytopathology. 152: 21-27.
- 2. Barcelos, Q.L. (2007). Análise de grupos de compatibilidade vegetativa e marcadores RAPD em Colletotrichum lindemuthianum. Lavras, Brasil, 50p. (M.Sc. Dissertation. Genética e Melhoramento de Plantas. UFLA).
- 3. Barcellos, F.G. (2002). Caracterização genética e citológica da recombinação somática em Trichoderma pseudokoningii São Paulo, Brasil, 110p. (D. Sc. Thesis. Genética e Melhoramento de Plantas. USP).
- 4. Brooker, N.L.; Leslie, J.F.; Dickman, M.B. (1991). Nitrate Non-Utilizing Mutants of Colletotrichum and their Use in Studies of Vegetative Compatibility and Genetic Relatedness. Phytopathology 81: 672-676.
- 5. Castro-Prado, M.A.A.; Querol, C.B.; Sant'anna, J.R.; Miyamoto, C.T.; Franco, C.C.S.; Mangolin, C.A.; Machado, M.F.P.S. (2007). Vegetative compatibility and parasexual segregation in Colletotrichum lindemuthianum, a fungal pathogen of the common bean. Genetics and Molecular Research. 6 (3): 634-642.
- 6. Correl, J.C.; Klittich, C.J.R.; Leslie, J.F. (1987). Nitrate nonutilizing mutants of Fusarium oxysporum and their use in vegetative compatibility tests. Phytopathology 77: 1640-1646.
- 7. Elmer, W.H.; Stephens, C.T. (1989). Classification of Fusarium oxysporum f. sp. aspargii into vegetatively compatible groups. Phytopathology 79: 88-93.
- 8. Fávaro, L.C.L.; Araújo, W.L.; Souza-Paccola, E.A.; Azevedo, J.L.; Paccola-Meirelles, L.D. (2007). Colletotrichum sublineolum genetic instability assessed by mutants resistant to chlorate. Mycological Research 111: 93-105.
- 9. Gichuru, E.K.; Várzea, V.M.P.; Rodrigues JR, C.J.; Masaba, D.M. (2000). Vegetative Compatibility Grouping of Colletotrichum kahawae in Kenia. J.Phytopathology 148: 233-237.
- 10. Glass, N.L.; Jacobson, D.J.; Shiu, P.K.T. (2000). The Genetics of Hyphal Fusion and Vegetative Incompatibility in Filamentous Ascomycete Fungi. Annu. Rev. Genetic 34: 165-186.
- 11. Junqueira, N.T.V.; Chaves, G.M.; Zambolim, L.; Romeiro, R. da S.; Gasparoto, L. (1984). Isolamento, cultivo e esporulação de Mycrocyclus ulei, agente etiológico do mal das folhas da seringueira. Revista Ceres 31: 322-331.
- 12. Leslie, J.F. (1990). Genetic exchange within sexual and asexual populations of the genus Fusarium. In: Ploetz, R.C. (eds). Fusarium wilt of banana, St. Paul, Minnesota, USA, p. 37-48.
- 13. Leslie, J.F. (1993). Fungal vegetative compatibility. Annu. Rev. Phytopathol, 31: 127 -150.
- 14. Leslie, J.F.; Summerell, B.A.; Bullock, S. (2006). The Fusarium laboratory manual. Blackwell Publishers, USA.
- 15. Monteiro, J.H.A. (2006). Grupos de compatibilidade vegetativa em isolados de Fusarium associados à malformação da mangueira no Brasil. Lavras, Brasil, 47p. (M.Sc. Dissertation. Fitopatologia. UFLA).
- 16. Oliveira, V.C. de; Costa, J.L. (2003). Compatibilidade vegetativa de nitmutantes de Fusarium solani patogênicos e não-patogênicos ao feijoeiro e à soja. Fitopatologia Brasileira 28: 89-92.
- 17. Rodriguez-Guerra, R.; Ramírez-Rueda, M.T.; Martínez De La Vega, O.; Simpson, J. (2003). Variation in genotype, pathotype and anastomosis groups of Colletotrichum lindemuthianum isolates from México. Plant Pathology 52: 228-235.
- 18. Varzea, V.M.P.; Rodrigues, J.R.; Lewis, B.G. (2002). Distinguishing characteristics and vegetative compatibility of Colletotrichum kahawae in comparison with other related species from coffee. Plant Pathology 51: 202-207.
- 19. Xiang, Q.; Glass, N.L. (2004). The control of mating type heterokaryon incompatibility by vib-1, a locus involved in het-c hetekaryon incompatibility in Neurospora crassa. Fungal Genetics and Biology 41: 1063-1076.
- 20. Zamani, M.R.; Motallebi, M.; Rostamian, A. (2004). Characterization of Iranian Isolates of Fusarium oxysporum on the Basis of RAPD Analysis, Virulence and Vegetative Compatibility. J. Phytopathology 152: 449-453.
Publication Dates
-
Publication in this collection
10 Jan 2011 -
Date of issue
Mar 2011
History
-
Received
23 May 2010 -
Accepted
21 June 2010