Abstract
A novel thermoacidophilic iron and sulfur-oxidizing archaeon, strain YN25, was isolated from an in situ enriched acid hot spring sample collected in Yunnan, China. Cells were irregular cocci, about 0.9-1.02 µm×1.0-1.31 µm in the medium containing elemental sulfur and 1.5-2.22 µm×1.8-2.54 µm in ferrous sulfate medium. The ranges of growth and pH were 50-85 (optimum 65) and pH 1.0-6.0 (optimum 1.5-2.5). The acidophile was able to grow heterotrophically on several organic substrates, including various monosaccharides, alcohols and amino acids, though the growth on single substrate required yeast extract as growth factor. Growth occurred under aerobic conditions or via anaerobic respiration using elemental sulfur as terminal electron acceptor. Results of morphology, physiology, fatty acid analysis and analysis based on 16S rRNA gene sequence indicated that the strain YN25 should be grouped in the species Acidianus manzaensis. Bioleaching experiments indicated that this strain had excellent leaching capacity, with a copper yielding ratio up to 79.16% in 24 d. The type strain YN25 was deposited in China Center for Type Culture Collection (=CCTCCZNDX0050).
isolation; identification; Acidianus manzaensis; bioleaching
ENVIRONMENTAL MICROBIOLOGY
A novel acidophilic, thermophilic iron and sulfur-oxidizing archaeon isolated from a hot spring of tengchong, yunnan, China
Jiannan DingI, II, * * Corresponding Author. Mailing address: Key Laboratory of Biometallurgy of Ministry of Education, School of Minerals Processing and Bioengineering, Central South University, 932 Lushang Road, Changsha, Hunan 410083, China.; E-mail: jiannanding@yahoo.cn / jlxia@mail.csu.edu.cn ; Ruiyong ZhangI; Yizun YuII; Decai JinI; Changli LiangI; Yang YiI; Wei ZhuI; Jinlan XiaI, * * Corresponding Author. Mailing address: Key Laboratory of Biometallurgy of Ministry of Education, School of Minerals Processing and Bioengineering, Central South University, 932 Lushang Road, Changsha, Hunan 410083, China.; E-mail: jiannanding@yahoo.cn / jlxia@mail.csu.edu.cn
IKey Laboratory of Biometallurgy of Ministry of Education of China, School of Minerals Processing and Bioengineering, Central South University, Changsha, Hunan 410083, China
IIBiological Resources Institute, Jiangxi Academy of Sciences, Nanchang, Jiangxi 330029, China
ABSTRACT
A novel thermoacidophilic iron and sulfur-oxidizing archaeon, strain YN25, was isolated from an in situ enriched acid hot spring sample collected in Yunnan, China. Cells were irregular cocci, about 0.9-1.02 µm×1.0-1.31 µm in the medium containing elemental sulfur and 1.5-2.22 µm×1.8-2.54 µm in ferrous sulfate medium. The ranges of growth and pH were 50-85 (optimum 65) and pH 1.0-6.0 (optimum 1.5-2.5). The acidophile was able to grow heterotrophically on several organic substrates, including various monosaccharides, alcohols and amino acids, though the growth on single substrate required yeast extract as growth factor. Growth occurred under aerobic conditions or via anaerobic respiration using elemental sulfur as terminal electron acceptor. Results of morphology, physiology, fatty acid analysis and analysis based on 16S rRNA gene sequence indicated that the strain YN25 should be grouped in the species Acidianus manzaensis. Bioleaching experiments indicated that this strain had excellent leaching capacity, with a copper yielding ratio up to 79.16% in 24 d. The type strain YN25 was deposited in China Center for Type Culture Collection (=CCTCCZNDX0050).
Key words: isolation, identification, Acidianus manzaensis, bioleaching
INTRODUCTION
It is well known that bioleaching, recovering metals from low-grade sulfidic ores by microorganisms, has developed into a successful commercial biotechnology. In many cases, it offers environmental and technical advantages over other available technologies (13, 21). This process is driven by consortia of chemolithotrophic iron and sulfur-oxidizing bacteria and archaea that are ubiquitous at sites of mineral oxidation. The most studied acidophilic metal sulfide oxidizing microorganisms belong to the mesophilic and moderately thermophilic bacteria. Thermophilic and acidophilic sulfur/iron oxidizers dominating at a temperature range of 40-60 are the typically rod-shaped, Sulfobacillus species (15, 22), although Jiannan Ding and Ruiyong Zhang are both first author other species, such as Leptospirillum ferriphilum Acidimicrobium ferrooxidans, Acidithiobacillus caldus, and Hydrogenobacter acidophilus, are also commonly present (15, 19, 33, 34). Thermophilic sulfur/iron oxidizers, which thrive above 60, usually belong to Archaea domain, genera Sulfolobus, Acidianus, or Metallosphaera (15, 22, 32).
Biological regeneration of FeP3+P from FeP2+P is the key to chemical attack of metal sulfides. However, biooxidation of reduced inorganic sulfur compounds (RISCs) is also important to prevent the accumulation of passivating sulfur particulate on metal surfaces that can limit metal mobilization rates. Thermoacidophiles grow at temperatures (60-85!) where mesoacidophilic or morderate biocatalysts (or contaminants from nonsterile substrates) are not able to exist, and where passivation from RISCs is nearly eliminated, lead to more effective leaching rates (23).
Sulfur-oxidizing thermoacidophilic archaea Acidianus brierleyi was ûrstly isolated from an acidic thermal spring in Yellowstone National Park, Wyoming, USA (2, 26), whose excellent ferrous oxidation ability revealed a potential in industrial application. The archaeon demonstrated a remarkable capacity in chalcopyrite bioleaching (the most refractory primary copper sulûde), which possessed a significant advantage over mestrophic leaching process.
This study focused on screening of thermophilic microorganisms that oxidize iron and/or sulfur compounds from hot acidic water samples. Strain YN25, a novel thermophilic iron and sulfur-oxidizing archaeon, from a hot spring (> 80) located on Tengchong, Yunnan, China, was identified as Acidianus manzaensis and its iron and sulfur oxidation and chalcopyrite leaching ability has been further investigated.
MATERIALS AND METHODS
Microbial enrichment and Isolation
The water samples from a hot spring located inTengchong were in situ enriched by medium A with an initial pH 1.8 at 70. The medium A contains (g·LP-1P): (NHB4B)B2BSOB4B (1.5), MgSOB4B·7HB2BO (0.25), KHB2BPOB4B (0.25), Yeast Extract (0.1), FeSOB4B·7HB2BO (5-20), pH 1.8. Two thermophilic microbial cultures (one of which is Strain YN25) were obtained. by serial dilution method.
Growth conditions
Strain YN25 was cultivated in basic salts (medium A with FeSOB4B as energy source and modified 9K with SP0P, NaB2BSB2BOB3 Bor KB2BOB6BSB4 as energy source), trace elements (7) and 0.02% YE. Modified 9K contains (g·LP-1P): (NHB4B)B2BSOB4 B(3.0), KHB2BPOB4B (0.5), KCl (0.1), MgSOB4B·7HB2BO (0.5) and Ca(NOB3B)B2B (0.01). Trace elements (mg·LP-1P) compose: FeClB3B·6HB2BO (11.0), CuSOB4B·5HB2BO (0.5), NaB2BSOB4 (50), HB3BBOB3 (2.0), MnSOB4B (2.0), NaB2BMoOB4B·2HB2BO (0.8), CoClB2B·6HB2BO (0.6), ZnSOB4B·7HB2BO (0.9) and NaB2BSeOB4B (0.1). The pH of medium 9K-S0 and medium A-FeSO4 were adjusted with 1 M sulfuric acid to 2.5 and 2.0, respectively. Cultures were incubated in rotary shakers at the indicated temperatures.
The optimum temperature and pH were detected by temperature- and pH- controlled experiments in 250 mL shaking flasks with 100 mL medium. The purified strain was inoculated into liquid medium with a final cell density of 1×10P6P cells mLP-1P. The temperature optimization was conducted under, initial pH 1.5 in medium A-FeSOB4B and pH 2.5 in 9K-SP0P, while the initial pH test was carried out at a constant temperature at 65°C, which was given in the former one. The cell densities of cultures in the flasks were monitored by a cell counting chamber directly.
To determine the optimal concentration of YE, a series of tests with the following different concentration of YE (w/v): 0.005%, 0.01%, 0.015%, 0.02%, 0.025%, 0.03%, 0.035%, 0.04%, 0.045%, 0.05%, 0.06%, 0.07%, 0.08%, 0.085%, 0.09%, 0.1%, were implemented.
Microscopy
The morphology and motility of the strain YN25 were observed with optical microscope (Olympus CX-31). Surface micro-structural features of the cells in the logarithm growth phase were examined with scanning electron microscope (SEM, JEOL JSM-6360 LV), following fixation, dehydration and critical point drying of samples (12).
16S rRNA gene profiling
Chromosomal DNA was purified in accordance with the manufacturer's instructions by DNA extraction kit (Tiangen Biotech), and was used as a template in PCR. The 16S rRNA genes of YN25 were amplified by polymerase chain reaction (PCR) using the forward primer 21F: 5′-TTCCGGTTGATCCTG-3′ and reverse primer Ar958R: 5′-TCCGGCGTTGAGTCC-3′ (4). The PCR program was 94° C for 3 min, followed by 30 cycles of 94° C for 30 s, 52° C for 30 s, and 72° C for 60 s, and finally 72° C for 10 min. PCR products of the expected size (approximately 1.5 kb) were excised from 1.0% agarose gels and purified with the purification columns (Promega), following the manufacturer's recommendations. The PCR products were ligated to the pGEM-T vector and transformed into Escherichia coli DH5α. The white colonies on the Luria-Bertani (LB) plates containing ampicillin (100 µg·mLP-1), X-gal (20 mg·mLP-1) and IPTG (16 mg·mLP-1) (31) were selected and sent to Sunbiotech Co.Ltd for sequencing. The nucleotide sequence of the inserts was determined by cycle sequencing with an ABI PRISM Big Dye terminator cycle sequencing Ready Reaction kit (Applied Biosystems, Foster City, California) and run in an ABI PRISM 3700 DNA analyzer (Applied Biosystems).
The phylogenetic tree showing the relationship of YN25 to other Acidianus species was elaborated with related sequences obtained from public databases (http://www.ncbi.nlm.nih.gov/). The sequences were aligned with YN25 sequence using Clustal X 1.80, which was used to make a distance matrix, followed by a neighbor-joining tree. Bootstrap analysis was carried out on 1000 replicates input data sets, and Phylogenetic trees were generated by Treeview software MEGA 3.1.
Nutrition growth
In Chemoorganotrophic growth tests, the following organic compounds were tested without FeSOB4B or SP0P, which were: Yeast extract (YE), Casamino acids, Tryptone, D-glucose, Maltose, Sucrose, Lactose, Starch, Sorbitol, Galactose, Fructose, Xylose, Mannitol, Serine, Cysteine, Tyrosine, Arginine, Tryptophan, Phenylalanine, Histidine, Methionine, Leucine, Threonine, Glycine, Valine, Glutamine. The concentrations of YE, Casamino acids and Tryptone were 0.2, 0.1 and 0.2 g·LP-1P, respectively. The other test substrates concentration was 1.0 g·LP-1P.The isolate was grown in 9K-base medium with the culture maintained at pH 2.5, 65°C. Growth was estimated by detecting the total protein concentration after incubation for 72 h (8).
Medium A-FeSOB4B and 9K-base medium were used for chemomixotrophic growth. 30 g·LP-1P FeSOB4B·7HB2BO for medium A, 10 g·LP-1P SP0P, NaB2BSB2BOB3 Bor 2 g·LP-1P KB2BOB6BSB4B for 9K-base medium, were added as energy. The only organic source was 0.2 g·LP-1P YE.
Chemoautotrophic growth tests were performed in the same way without YE.
Anaerobic growth
Anaerobic growth was tested in anaerobic tank with of 21 kPa COB2B and 79 kPa NB2ÿcontaining basic salts, trace elements and YE (0.2 g·LP-1) with elemental sulfur (10 g·LP-1P) or ferric iron (10 mmol·LP-1) B. The initial inoculation concentration was adjusted to 1×10P6P cells·mLP-1 with cells washed in basal salts medium thoroughly. To assure the anaerobic circumstance, the media were sprinkled with NB2B for 5 min and oxygen indicators were placed in the anaerobic tank. To monitor the sulfate reduction reaction lead acetate papers was used in the anaerobic tank. Concentration of total iron was analyzed by atomic absorption spectrometry, and that of ferric iron was calculated by the concentrations of total iron and ferrous iron (34). Cell growth was determined by the protein concentration started from the stationary phase.
Sensitivity to antibiotics and tolerance to heavy metals
The antibiotic sensitivity to antibiotics and tolerance to several heavy metals of strain YN25 were monitored in sulfur-containing media (as described above) different concentrations of ampicillin, chloramphenicol, kanamycin, rifampin, tetracycline and gentamicin (35) and varying concentrations of CuSOB4B·5HB2BO, NiSOB4B·6HB2BO, ZnSOB4B·7HB2BO, AlB2B(SOB4B)B3B·18HB 2BO, CoSOB4B·7HB2BO and 3CdSOB4B·8HB2BO (33). Growth was estimated by detecting the total protein concentration in 72 h of incubation (8).
Whole-cell lipid fatty acid analysis
Cells of strain YN25 were harvested by centrifugation, and then transferred directly to a screw-cap vial. The fatty acid methyl esters (FAMEs) were obtained by methylation, saponification and extraction, as described previously (18). The separation of FAMEs was performed by a gas chromatography (model DNAI 6500-HR) equipped with a flame ionization detector (FID) and a 25-m fusedsilica column cross-linked with an SE-30 liquid phase (SGE; Ringwood, Victoria, Australia), in a carrier gas (HB2B) flow rate of 1.2 mL·minP-1P, an injector temperature of 250, a detector temperature of 300, and an oven temperature program of 120 to 280 at 4 minP-1P. The FAMEs were identified by comparison with the retention times of standards and their proportions were calculated on a Chromatography Data System.
Bioleaching of chalcopyrite
Chalcopyrite used in this experiment was provided by Institute of Mineral Processing Engineering, School of Resources Processing and Bioengineering, Central South University, China. The chalcopyrite contains 30.6% of Cu, 22.64% of Fe, 29.6% of S, 1.72% of Zn and 8.95% of Pb.
Bioleaching tests were carried out in 250 mL flasks with 100 mL 9K-base medium, in a mineral concentration of 3% (w/v). The initial concentration of YN25 was 2-4×106 cells·mLP-1, and all the experiments were carried out in triclicates. Abiotic controls were also implemented at same conditions by replacing the bacterial inoculum with an equal volume of medium. Aliquots of solution were sampled, and the concentration of CuP2+ Pwas determined by atomic absorption spectrometry (Hatichi Z-8000). The evaporated water in the medium was supplemented with sterilized deionized water.
RESULTS AND DISSCUSSION
Morphological characterization
The cell morphology was similar to other members in the order of Sulfolobales, with slightly aspherical shape (Figure 1). The cell shape and size were significantly varied in different energy resource. The dimension of strain YN25 was about 0.9-1.02 µm×1.0-1.31 µm in the medium containing elemental sulfur and 1.5-2.22 µm×1.8-2.54 µm in the medium containing ferrous sulfate. The cell surface was crimpy but smooth in medium 9K-SP0 while its counterpart in medium A-FeSOB4B was round and, rough.
Growth conditions
The strain YN25 was able to grow on several organic substrates, including various monosaccharides, alcohols and amino acids (Table 1), while no growth was observed when it was cultivated in inorganic substrates alone. However, in the presence of YE, peptone or other organics, the strain YN25 could grew well in inorganic substrates. These results suggested that strain YN25 is a facultative autotrophic microorganism, in absence of the evidence from carbon isotope experiment.
Bacterial growth was observed at temperatures between 50 and 85, with an optimum status at 65 (Figure 2a). No growth occurred when temperature was below 30 or above 90 (data not shown). Growth occurred at initial pH of 1.0 to 5.0, with an optimum pH of 1.5 in the medium A-FeSOB4, and, at initial pH of 1.0 to 6.0 with an optimum pH of 2.5 in 9K-SP0P medium (Figure 2b).
The growth of strain YN25 at different YE concentrations was determined. The optimum concentration of YE for YN25 in medium A-FeSO4 and 9K-S0 was 0.02% and 0.45%, respectively. Growth was strongly inhibited by the presence of YE in concentration above 0.10% (Figure 2c), which may act as growth factors in the process.
It can be seen from Figure 2d, FeP2+P was completed oxidized after 35 h when FeSOB4B·7HB2BO concentration varied between 10-40 g·LP-1, andand largest cell density was gained with a ferrous iron concentration of 35 g·LP-1P. Cell density and FeP2+P oxidizing activity significantly decreased when FeSOB4B·7HB2BO concentration was above 45 g·LP-1, while cells reproduction was reduced in a FeSOB4B·7HB2BO concentration below 25 g·LP-1P. Generally, compared with other iron-oxidizing bacteria, strain YN25 had higher ferrous iron oxidizing activity (2, 8, 11, 33, 34).
The sulfur oxidizing activity of strain YN25 was determined in the optimum conditions (initial pH 2.5, 10 g·L-1 S0, 0.2 g·L-1 YE), where the concentration of SOB4PB2- P reached 25 g·L-1 after 70 hours' cultivation.
Anaerobic Metabolisms
The biggest differents in characteristics of genus Acidianus were the two chemolithotrophic cultivations. Under aerobic conditions, Acidianus grew aerobically with OB2B as the final electron acceptor. At extremely anaerobic conditions, Acidianus grew anaerobically, forming HB2BS or FeP2+P, in which HB2B or organic components served as electron donor, and SP0 Por FeP3+P served as the final electron acceptor (16, 20, 26). Electron donor and acceptor were important basis for this archaea system classification.
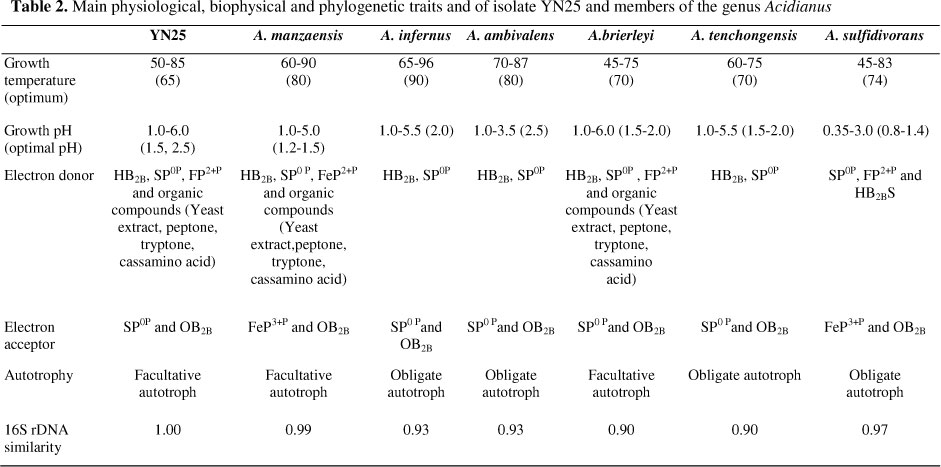
YN25 has high elemental sulfur reducing capacity at extremely anaerobic conditions, generating a significant amount of HB2BS. In the anaerobic experiment, the lead acetate filter paper suspended in the experimental bottle began to change color after 40 h cultivation, and became completely black after 60 h. Meanwhile, we found strain YN25 can not reduce the ferric iron. At anaerobic conditions, YN25 shared the same electron donor and acceptor, HB2B and SP0P, respectively, with A. infernus, A. ambivalens, A. brierleyi and A. tenchongensis. A. manzaensis, and had the same electron acceptor of ferric iron as A. sulfidivorans, but different electron donor, which were HB2B and HB2BS, respectively (Table 2). Based on the characterization of anaerobic metabolism, strain YN25 had the same electron donor with A. manzaensis ATCC BAA 1057 but clear differences in electron acceptor (32).
Antibiotic sensitivity and heavy metal tolerance
Strain YN25 was sensitive to all antibiotics except gentamicin in a limited concentration (Table 3). It presented little sensitivity to gentamicin (100 mg·L-1) and high sensitivity to rifampin (5 mg·L-1), tetracycline (20 mg·L-1), Kanamycin (50 mg·L-1), (Chloramphenicol 50 mg·L-1) and Ampicillin (50 mg·L-1).
Generally, the strain YN25 growth was inhibited with the increase of heavy metal concentrations. CoP2+P, NiP2+P and CuP2+P were deleterious for the strain growth However, YN25 TexhibitTed tolerance in different levels to a range of heavy metals and showed strongest resistance to AlB2B(SOB4B)B3B·18HB 2BO (Table 4). Growth was significantly enhacnced with 20-40 mM AlB2B(SOB4B)B3B·18HB 2BO and the biomass increased by 61% in the presence of 40 mM AlB2B(SOB4B)B3B·18HB 2BO. In addition, low concentration of ZnSOB4B·7HB2BO (<4 mM) and 3CdSOB4B·8HB2BO (<0.4 mM) could slightly promote the growth of YN25. The heavy metals tolerance granted strain YN25 a special advantage in bioleaching.
Fatty acid analysis
Thermophilic archaea possess unique fatty acid composition which enable it to grow in high temperature (17), which is also an important method for phylogenetic study such as archaea identification and classification of (3).
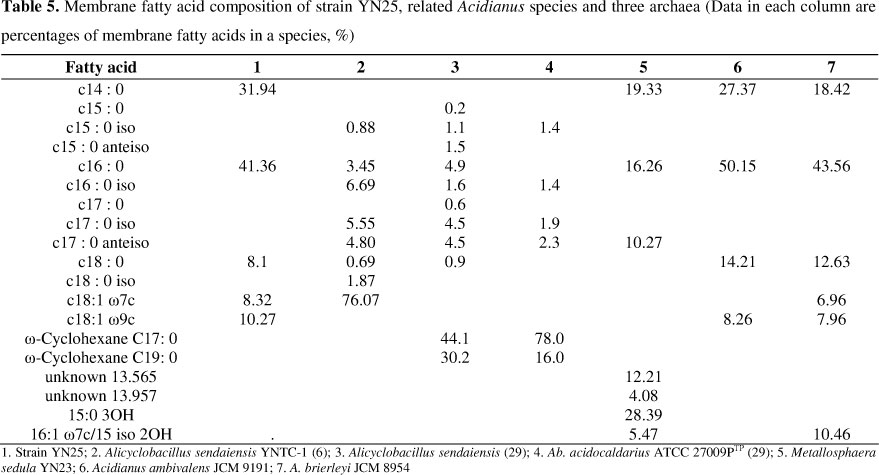
Whole-cell fatty acid compositions were shown in Table 5. There were five groups of fatty acids found in strain YN25 by gas chromatograph. The predominant one was C16:0, accounting for 41.36% of the total fatty acids. The other four groups included C14:0, C18:0, C18:1Ω7c and C18:1Ω9c, which account for 31.94%, 8.1%, 8.32% and 10.27% respectively. Strain YN25 had similar composition of membrane fatty acid with strain A. ambivalens JCM 9191 and A. brierleyi JCM 8954. However, compared with YN25, A. ambivalens JCM 9191 does not contain C18:1Ω7c monounsaturated fatty acid while A. brierleyi JCM 8954 possesses a unique fatty acid C16:1 Ω7c/15 iso 2OH.
According to the characteristics of Alicyclobacillus, Ω-alicyclic acids are the predominant membrane fatty acids in this genus (14, 29). Membrane lipid composition of Metallosphaera sedula YN23 (accession number of GenBank: EF142855) was more complex than YN25. As shown in Table 5, strain YN25 was substantially different from Alicyclobacillus sendaiensis, Sulfolobus metallicus and Metallosphaera sedula. According to the analysis of fatty acid, strain YN25 was closed related to the genus Acidianus.
Molecular phylogenetic analysis
The phylogenetic position of the new isolate was evaluated by 16S rRNA gene sequence information, in which a total of 1416 nucleotides were sequenced (accession number of GenBank EF522787). The nearest phylogenetic relative to the isolate was Acidianus manzaensis ATCC BAA 1057 with exactly 99% of similarity. A neighbour-joining phylogenetic tree was constructed based on the distance matrix data of the isolate and several reference archaea. As described in Figure 3, the species of Acidianus chosen were dividied into six groups based on the 16S rRNA gene sequences, and strain YN25 clustered with Acidianus manzaensis ATCC BAA 1057.
Based on morphological, biochemical, physiological characterizatics and the molecular biology analysis, strain YN25 could be classified into Acidianus manzaensis.
Bioleaching experiments
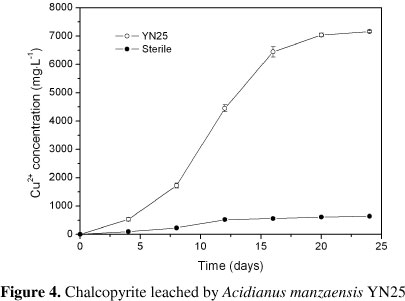
The bioleaching results by Acidianus manzaensis YN25 were as shown in Figure 4. In the leaching process, where more than 70% copper was extracted during the first 16 days, the copper extraction ratio continuously increases with a final concentration of 7.16 g·L-1 after 24 days. Comparatively, almost no soluble copper was detected in sterile controls. This was the first report in bioleaching by the an Acidianus species.
As a thermophilic microbe, Acidianus manzaensis YN25 showed strong capacity of chalcopyrite bioleaching compared with moderate thermophiles (Acidithiobacillus caldus, Sulfobacillus thermosulfidooxidans, Sulfobacillus acidophilus, Leptospirillum ferriphilum and Ferroplasma thermophilum) (11, 30, 33, 34, 35) or mesophiles (Leptospirillum ferrooxidans, Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans, Acidithiobacillus albertensis) (5, 9, 27, 31). The sulfur particles formed during the bioleaching were readily to accumulate on the surface of solid phase and form passivation layer,which can significantly inhibit the contaction of solid and liquid phases thus hinder the leaching process (10, 24, 25, 28). Acidianus manzaensis YN25 showed strong capacity of oxidizing FeP2+P and SP0P in our previous study. It can be inferred that, Acidianus manzaensis YN25 thriving at temperatures of 65!, which is lethal to moderate thermophiles and mesophiles,could effectively limit passivation, consequently, leading to a better leaching kinetics.
Pure culture of Acidianus manzaensis was first discovered in 2007 by Yoshida et al, named Acidianus manzaensis strain NA-1T (strain NA-1T = NBRC 100595 = ATCC BAA 1057).
The strain isolated from a hot fumarole in Manza, Japan is a facultative autotrophic archaeon in both anaerobic and aerobic conditions (32). Acidianus manzaensis YN25 was the second strain of this species. Strain YN25 have new features in cell morphology, growth conditions and anaerobic metabolisms compared with Acidianus manzaensis ATCC BAA 1057, which could be relevant to its excellent leaching ability and prosperous prospective in industrial use.
ACKNOWLEDGEMENTS
This work was supported by the China National Basic Research Program (2010CB630902) and China National Nature Science Foundation (50974140).
Submitted: January 11, 2010; Returned to authors for corrections: February 08, 2010; Approved: January 13, 2011.
- 1. Acevedo, F.; Gentina, J.C.; Valencia, P. (2004). Optimization of pulp density and particle size in the biooxidation of a pyritic gold concentrate by Sulfolobus metallicus World J. Microbiol. Biotechnol. 20(8), 865-869.
- 2. Brierley, C.L.; Brierley, J.A. (1973). A chemoautotrophic and thermophilic microorganism isolated from an acid hot spring. Can. J. Microbiol. 19, 183-188.
- 3. Claus, H.; Akca, E.; Debaerdemaeker, T.; Evrard, C.; Declercq, J.P.; Konig, H. (2002). Primary structure of selected archaeal mesophilic and extremely thermophilic outer surface layer proteins. Syst. appl. microbiol. 25(1), 3-12.
- 4. Cytryn, E.; Minz, D; Oremland, R.S.; Cohen, Y. (2000). Distribution and diversity of archaea corresponding to the limnological cycle of a Hypersaline Stratified Lake (Solar Lake, Sinai, Egypt). Appl. Environ. Microbiol. 66(8), 3269-3276.
- 5. Dempers, C.J.N.; Breed, A.W.; Hansford, G.S. (2003). The kinetics of ferrous-iron oxidation by Acidithiobacillus ferrooxidans and Leptospirillum ferrooxidans: effect of cell maintenance. Biochem. Eng. J. 16(3), 337-346.
- 6. Ding, J.; He, H.; Zhang, C.; Yu, Y.; Qiu, G. (2008). Isolation and characterization of YNTC-1, a novel Alicyclobacillus sendaiensis strain. J. Cent. South Univ. T. 15(4), 508-514.
- 7. Dopson, M.; Lindstrom, E.B. (1999). Potential role of Thiobacillus caldus in arsenopyrite bioleaching. Appl. Environ. Microbiol. 65(1), 36-40.
- 8. Dopson, M.; Baker-Austin, C.; Hind, A.; Bowman, J.P,; Bond, P.L. (2004). Characterization of Ferroplasma isolates and Ferroplasma acidarmanus sp. nov., extreme acidophiles from acid mine drainage and industrial bioleaching environments. Appl. Environ. Microbiol. 70(4), 2079-2088.
- 9. Falco, L.; Pogliani, C.; Curutchet, G.; Donati, E. (2003). A comparison of bioleaching of covellite using pure cultures of Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans or a mixed culture of Leptospirillum ferrooxidans and Acidithiobacillus thiooxidans Hydrometallurgy 71(1-2), 31-36.
- 10. Fowler, T.A.; Crundwell, F.K. (1999). Leaching of zinc sulfide by Thiobacillus ferrooxidans: bacterial oxidation of the sulfur product layer increases the rate of zinc sulfide dissolution at high concentrations of ferrous ions. Appl. Environ. Microbiol. 65(12), 5285-5292.
- 11. Gao, J.; Zhang, C.G.; Wu, X.L.; Wang, H.H.; QIU, G.Z. (2007). Isolation and identification of a strain of Leptospirillum ferriphilum from an extreme acid mine drainage site. Ann. Microbiol. 57(2), 171-176.
- 12. Geng, A.; Soh, A.E.W.; Lim, C.J.; Loke, L.C.T. (2006). Isolation and characterization of a phenol-degrading bacterium from an industrial activated sludge. Appl. Microbiolm. Biotechnol. 71(5), 728-735.
- 13. Gomez, E.; Ballester, A.; Blazquez, M.L.; Gonzalez, F. (1999). Silver-catalysed bioleaching of a chalcopyrite concentrate with mixed cultures of moderately thermophilic microorganisms. Hydrometallurgy 51(1), 37-46.
- 14. Goto, K.; Matsubara, H.; Mochida, K.; Matsumura, T.; Hara, Y.; Niwa, M.; Yamasato, K. (2002). Alicyclobacillus herbarius sp. nov., a novel bacterium containing omega-cycloheptane fatty acids, isolated from herbal tea. Int. J. Syst. Evol. Microbiol. 52(1), 109-113.
- 15. Hallberg, K.B.; Johnson, D.B. (2001). Biodiversity of acidophilic prokaryotes. Adv. appl. Microbiol. 49, 37-84.
- 16. He, Z.G.; Zhong, H.; Li, Y. (2004). Acidianus tengchongensis sp. nov., a new species of acidothermophilic archaeon isolated from an acidothermal spring. Curr. Microbiol. 48(2), 159-163.
- 17. Ivanov, I.T. (2002). Derivative conductometry profile of thermal alterations in cellular membranes-a possible relationship between membrane alterations, cellular proliferation capacity and maximum temperature of growth. J. Therm. Biol. 27(2), 137-149.
- 18. Jantzen, E.; Sonesson, A.; Tangen, T.; Eng, J. (1993). Hydroxy-fatty acid profiles of Legionella species: diagnostic usefulness assessed by principal component analysis. J. Clin. Microbiol. 31(6), 1413-1419.
- 19. Johnson, D.B.; Hallberg, K.B. (2003). The microbiology of acidic mine waters. Research in Microbiology 154(7), 466-473.
- 20. Plumb, J.J.; Haddad, C.M.; Gibson, J.A.E.; Franzmann, P.D. (2007). Acidianus sulfidivorans sp. nov., an extremely acidophilic, thermophilic archaeon isolated from a solfatara on Lihir Island, Papua New Guinea, and emendation of the genus description. Int. J. Syst. Evol. Microbiol. 57(7), 1418-1423.
- 21. Poulin, R.; Lawrence, R.W. (1996). Economic and environmental niches of biohydrometallurgy. Miner. Eng. 9(8), 799-810.
- 22. Rawlings, D.E. (2002). Heavy metal mining using microbes. Annu. Rev. Microbiol. 56, 65-91.
- 23. Rawlings, D.E.; Johnson, D.B. (2007). The microbiology of biomining: development and optimization of mineral-oxidizing microbial consortia. Microbiology 153(2), 315-324.
- 24. Rohwerder, T.; Gehrke, T.; Kinzler, K.; Sand, W. (2003). Bioleaching review part A. Appl. Microbiol. Biotechnol. 63(3), 239-248.
- 25. Schippers, A.; Sand, W. (1999). Bacterial leaching of metal sulfides proceeds by two indirect mechanisms via thiosulfate or via polysulfides and sulfur. Appl. Environ. Microbiol. 65(1), 319-321.
- 26. Segerer, A.; Neuner, A.; Kristjansson, J.K.; Stetter, K.O. (1986). Acidianus infernus gen. nov., sp. nov., and Acidianus brierleyi comb. nov.: facultatively aerobic, extremely acidophilic thermophilic sulfur-metabolizing archaebacteria. Int. J. Syst. Evol. Microbiol. 36(4), 559-564.
- 27. Shi, S.; Fang, Z. (2004). Bioleaching of marmatite flotation concentrate by Acidithiobacillus ferrooxidans Hydrometallurgy 75(1-4), 1-10.
- 28. Shi, S.; Fang, Z.; Ni, J. (2005). Bioleaching of marmatite flotation concentrate with a moderately thermoacidophilic iron-oxidizing bacterial strain. Miner. Eng. 18(11), 1127-1129.
- 29. Tsuruoka, N.; Isono, Y.; Shida, O.; Hemmi, H.; Nakayama, T.; Nishino, T. (2003). Alicyclobacillus sendaiensis sp. nov., a novel acidophilic, slightly thermophilic species isolated from soil in Sendai, Japan. Int. J. Syst. Evol. Microbiol. 53(4), 1081-1084.
- 30. Watling, H.R.; Perrot, F.A.; Shiers, D.W. (2008). Comparison of selected characteristics of Sulfobacillus species and review of their occurrence in acidic and bioleaching environments. Hydrometallurgy 93(1-2), 57-65.
- 31. Xia, J.L.; Peng, A.A.; He, H.; Yang, Y.; Liu, X.D.; Qiu, G.Z. (2007). A new strain Acidithiobacillus albertensis BY-05 for bioleaching of metal sulfides ores. T. Nonferr. Metal. Soc. 17(1), 168-175.
- 32. Yoshida, N.; Nakasato, M.; Ohmura, N.; Ando, A.; Saiki, H.; Ishii, M.; Igarashi, Y. (2006). Acidianus manzaensis sp. nov., a novel thermoacidophilic Archaeon growing autotrophically by the oxidation of HB2B with the reduction of FeP3+P Curr. Microbiol. 53(5), 406-411.
- 33. Zhang, R.; Xia, J.; Peng, J.; Zhang, Q.; Zhang, C.; Nie, Z.; Qiu, G. (2010). A new strain Leptospirillum ferriphilum YTW315 for bioleaching of metal sulfides ores. Trans. Nonferrous Met. Soc. China 20(1), 135-141.
- 34. Zhou, H.; Zhang, R.; Hu, P.; Zeng, W.; Xie, Y.; Wu, C.; Qiu, G. (2008). Isolation and characterization of Ferroplasma thermophilum sp. nov., a novel extremely acidophilic, moderately thermophilic archaeon and its role in bioleaching of chalcopyrite. J. Appl. Microbiol. 105(2), 591-601.
- 35. Zhou, Q.G.; Bo, F.; Hong Bo, Z.; Xi, L.; Jian, G.; Fei Fei, L.; Xin Hua, C. (2007). Isolation of a strain of Acidithiobacillus caldus and its role in bioleaching of chalcopyrite. World J. Microbiol. Biotechnol. 23(9), 1217-1225.
Publication Dates
-
Publication in this collection
06 June 2011 -
Date of issue
June 2011
History
-
Accepted
13 Jan 2011 -
Reviewed
08 Feb 2010 -
Received
11 Jan 2010