Abstract
Kluyveromyces aestuarii was found in sediments from 7 of 8 mangroves in Rio de Janeiro; and absent only at one site with heavy plastic bag pollution. Its presence suggests influence in other habitats from a mangrove and its absence in a mangrove suggests some non-fecal pollution or other habitat alteration.
Kluyveromyces aestuarii; mangroves; environmental indicator
ENVIRONMENTAL MICROBIOLOGY
Kluyveromyces aestuarii, a potential environmental quality indicator yeast for mangroves in the State of Rio de Janeiro, Brazil
Araujo, F.V.I,* * Corresponding Author. Mailing address: Faculdade de Formação de Professores da Universidade do Estado do Rio de Janeiro; Rua Dr. Francisco Portela 1470, Patronato, São Gonçalo, RJ, CEP 24435-005.; E-mail: fvaraujo@uol.com.br ; Hagler, A. NII
IFaculdade de Formação de Professores, Universidade do Estado do Rio de Janeiro, São Gonçalo, RJ, Brasil
IIInstituto de Microbiologia Professor Paulo de Góes, Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ, Brasil
ABSTRACT
Kluyveromyces aestuarii was found in sediments from 7 of 8 mangroves in Rio de Janeiro; and absent only at one site with heavy plastic bag pollution. Its presence suggests influence in other habitats from a mangrove and its absence in a mangrove suggests some non-fecal pollution or other habitat alteration.
Key words:Kluyveromyces aestuarii; mangroves; environmental indicator
Estuaries have more yeast cells per volume and also greater richness of species than adjacent aquatic areas (10, 28). Some yeasts have been isolated mostly from these environments, especially in mangrove forests of tropical and subtropical estuaries and temperate salt marshes. Scheffersomyces spartinae has been reported under the name Pichia spartinae from southeastern U. S. salt marshes and associated with the marsh grass Spartina alterniflora, (2, 9, 20, 21), and various new species including some in the Saccharomyces clade have been described recently from mangroves confirming the yeast species richness of this biome (4, 5, 13, 19). Yeasts of the genus Kluyveromyces have been found frequently in these environments. Kluyveromyces lactis (syn. K. drosophilarum) has been found in large populations in estuarine sediments in Louisiania and salt marches in Georgia U.S.A. (9, 20). Kluyveromyces lactis strains that were found in estuarine salt marsh environments by Meyers and colleagues (20) produced more pulcherriminic acid than the type strain of the same species, suggesting that this species plays an ecological role in iron cycle in estuaries. Kluyveromyces aestuarii also produces this pigment in its ascospores and can often be noted on isolation plates by the pink to deep ochre color frequently formed in colonies (18). Yeasts in mangrove ecosystems may play an important role in the detrital food web in that they may be a food source for some marine invertebrates and zooplankton species (23). Kluyveromyces aestuarii has been considered a marine yeast (18), and although frequently isolated from mangroves, this species is rare in other habitats including temperate estuaries and salt marches (10, 16, 20). Am-In et al. (4) described Kluyveromyces siamensis as sibling species isolated from water in a mangrove forest in Ranong Province, Thailand. However, this species is only marginally separated from K. aestuarii based on the D1/D2 region partial rDNA sequence, and there may reason to doubt its validity as an ecological species because of the similarity in habitat and phenotypic characteristics. Although the genus Kluyveromyces is more known to be associated with forest substrates and insect vectors like Drosophila, Kluyveromyces nonfermentans has been described based on isolates from deep sea habitats (22). So the genus is clearly present also in marine habitats.
First described as Saccharomyces aestuarii based on a culture isolated by J. W. Fell from sediments of Biscayne Bay in Miami Florida (11, 12), K. aestuarii was then isolated 14 times from marine, but not fresh water sites, in the Florida Everglades by Ahearn and colleagues (1, 14). Araujo et al. (6) and Soares et al. (26) observed very high levels of yeast species diversity in a mangrove in Rio de Janeiro, but K. aestuarii was the prevalent species. A study of the yeast community in the Coroa Grande mangrove in Rio de Janeiro found K. aestuarii as the prevalent species associated especially with detritivores animals, but not herbivores and carnivores (6), and with sediments directly under mangrove vegetation (26). There is no published data on yeast community structure of mangroves from other continents, but some isolates of K. aestuarii from Australia and Asia have been deposited in culture collections showing that distribution is not limited to the Americas (http://www.cbs.knaw.nl/yeast/BioloMICS.aspx & http://www.nbrc.nite.go.jp/NBRC2/NBRCCatalogueDetailServlet?ID=NBRC&CAT=00010952). Our objective in this work was to see if K. aestuarii was consistently present in different mangrove areas in Rio de Janeiro to support that it has potential as an indicator of environmental quality of mangroves.
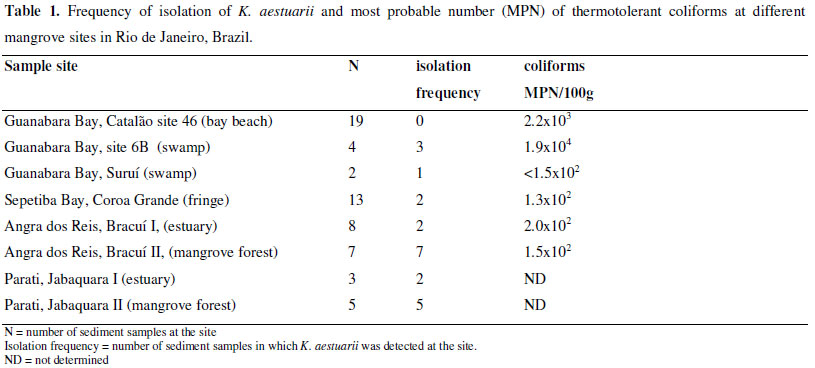
Sediments were collected from 8 different mangrove areas in Rio de Janeiro, Brazil. Suruí is located in a swamp near Magé (22º 41' 43.9" S; 43º 6' 49.2" W). Sites 6B in a swamp (22º 50' 16" S; 43º 13' 50" W) and 46-Catalão on a beach (22º 50' 29" S; 43º 13' 35" W) of the Ilha do Fundão in Guanabara Bay. The Coroa Grande site was located in a mangrove fringe along a mudflat in Sepetiba Bay (22º 54' 32.6" S; 43º 52' 37.2" W). The other sites were located in Angra do Reis Bay with 2 at Ilha do Jorge near Bracuí; Bracuí I in an estuary (22º 57' 6.6" S; 44º 24' 26.5" W) and Bracuí II in a mangrove forest (22º 56' 50.0" S; 44º 24' 24.6" W); and 2 near Parati; Jabaquara I in an estuary (23º 12' 43,4" S; 44º 43' 1.8" W) and Jabaquara II in a mangrove fringe (23º 12' 1.7" S; 44º 43' 18.8" W). All the stations had typical mangrove vegetation (Rhizophora mangle, Avicenia schaueriana and Laguncularia racemosa) and fine dark intertidal sediments. The number of samples taken at each site is indicated in Table 1. Sampling on Ilha do Fundão was done at site 6B in March of 2008 and 2009 and at site 46 in March from 2001 through 2004, 2008 and 2009. The other sites were sampled between October 1995 and June of 1997.
Samples of 100 to 500 cm3 of the upper 5 cm layer of sediment were collected aseptically with a sterilize spatula, placed in sterile bottles and returned to the laboratory on wet ice to be processed (25, 26). Sub-samples of 5g from each well mixed sediment sample were diluted in 2 volumes of sterile 0.85% NaCl and agitated vigorously for 30 seconds. Aliquots of 0.1mL and serial dilutions of these suspensions were inoculated on YM agar (yeast extract 0.3%, malt extract 0.3%, peptone 0.5%, glucose 1%, agar 2% and chloramphenicol 100 mg%). An Aliquot of 1mL from each sample was also inoculated into tubes of a selective broth containing sodium propionate 0.1%, lactose 0.5%, ammonium sulphate 0.5%, yeast extract 0.1%, NaH2PO4 0.2%, chloramphenicol 200 mg/L, pH 4.5 with HCl. After one week of incubation, aliquots of enrichment tubes showing probable yeast growth were streaked out on YM agar and selected colonies from these and spread plates were streaked on YM agar to obtain pure cultures. These isolates were characterized according to methods by Yarrow (29), and identified using the taxonomic keys of Kurtzman and Fell (17). Amplification of ITS region of rDNA of some strains was carried out in order to confirm similarity of the cultures (27), and some of the K. aestuarii from Coroa Grande, were deposited in the CBS collection and their identifications confirmed by rDNA partial sequences (CBS 7767, 7768, 7775 & 7776 http://www.cbs.knaw.nl/yeast/BioloMICS.aspx consulted on Oct. 30 2010). Presumptive thermotolerant coliforms counts were determined by the APHA standard 3 tube MPN test (3).
From a total of 61 samples from mangrove sediments, 22 yielded 1 or more cultures of K. aestuarii (Table 1). Some of our K. aestuarii isolates were different from the standard description in the assimilation of sorbose and xylose, but this was also observed previously by Soares et al. (26) and these abilities were noted in the original description of the species so they may have been lost during storage in culture collections (6, 11, 26). Kluyveromyces aestuarii was isolated from all mangrove sites studied except for the Catalão Point site 46 on Ilha do Fundão in spite of being the most extensive sampling with 19 taken from it between 2001 and 2009. The highest frequencies of this species were in Bracuí II and Jabaquara II, sites within the mangrove vegetation areas, but without extensive anthropic impact. The lowest frequency was from the Bracuí I estuary site where there was a construction project in progress that was perturbing the sediments and Ilha do Fundão site 46 which has heavy pollution from drift material. The site with the highest coliform level had a high frequency of K.aestuarii isolations so fecal pollution is apparently not an important factor in distribution of the species. In previous studies of dark mangrove mud from sites not directly associated with mangrove vegetation this species was not isolated (24, 26); but from the same region in Sepetiba Bay it had been found previously to be the prevalent yeast species from samples taken within sediments under mangrove vegetation of Coroa Grande (6, 26). However, K. aestuarii was found in only 2 of 5 sediment samples and none in the mangrove mussels sampled in 1995 and 1996 from Coroa Grande (7, 8). A flood caused by torrential rains in Coroa Grande could have influenced the mangrove biota including the yeast community associated with sediments during 1996.
The role of this species in mangrove ecosystems is still not clear, but it appears to be associated with degrading leaves within the mangrove vegetation areas. This yeast was found in detritus feeding invertebrates and sediments within the mangrove vegetation area, but was not common in invertebrates feeding on fresh leaves or other animals, or in the sediments of a tide flat bordering on, but not covered by mangrove vegetation (6, 26). There were changes in the dominance of this species over time at Coroa Grande, but it was generally more frequent in the more marine influenced sites and this in agreement with it being considered a marine organism. Other factors appear to be involved in its distribution. Although K. aestuarii was present in all the other mangrove sites, especially those with stronger marine influence, it was conspicuously absent in 19 samples taken over a decade from the Ilha do Fundão, Catalão beach site 46. This site is directly exposed to seawater in Guanabara Bay and always had a thick layer of plastic bags deposited in the top few cm layer of mud that had to be removed to get sediment samples from the intertidal zone. Coliform levels at site 46 were typically close to the limits of bathing quality standards and salinity close to that of seawater, although the location is within an otherwise heavily polluted urban area. Impact from the plastic bag pollution at Catalão beach site 46 and from alterations of the sediments due to flooding at Coroa Grande and construction at Bracuí I may have inhibited populations of this otherwise typical mangrove yeast. Santos and colleagues (25) have suggested the use of microeukaryotes detected by noncultivation methods as environmental quality indicators, and Hagler (15) has indicated previously that K. aestuarii could serve as an indicator for mangroves. Our data suggests the species could be used in environmental quality monitoring of mangroves for factors other than recent fecal contamination. This could be made more practical by using more selective cultivation methods as suggested by Hagler (15), or non-cultivation PCR based methods employing a specific primer as suggested recently by Santos et al. (25). Clearly the presence of K. aestuarii in other habitats suggests influence from a mangrove habitat, and the absence in the site heavily impacted by plastic bags suggests that this kind of pollution does affect the microbial communities of mangrove sediments.
ACKNOWLEDGMENTS
We thank Renato F. de Oliveira and Antônio Carlos Cardoso for their dedicated field and laboratory assistance. This work was supported by CNPq and Pronex.
Submitted: November 27, 2010; Returned to authors for corrections: January 18, 2011; Approved: May 16, 2011.
- 1. Ahearn, D.G.; Roth Jr, F.J.; Meyers, S.P. (1968). Ecology and Characterization of Yeasts from Aquatic Regions of South Florida. Marine Biology I: 291-308.
- 2. Ahearn, D.G.; Yarrow, D.; Meyers, S.P. (1970). Pichia spartinae sp n. from Louisiana Marshland Habitats. Antonie van Leeuwenhoek, 36(4):503-508.
- 3. A.P.H.A. (1976). Standard Methods for the Examination of Water and Wastewater, 14th Edition. American Public Health Association, Washington D.C., P 922-926.
- 4. Am-In, S.; Yongmanitchai, W.; Limtong, S. (2008). Kluyveromyces siamensis sp. nov., an ascomycetous yeast isolated from water in a mangrove forest in Ranong Province, Thailand. FEMS Yeast Res, 8:823-828.
- 5. Am-In, S.; Limtong, S.; Yongmanitchai, W.; Sasitorn, J. (2010). Candida andamanensis sp. nov., Candida laemsonensis sp. nov., and Candida ranongensis sp. nov., three anamorphic yeast species isolated from estuarine waters in a mangrove forest in Ranong Province, Thailand, Int J Syst Evol Microbiol, In Press.
- 6. Araujo, F.V.; Soares, C.A.G.; Hagler, A.N.; Mendonça-Hagler, L.C. (1995). Ascomycetous yeast communities of marine invertebrates in a southeast Brazilian mangrove ecosystem. Antonie van Leeuwenhoek 68:91-99.
- 7. Araujo, F.V.; Miguel, M.A.L.; Hagler, A.N. (2006). Leveduras, incluindo patógenos oportunistas associadas ao mexilhão Mytella guyanensis no manguezal de Coroa Grande, Baía de Sepetiba, RJ. Higiene Alimentar, v. 20 (142), p. 92-96.
- 8. Araujo, F.V.; Hagler, A.N. (2010). Leveduras associadas a sedimentos em diferentes manguezais fluminenses. Gerenciamento Costeiro Integrado Ano 4, n. 6 (In Press)
- 9. Buchan, A.; Newel, S.Y.; Moreta, J.I.L.; Moran, M.A. (2002). Analysis of Internal Transcribed Spacer (ITS) Regions of rRNA Genes in Fungal Communities in a Southeastern U.S. Salt Marsh. Microbial Ecology 43,329-340.
- 10. Coelho, M.A.; Almeida, J.M.; Martins, I.M.; da Silva, A.J.; Sampaio, J.P. (2010). The dynamics of the yeast community of the Tagus river estuary: testing the hypothesis of the multiple origins of estuarine yeasts. Antonie Van Leeuwenhoek. 98(3):331-42.
- 11. Fell, J.W. (1961). A. new species of Saccharomyces isolated from a subtropical estuary. Antonie van Leuwenhoeck 27:27-30.
- 12. Fell, J.W.; Ahearn, D.G.; Meyers, S.P.; Roth, E.J.Jr, (1960). Isolation of Yeasts from Biscayne Bay Florida and Adjacent Benthic Areas. Limnol. Oceanogr, 5:366-371.
- 13. Fell, J.W.; Statzell-Tallman, A.; Kurtzman C.P. (2004). Lachancea meyersii sp. nov., an ascosporogenous yeast from mangrove regions in the Bahama Islands. Studies in mycology 50:359-363.
- 14. Fell, J.W.; Statzell-Tallman, A.; Scorzetti, G.; Marcelo, H.; Gutiérrez, M.H. (2010). Five new species of yeasts from fresh water and marine habitats in the Florida Everglades. Antonie van Leeuwenhoek DOI 10.1007/s10482-010-9521-6
- 15. Hagler, A.N. (2006). Yeasts as indicators of environmental quality. In Rosa, C.A.; Gabor, P. editors Biodiversity and Ecophysiology of Yeasts, Springer, PP. 519-536.
- 16. Hagler, A.N.; Ahearn, D.G. (1987). Ecology of Aquatic Yeasts. In Rose, A.H.; Harrison, J.S. eds. The Yeasts, second edition, Vol. 1. Academic press, London. 181-206.
- 17. Kurtzman, C.P.; Fell, J.W. (1998). The Yeasts, A Taxonomic Study 4th edn. Elsevier Science B.V., Amsterdam.
- 18. Lachance, M.A. (1998). Kluyveromyces van der Walt emend. van der Walt. In The Yeasts. A taxonomic Study, 4th ed. (Kurtzman CP and Fell JW, eds), pp.227-247. Elsevier, Amsterdam, the Netherlands.
- 19. Limtong, S.; Imanishi, Y.; Jindamorakot, S.; Ninomiya, S.; Yongmanitchai, W.; Nakase, T. (2008). Torulaspora maleeae sp. nov., a novel ascomycetous yeast species from Japan and Thailand. FEMS Yeast Res 8(2):337-43.
- 20. Meyers, S.P.; Ahearn, D.G.; Miles, P. (1971). Characterization of Yeasts in Baratara Bay. La St. Univ. Coastal Stud. Bull, 6:7-15.
- 21. Meyers, S.P.; Ahearn, D.G.; Alexander, S.K.; Cook, W.L. (1975). Pichia spartinae, a Dominant Yeast of the Spartina salt marsh. Dev. In Indust. Microbiol, 6:261-267.
- 22. Nagahama, T.; Hamamoto, M.; Nakase, T.; Horikoshi, K. (1999). Kluyveromyces nonfermentans sp. nov., a new yeast species isolated from the deep sea. Int J Syst Bacteriol 49:1899-1905.
- 23. Nagahama, T. (2006) Yeast Biodiversity in Freshwater, Marine and Deep-Sea Environment. In; The Yeast Handbook, Biodiversity and Ecophysiology of Yeasts (Rosa Ca & Peter G, eds), pp. 241-262. Springer-Verlag, Berlin, Germany.
- 24. Pagnocca, F.C.; Mendonça-Hagler, L.C.; Hagler, A.N. (1989). Yeasts Associated with the White Shrimp Penaeus schmitti, Sediment and Water of Sepetiba Bay, Rio de Janeiro, Brazil. Yeast, 5S:479-483.
- 25. Santos, H.F.; Cury, J.C.; Carmo, F.L.; Rosado, A.S.; Peixoto, R.S. (2010) 18S rDNA Sequences from Microeukaryotes Reveal Oil Indicators in Mangrove Sediment. PLoS ONE 5(8): e12437. doi:10.1371/journal.pone.0012437
- 26. Soares, C.A.G.; Maury, M.; Pagnocca, F.C.; Araujo, F.V.; Mendonça-Hagler, L.C.; Hagler, A.N. (1997). Ascomycetous yeasts from tropical intertidal dark mud of southeast Brazilian estuaries. J. Gen. Appl. Microbiol, 43,265-272.
- 27. Valente, P.; Gouveia, F.C.; Lemos, G.A.; Pimentel, D.; Van Elsas, D.; Mendonça-Hagler, L.C.; Hagler, A.N. (1996). PCR amplification of the rDNA internal transcribed spacer region for differentiation of Saccharomyces cultures. FEMS Microbiology letters, 137:253-256.
- 28. Van Uden, N. (1967). Occurence and origin of yeasts in estuaries, In: Lauff, G.H. (Ed.) Estuaries. American Association for the Advancement of Science Publication, 83: pp. 306-310.
- 29. Yarrow, D. (1998). Methods for the isolation, maintenance and identification of Yeasts. In The Yeasts. A taxonomic Study, 4th ed (Kurtzman CP and Fell JW, eds), pp.77-100. Elsevier, Amsterdam, the Netherlands.
Publication Dates
-
Publication in this collection
21 Dec 2011 -
Date of issue
Sept 2011
History
-
Reviewed
18 Jan 2011 -
Received
27 Nov 2010 -
Accepted
16 May 2011