Abstract
Lactobacillus reuteri LPB P01-001 was isolated from the gastrointestinal tract of wild swine and was characterised by biochemical testing and sequencing of gene 16S rRNA. A simple and low-cost culture medium based on cane sugar (2.5% p/v) and yeast extract (1% p/v) was used in the production of this probiotic. The fermentative conditions were a) pH control at 6.5 and b) no pH control; both were set at 37°C in a 12 L slightly stirred tank bioreactor. Fermentation parameters such as the specific growth rate, productivity and yield of biomass, lactic and acetic acid levels were determined. L. reuteri LPB P01-001 behaves as an aciduric bacteria because it grows better in a low pH medium without pH control. However, the lactic acid production yield was practically half (9.22 g.L-1) of that obtained under a constant pH of 6.5, which reached 30.5 g.L-1 after 28 hours of fermentation. The acetic acid production was also higher under pH-controlled fermentation, reaching 10.09 g.L-1 after 28 hours of fermentation. These parameters may raise the interest of those committed to the efficient production of a probiotic agent for swine.
Probiotic; Lactobacillus reuteri; Molecular characterisation; Fermentation parameters; Lactic and acetic acids
INDUSTRIAL MICROBIOLOGY
Molecular characterisation and biomass and metabolite production of Lactobacillus reuteri LPB P01-001: a potential probiotic
Elizete de F. R. PancheniakI; Maike T. MazieroI; José A. Rodriguez-LeónII; José L. ParadaII; Michele R. SpierII; Carlos R. SoccolII,* * Corresponding Author. Mailing address: Departamento Engenharia de Bioprocessos e Biotecnologia, Universidade Federal do Paraná, Curitiba, PR, Brasil, Caixa Postal 19011 CEP 81531-990.; E-mail: soccol@ufpr.br
IPrograma de Pós-Graduação em Tecnologia de Alimentos, Universidade Federal do Paraná, Curitiba, PR, Brasil
IIDepartamento Engenharia de Bioprocessos e Biotecnologia, Universidade Federal do Paraná, Curitiba, PR, Brasil
ABSTRACT
Lactobacillus reuteri LPB P01-001 was isolated from the gastrointestinal tract of wild swine and was characterised by biochemical testing and sequencing of gene 16S rRNA. A simple and low-cost culture medium based on cane sugar (2.5% p/v) and yeast extract (1% p/v) was used in the production of this probiotic. The fermentative conditions were a) pH control at 6.5 and b) no pH control; both were set at 37°C in a 12 L slightly stirred tank bioreactor. Fermentation parameters such as the specific growth rate, productivity and yield of biomass, lactic and acetic acid levels were determined. L. reuteri LPB P01-001 behaves as an aciduric bacteria because it grows better in a low pH medium without pH control. However, the lactic acid production yield was practically half (9.22 g.L-1) of that obtained under a constant pH of 6.5, which reached 30.5 g.L-1 after 28 hours of fermentation. The acetic acid production was also higher under pH-controlled fermentation, reaching 10.09 g.L-1 after 28 hours of fermentation. These parameters may raise the interest of those committed to the efficient production of a probiotic agent for swine.
Key words: Probiotic, Lactobacillus reuteri, Molecular characterisation, Fermentation parameters, Lactic and acetic acids.
INTRODUCTION
Lactobacillus reuteri is an obligatorily heterofermentative lactic acid bacteria, a microaerophilic, and is a common inhabitant of the gastrointestinal tract of humans (28, 36) and animals such as pigs (12, 24, 33), turkeys, chickens, and monkeys (24). L. reuteri also belongs to the predominant microflora of fermented cereal products and meat (14, 15, 25).
Some species of L. reuteri produce the enzyme invertase, which is used in converting sugar from sucrose (17, 26). In addition, L. reuteri also produces a large amount of glucan and fructan exopolysaccharides, which are considered prebiotics (22). These prebiotics have been investigated with regards to antitumour activity (52), immunomodulation (55), and cholesterol reduction (50). In recent years, there has been considerable interest in the use of probiotic microorganisms and organic acids as alternatives to antibiotics in feeds to reduce antibiotic residues in the carcass, among other excellent benefits such as diarrhea control and immunostimulation (2, 37, 42, 48, 54, 61). The antimicrobial effect exerted by Lactobacillus arises from its production of many compounds, mainly organic acids, peroxide hydrogen, bacteriocins and reuterin (1, 16, 45, 47, 58, 49).
Lactobacilli strains require a nutritional complex frequently found in media containing fermentable carbohydrates, amino acids, vitamins, nucleic acids precursors, and minerals to produce significant biomass (8, 11, 25), which results from a series of highly coordinated enzyme-catalysed events. Lactic acid bacteria are generally cultivated on MRS medium (11).
One of the most important parameters for cultivating acid lactic bacteria is the pH of the fermentation environment (7, 56). Lactobacilli are microorganisms with an optimal pH around 5.5 6.2, and their growth generally occurs at pH 5.0 or lower; their growth rate is often reduced at a neutral pH or in media that are initially alkaline (25). The limitations of growth and acid production by the end-product are well known. Kashket (27) reported growth inhibition in Lactobacillus by cytoplasm acidification via the produced acid. Additionally, the energy gained by lactate production is no longer available for cell growth, but it is used to some extent for the maintenance of pH homeostasis.
Some of the important aspects for industrial production of probiotics are related to the microorganism itself, the cost of nutrient substrates, and the processes used in their production and recovery are a few important aspects of Lactobacilli in the industrial production of probiotics. In addition, parameters for scale optimisation and amplification are necessary. According to Schmidell (56), different phases of the process must be evaluated, such as the kinetics of growth, and primary or secondary metabolite production, as well as separation, recovery and formulation of the products. The fermentative process requires monitoring parameters of the culture system as a function of the fermentation time (23).
In a spontaneous fermentation process, lactic acid bacteria evolve in non-pH-controlled conditions, but for other applications (e.g., lactic acid or biomass production), it may be necessary to achieve pH control (20). The optimal pH for the growth of various strains of lactic bacteria has been previously determined as has the correlations between pH and lactic acid concentration (18, 38).
L. reuteri LPB P01-001 has been isolated from the gastrointestinal tract of wild swine and fulfils some important requirements for use as a probiotic: it is non-pathogenic, non-toxigenic, bile-resistant, and tolerant to gastric acidity, and it produces antimicrobial compounds with the ability to reduce pathogens, normal inhabitants of the gut, that are host-specific. Furthermore, technological aspects include the ability of L. reuteri LPB 01-001 to withstand lyophilisation, freeze-drying processes and the final formulation of the product (43, 44). However, more criteria presented by FAO/WHO (13) must be determined, which include the following: antioxidant activity, ability to modulate the immune response and adherence in intestinal tissue.
Thus, the present work aimed to develop a low-cost culture medium and to determine convenient growth conditions for the potential probiotic L. reuteri LPB P01-001 strain, to perform its molecular identification, and to evaluate fermentation parameters such as biomass yield and production of lactic and acetic acids, which are also considered to be inhibitory substances for pathogens.
MATERIALS AND METHODS
Isolation and biochemical characterisation of L. reuteri LPB P01-001
Lactobacillus strains were obtained from the gastrointestinal tract of swine by plating on MRS agar (Merck) with 5% bile (40), and the colonies were submitted to Gram staining and catalase testing. Their biochemical phenotypic properties were studied by means of sugar fermentation and other biochemical reactions using the API 50 CH gallery system (Biomerieux) for Lactobacilli identification (5, 35). The strain was characterised as an acid and bacteriocin producer (43, 44), according to growth inhibition tests.
Antimicrobial activity
To study its antimicrobial activity, the isolated L. reuteri LPB P01-001 was grown in MRS broth (Merck) and centrifuged. The supernatant, after being sterilised by filtration, was tested against two pathogenic strains: Staphylococcus aureus coagulase positive (ATCC 14458) obtained from CEPPA, Brazil, and a swine haemolytic Escherichia coli LME21, from Enrietti's Lab, Brazil. These indicator strains were grown in Tryptone Soy Broth at 37ºC for 24 h and were used for testing the supernatant in Muller Hinton (Difco) broth and on agar plates.
Fermented MRS broth was centrifuged to remove bacterial cells (6000 rpm for 30 min), and the resulting supernatant was concentrated to 10% of the original volume under vacuum at 45ºC. The pH of the material was adjusted to 5.5, and the sample was filtered through a sterile 0.22 µm membrane (44).
Inhibition of indicator pathogenic strains in liquid media
Sterile supernatant was added in the same amount to double-concentration Mueller-Hinton broth and then inoculated with the above-mentioned testing strains (OD 0.040). The absorbance at 620 nm was periodically recorded to monitor the growth inhibition effect (44).
Molecular identification of the L. reuteri LPB P01-001 strain
Confirmatory molecular tests were carried out according to the following protocol: total genomic DNA was isolated following Young and Blakesley's method (63), and the oligonucleotide primers p27f (31) and p1401r (21) were used for PCR. The reaction was carried out in a BioRad (Thermal Cycler) with 50 µL of DNA (50-100 ng), 0.2 mmol L-1 dNTP mixture, 1.5 mmol L-1 MgCl2, 0.4 µmol L-1 of each primer and 2 U of Taq DNA polymerase (Invitrogen). Amplification was conducted using an initial denaturation step at 95ºC for 2 min, followed by 30 cycles of 1 min at 94ºC, 1 min at 55ºC, and 3 min at 72ºC, and a final step of 5 min at 72ºC.
Fragments of amplified 16S rRNA were purified using a column (GFX PCR DNA and Gel Band Purification Kit, Amersham Biosciences), and the DNA was eluted in 30 µL of sterile ultrapure water. The samples were submitted to a sequencing process in a mega BACE 1000 apparatus (Amersham Biosciences). The primers used were p10f, p1100r (30), p765f (60), and p782r (9).
Partial sequences of 16S rRNA were compared with the 16S rRNA of related microorganisms included in the RDP (Ribosomal Database Project, Wisconsin, USA; 10; 34) and Genbank (41). Matrices of evolution distances were calculated according to Kimura's model (29), and a phylogenetic tree was constructed by following the Neighbor-Joining's method (53) using the RDP software.
Inoculum preparation and bioreactor fermentation
The preparation of the inoculum began with its reactivation from MRS agar. The strain was transferred to MRS broth and kept at 37°C for 48 hours. After its reactivation, the inoculum was transferred at a proportion of 10% (v/v) in relation to the total volume of the culture medium. After preliminary studies in flasks (44), the composition of the chosen E3 medium was 1% p/v yeast extract and 2.5% v/v total cane sugar, due to its low cost as compared to commercial MRS medium (unpublished data). The dry weight of biomass obtained using the E3 medium was similar to the biomass achieved in commercial MRS medium, approximately 1.3 g.L-1, but E3 medium is 33 times cheaper than commercial medium MRS. Bench fermentation experiments were conducted under the same temperature and inoculation conditions; the fermentation period was 28 hours.
Fermentation experiments were carried out in a 12 L stirred tank bioreactor (New Brunswik) under the following conditions: a) one at a constant pH of 6.5 and another without pH control, starting at pH 4.65 in the first case; to maintain a constant pH, 3N sodium hydroxide was added automatically; b) slight agitation at 80 rpm was used in both cases, to prevent cells from settling at the bottom of the bioreactor and to incorporate the fewest number of air bubbles, considering the microaerophilic requirements of the strain; c) the temperature was kept at 37°C. The experiments were done in triplicates and statistically analysed.
Evaluation of kinetic parameters
Fermented samples were collected to determine the following parameters: pH, sugar consumption, biomass, and organic acid levels.
Determination of reducing sugar
The reducing sugar levels in the fermented samples were determined by following Somogyi-Nelson's method (39, 57), which is based on the colourimetric reaction of sugars with a cupro-alkaline reactive, which in presence of molybdic arsenic forms a blue-coloured compound whose maximal absorbance occurs at 535 nm. For the standard curve, a glucose solution containing 100 mg of sugar per mL of solution was used. The non-reducing sugar levels (sucrose) in the fermented samples were determined after sample hydrolysis (4) and were then determined again as described above.
Biomass determination
The biomass concentration in fermented samples was determined gravimetrically after filtration through a 0.22 µm pore PVDF membrane and a drying step at 80°C for 24 hours until a constant weight was reached (40).
Organic acid identification and quantification
Lactic and acetic acid levels were determined using HPLC chromatography with an HPX87H column, operated under the following conditions: column temperature: 60ºC, mobile-eluent phase: H2SO4, mobile phase concentration: 5 mM, outflow of the mobile phase: 0.6 mL.min-1, pump pressure: 48 kg/cm2, volume sample: 50 μL, dilution: 1:5, time retention of standards: glucose: 9.38 min, lactic acid: 12.74 min, and acetic acid: 14.92 min.
Analyses of biomass and metabolite production during the fermentative process
The specific growth rate µ (h-1) was determined using the angular coefficient of the best correlation of the exponential phase of biomass growth, where the neperian logarithms of the biomass (X) concentration (LnX) (g.L-1) versus fermentation time (h) were plotted (3).
The yield of biomass in relation to the consumed substrate (YX/S) a substrate conversion factor can be obtained from Equation 1:
YX/S (g.g-1) = (Xf-Xi)/(Si-Sf)
where: Xf = final biomass concentration; Xi = initial biomass concentration; Si = initial sugar concentration; Sf = final sugar concentration.
The productivity of biomass (PX) is defined by Equation 2:
PX (g.L-1.h-1) = µ.Xf
where: µ = specific growth rate; Xf = final biomass concentration.
The yield of metabolic products (YP/S) was calculated using Hiss' formula (23), expressed by Equation 3:
YP/S (g.g-1) = (Pf-Pi)/(Si-Sf)
where: Pf = final product (lactic acid + acetic acid concentrations); Pi = initial product (lactic acid + acetic acid concentrations); Si = initial sugar concentration; Sf = final sugar concentration.
RESULTS AND DISCUSSION
Isolation and biochemical characterisation of L. reuteri LPB P01-001
The L. reuteri LPB P01-001 strain has probiotic potential due to its bile resistance (5% bile) and phenol resistance (0.4%), isolated from healthy swine gut, and such features are important characteristics for surviving in the intestine (unpublished data). Tolerance to bile and phenol (phenols can be formed in the gut by bacteria that have deaminated aromatic amino acids from the diet or can be produced by endogenous proteins) is important for improved survival rates, but not necessarily for multiplication in the intestine (62).
This strain has a bacillary morphology and reacts positively to Gram staining. After biochemical characterisation, the strain was presented as catalase (-); thus, it belongs to the Lactobacillus genus, which does not produce catalase to decompose hydrogen peroxide (25). The strain is a lactic acid bacteria because it is a lactic acid producer, as detected by HPLC. It is heterofermentative due to its production of products other than lactic acid, including acetic acid and ethanol from glucose (25), substances with possible antimicrobial activity. L. reuteri LPB P01-001 produces CO2 from hexose and presents better growth at 37-45°C with slight growth at 15°C. The results of the API 50 CH gallery system (Biomerieux) are not conclusive regarding identification of the Lactobacillus fermentum species, due to limitations of the biochemical method (5). According to Bergey's Manual (25), the Lactobacillus fermentum species cannot be distinguished from the Lactobacillus reuteri species by means of simple physiological tests. Other parameters may distinguish the species, such as: % mol guanine and cytosine, diamino acid levels of peptidoglycan or the electrophoretic mobility of lactic acid dehydrogenase.
Molecular characterisation
The isolated strain was molecularly identified, and the sequence was analysed using the BLAST routine of the Genbank and the Sequence Match of the RDP. The partial sequence of 16S rRNA from LPB P01-001 and the phylogenetic tree are presented in Figures 1 and 2. The partial sequence of 16S rRNA from sample LPB P01-001 is 98% similar to sequences of 16S rRNA from the Lactobacillus reuteri lineages available in the databases. Additionally, it presented similar but lower percentages (i.e., 96 to 97%) when compared to other Lactobacillus species, such as Lactobacillus vaginalis (ATCC49540T), Lactobacillus pontis (LTH 2587T), Lactobacillus panis (DSM 6035T) and Lactobacillus antri (DSM 16041T). The phylogenetic analysis confirmed a closer phylogenetic proximity of the LPB-P01-001 sample with the Lactobacillus reuteri species. Lactobacillus reuteri LPB P01-001 has been deposited at the Bioprocess Engineering and Biotechnology Department.
Kinetic parameters of L. reuteri LPB P01-001
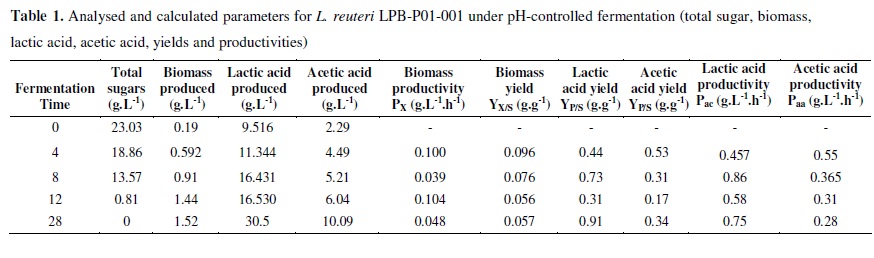
The growth kinetic parameters of L. reuteri LPB P01-001 in 12 L bench fermentation experiments with E3 medium under controlled and uncontrolled pH were determined for up to 12 hours at the end of the exponential growth phase, as can be seen in Figures 3A and B. The biomass increased after 2.8 h in both conditions, and the sugars were rapidly and almost completely consumed during the fermentation time with pH control (Figure 3A). The total sugar concentration decreased within 28 hours of fermentation, varying from 23.03 g.L-1 to 0 g.L-1. The initial biomass concentration was 0.19 g.L-1, and 1.52 g.L-1 of biomass was achieved after 28 hours of fermentation under pH control (Figure 3A), while 14.76 g.L-1 was obtained without pH-controlled fermentation after 26 hours (Figure 3B).
L. reuteri LPB P01-001 showed the usual growth behaviour, with duplication times from 2.8 4.0 h, depending on the initial pH and the control conditions, indicating the adequacy of the simple and low-cost E3 medium. In Figure 3B, the sugar consumption is shown to decrease slowly in the fermentation experiment without pH control when compared to fermentation with pH control.
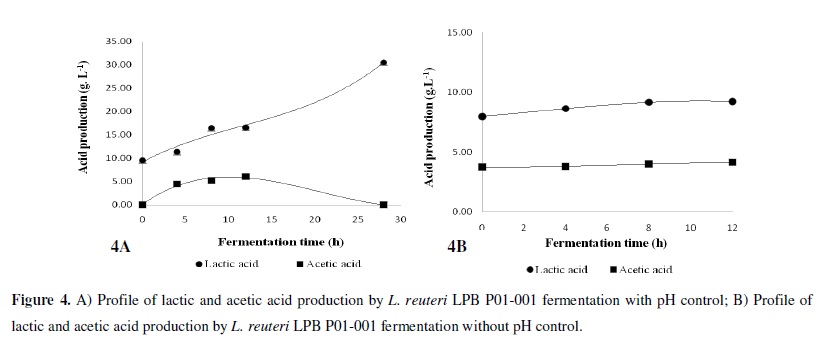
Figures 3A and 3B show the biomass production, sugar consumption, and pH variation during L. reuteri LPB P01-001 fermentation. In the experiment represented in Figure 3A, the pH was kept constant at 6.5 during the fermentation time. It was found that the sugars were readily consumed during 28 hours of fermentation, while in the uncontrolled pH experiment (Figure 3B), the pH changed from 4.65 to 3.9 after 8 h. Although the sugar was not completely consumed, similar biomass contents were obtained in the experiments with and without pH control (Tables 1 and 2). The kinetic parameters for biomass and lactic and acetic acid production are shown in Tables 1 and 2.
The specific growth rate μ (h-1) was determined using the angular coefficient of the best correlation of the neperian logarithm of biomass (X) concentration (LnX) (g.L-1) versus fermentation time (h) plot. The biomass and acid metabolite data obtained from bioreactor were statistically treated, and the corresponding kinetic parameters were determined, as can be seen in Tables 1 and 2. The specific growth rate (µ) for pH-controlled fermentation was 0.177 h-1, while for the non-pH controlled fermentation was 0.302 h-1. The production of lactic acid and acetic acid was higher in the pH control experiment, possibly due to the higher sugar consumption in this case (Figures 4A and B).
The kinetics of organic acid production under pH control and uncontrolled fermentation are presented in Figures 4A and B.
To obtain more accurate parameter values, some data were evaluated using an application of the general substrate consumption balance equation (23) shown in Equation 4:
∆S/∆t = (1/Yx/s) ∆X/∆t + mX (4)
The maintenance coefficient for pH-controlled fermentation (pH=6.5) was 0.50 g.L-1.h-1, while in uncontrolled fermentation, it was 0.13 g.L-1.h-1. The maintenance coefficient value obtained for L. reuteri LPB P01-001 in E3 medium was higher than that reported for Lactobacillus rhamnosus (6, 51). When the general substrate consumption balance is used, the resulting parameters are more accurate (23).
When extracellular metabolites are synthesised as in this work the parameter is of interest (46). From Equation 4, it can be deduced that:
∆S = 1/Yx/s ∆X + mX ∆X (5)
Considering Equation 5 and the values calculated, it was estimated that, for the pH-controlled fermentation, 59% of the energy was used for biomass synthesis and 41% was used for self-maintenance, including the synthesis of metabolic acids. Under an uncontrolled pH, the values were 70% and 30%, respectively.
Although the maintenance coefficient represents the production of metabolites and the energy required for their production, the value does not specifically express the type or kinetics of the metabolic acids produced. Therefore, we determined the kinetics of lactic and acetic acid production under different pH conditions, as shown in Tables 1 and 2. It has previously been reported that glucose consumption is lower at pH values lower than 5.5 and that a longer time is required to attain the maximum lactic acid concentration (59). A similar trend may occur for these fermentation conditions with cane sugar in E3 medium, since the growth at a constant pH of 6.5 produced about twice as much organic acid as that obtained at an uncontrolled pH (equivalent to 4.65) (Tables 1 and 2).
The results for L. reuteri LPB P01-001 show that, at an uncontrolled pH, a reduction in the rate of sugar consumption occurred (Figure 3B), and a poor production of lactic and acetic acids with reference to those obtained at a constant pH of 6.5 was observed, confirming other reported data (59).
Narayanan et al. (38), Giraud et al. (18), and Girauld et al. (19) reported a higher yield in lactic acid in relation to the yield in biomass for Lactobacillus during culture at pH 5-6. These results are in accordance with the yield observed for L. reuteri LPB P01-001 (Table 3).
Considering the results presented in Figures 4A and B, it is clear that the lactic and acetic acids present a zeroth-order kinetic pattern:
dA / dt = k
where: A: concentration (g.L-1); t: time (h); k: kinetic constant (g.L-1.h-1).
For the pH-controlled fermentation, the aciduric L. reuteri LPB P01-001 strain synthesised a higher quantity of lactic and acetic acids, corresponding to 20.98 g.L-1 and 7.8 g.L-1 after 28 hours of fermentation. These results are quite different from those obtained under an uncontrolled pH, in which case, the values for lactic and acetic acids were only 1.25 g.L-1 and 0.39 g.L-1, much lower than those obtained at a constant pH of 6.5. At a controlled pH, the lactic acid production was 16.78 times higher than under uncontrolled pH fermentation. The results are similar to those for acetic acid production, where at a controlled pH the production was 20 times higher than the acetic acid production during uncontrolled pH fermentation. Coincidentally, lactic acid production was predominant over that of acetic acid, a typical characteristic of heterofermentative lactic acid bacteria.
L. reuteri LPB P01-001 isolated from pigs can produce substances that produce inhibition of the frequent pathogens S. aureus (ATCC 14458) and haemolytic E. coli. The growth inhibition % for Escherichia coli haemolytic swine and Staphylococcus aureus determined using the supernatant of MRS broth fermentation for Lactobacillus reuteri LPB P01-001, in Mueller-Hinton medium adjusted to different pH values, was 96.7% at pH 5.5, the inhibition was 28.9% at pH 6.5 and 22.5% inhibition was observed at pH 7.0 for Escherichia coli haemolytic swine; and values of 96.1% at pH 5.5, 21.9% at pH 6.5, and 15.9% at pH 7.0 were found for Staphylococcus aureus (Table 4). A freeze-dried product containing viable lactic acid bacteria LPB P01-001 reuterin producer (unpublished data) and metabolic organic acids may be useful for swine nutrition and disease protection (42, 58, 61). At present, these types of agents are increasingly used to replace antibiotics as growth promoters in animals. The kinetics of L. reuteri LPB P01-001 fermentation with 1% yeast extract and 2.5% total cane sugar in a 12 L bioreactor at 37ºC were determined. Under controlled and uncontrolled pH, the biomass and lactic and acetic acid production were evaluated. Fermentation parameters such as the specific growth rate µ (h-1), productivity Px (g.L-1.h-1), yield of biomass Yx/s (g.g-1) and metabolic organic acid yields Y P/S (g.g-1) in lactic and acetic acid are shown below. Similar results regarding a pH effect on Lactobacillus microbial growth were reported by LeBlanc et al. (32), who indicated that the bacterial growth of Lactobacillus was not noticeably affected by the pH of the fermentation medium.
In fermentation carried out under a controlled pH of 6.5, greater organic acid production was obtained, although the biomass growth was similar in both experiments (Tables 1 and 2).
The pH effect on the total sugar consumption at uncontrolled pH is very strong and accounts for the low final yield of the lactic and acetic acids (19); this effect is displayed in Table 2.
The corresponding values for biomass yield (Yx/s) calculated after 12 h are 0.056 (pH-controlled fermentation) and 0.18 g.g-1 (uncontrolled fermentation), with the greater value obtained for the case in which the initial pH was 4.65 and the fermentation pH was not controlled. Growth rates are often reduced under neutral pH conditions (25), and this effect was observed when L. reuteri LPB P01-001 was grown at pH 6.5.
CONCLUSION
These results demonstrate that L. reuteri LPB-P01-001 is an interesting aciduric strain because it grows better under acidic conditions. The biomass yield was higher in the culture medium without pH control. However, the yield of lactic and acetic acids was higher in the fermentation experiments conducted at a constant pH of 6.5. Culture conditions with a constant pH of 6.5 are better for metabolic acid production, but when maximal biomass productivity is the goal, a lower pH should be used. Further studies may determine the optimal balance of these two parameters for use in industrial production of this potentially probiotic strain. Although the biomass production presented lower values, the biomass production cost is lower using sugar cane as compared to that of commercial media such as MRS synthetic medium; thus, sugar cane is a low-cost substrate alternative for L. reuteri probiotic production.
ACKNOWLEDGEMENTS
The authors thank the Coordination for the Improvement of Higher Education Personnel (CAPES, Brazil) and the company Ouro Fino Saúde Animal (Brazil) for their financial support.
REFERENCES
1. Bendall, F.; Gaillard-Martinie, B.; Hebraud, M.; Sadoun, D. (2008). Kinetic of production and mode of action of the Lactobacillus paracasei subsp. paracasei anti-listerial bacteriocin, an Algerian isolate. LWT Food Sci. Technol. 41, 1784-1792.
2. Bogovic Matijasic, B.; Stojkovic, S.; Salobir, J.; Malovrh, S.;Rogelj, I. (2004). Evaluation of the Lactobacillus gasseri K7 and LF221 strains in weaned piglets for their possible probiotic use and their detection in the faeces. Anim. Res. 53, 3544.
3. Bonomi, A.; Schmidell, W. (2001). Modelagem matemática e simulação de processos fermentativos. In: Biotecnologia Industrial. São Paulo: Edgard Blücher, 2, p.123-178.
4. Brasil (1981). Ministério da Agricultura. Secretaria Nacional de Defesa Agropecuária, Laboratório Nacional de Referência Animal. Métodos analíticos oficiais para controle de produtos de origem animal e seus ingredientes. II métodos físicos e químicos. Brasília.
5. Brolazo, E. M.; Leite, D. S.; Tiba, M. R.; Villarroel, M.; Marconi, C.; Simoes, J. A. (2011) Correlation between API 50 CH and multiplex polymerase chain reaction for the identification of vaginal lactobacilli in isolates. Braz. J. Microbiol. 42 (1), 225-232.
6. Bustos, G.; Moldes, A.B.; Cruz, J.M. Domínguez, J.M. (2004). Formulation of low-cost fermentative media for lactic acid production with Lactobacillus rhamnosus using vinification lees as nutrients. J. Agric. Food. Chem. 52, 801-808.
7. Carvalho, W.; Silva, D.D.V.; Canilha, L.; Mancilha, I.M. (2005). Aditivos alimentares produzidos por via fermentativa parte I: ácidos orgânicos. Rev. Anal., 18, 70-76.
8. Carr, F.J.; Chill, D.; Maida, N. (2002). The lactic acid bacteria: a literature survey. Crit. Rev.Microbiol. 28(4), 281-370.
9. Chun, J. (1995) Computer-Assisted Classification and Identification of Actinomycetes .UK. (PhD Thesis, University of Newcastle upon Tyne ).
10. CME - Center for Microbial Ecology. 2005. Available at: 1. http://www.cme.msu.edu/RDP/html/index.html Accessed 5 May 2005.
11. De Man, J.C.; Rogosa, M.; Sharpe, M.E. (1960). A medium for the cultivation of lactobacilli. J. App. Bacteriol. 23, 130-135.
12. El-Ziney, M.G.; Arneborg, G.N.; Uyttendaele, M.; Debevere, J.; Jakobsen, M. (1998). Characterization of growth and metabolite production of Lactobacillus reuteri during glucose/glycerol cofermentation in batch and continuous cultures. Biotechnol. Lett. 20 (10), 913-916.
13. FAO/WHO, Food and Agriculture Organization of the United Nations / World Health Organization. Guidelines for the evaluation of podsafety/fs_management/en/probiotic_guidelines.pdf, Access in 30 jun 2011.
14. Gänzle, M.G. (2004). Reutericyclin: biological activity, mode of action, and potential applications. Appl. Microbiol. Biotechnol., 64, 326-332.
15. Germany. DSMZ - Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig. 2005. Available at: http://www.dsmz.de/strains/no020016.htm.
16. Gil, N.F.; Martinez, R.C.R.; Gomes, B.C.; Nomizo, A.; Martinis, E.C.P. (2010). Vaginal lactobacilli as potencial probiotics against Candida spp. Braz. J. Microbiol. 41(1), 6-14.
17. Ginés, S.C.; Maldonado, M.C.; Valdez, G.F. (2000). Purification and characterization of invertase from Lactobacillus reuteri CRL 1100. Current Microbiol. 40, 181-184.
18. Giraud, E.; Braumana, A.; Keleke, S.; Lelong, B.; Raimbault, M. (1991). Isolation and physiological study of an amylolytic strain of Lactobacillus plantarum. Appl. Microbial Biotechnol, 36, 379-383.
19. Giraud, E.; Lelong, B.; Raimbault, M. (1991). Influence of pH and lactate concentration on the growth of Lactobacillus plantarum. Appl. Microbiol. Biotechnol., 36, 96-99.
20. Guyot, J.P.; Calderon, M.; Morlon-Guyot, J. (2000). Effect of pH control on lactic acid fermentation of starch by Lactobacillus manihotivorans LMG 18010T. J. Applied Microbiol. 88, 176-182.
21. Heuer, H.; Krsek, M.; Baker, P.; Smalla, K.; Wellington, E.M.H. (1997). Analysis of actinomycete communities by specific amplification of genes encoding 16S rRNA and gel-electrophoretic separation in denaturing gradients. Applied and Environ. Microbiol., 63, 3233-3241.
22. Hijum, S.A.F.T.; Bonting, K.; Maarel, M.J.E.C.; Dijkhuizen, L. (2001). Purification of a novel fructosyltransferase from Lactobacillus reuteri strain 121 and characterization of the levan produced. FEMS Microbiol. Lett. 205, 323-328.
23. Hiss, H. (2001). Cinética de processos fermentativos. In: Biotecnologia industrial. São Paulo: Edgard Blücher 2, 93-122.
24. Jonsson, H.; Ström, E.; Roos, S. (2001). Addition of mucin to the growth medium triggers mucus-binding activity in different strains of Lactobacillus reuteri in vitro. FEMS Microbiol. Lett. 204, 19-22.
25. Kandler, O.; Weiss, N. (1989). Genus Lactobacillus Beijerinck 1901. In: Bergey´s manual of systematic bacteriology. Baltimore: Williams & Wilkins, 2, p.1209-1234.
26. Kaplan, Ö.; Bakir, U. (1998). The effect of chemical crosslinking of invertase with dimethyl suberimidate on its pH stability. World J. Microbiol. & Biotechnol. 14, 277-280.
27. Kashket, E.R. (1987). Bioenergetics of lactic acid bacteria: cytoplasmic pH and osmotolerance. FEMS Microbiol. Rev. 46, 233-244.
28. Kawai, Y.; Ishii, Y.; Uemura, K.; Kitazawa, H.; Saito, T.; Itoh, T. (2001). Lactobacillus reuteri LA6 and Lactobacillus gasseri LA39 isolated from faeces of the same human infant produce identical cyclic bacteriocin. Food Microbiol. 18, 407-415.
29. Kimura, M. (1980) A simple model for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Molecular Evol., 16, 111-120. .
30. Lane, D.J.; Pace, B.; Olsen, G.J.; Stahl, D.A.; Sogin, M.L.; Pace, N.R. (1985) Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proceedings of the National Academy Sciences of the United States of America, 82(20), 6955-6959.
31. Lane, D.J. (1991). In: Stackebrandt, E. & Goodfellow, M. Nucleic Acid Techniques in Bacterial Systematics. John Wiley & Sons, Chichester, England.
32. Le Blanc, J. G.; Garro, M.S.; Savoy de Giori, G. (2004). Effect of pH on Lactobacillus fermentum growth, raffinose removal, α-galactosidase activity and fermentation products. Applied Microb. Cell Physiol. 65, 119-123.
33. Lee, D.Y.; Seo Y-S.; Rayamajhi N.; Kang, M.L.; Lee, S.I.; Yoo, H.S. (2009). Isolation, characterization, and evaluation of wild isolates of Lactobacillus reuteri from pig feces. The J. of Microbiol., 47(6), 663-672.
34. Maidak, B.L.; Cole, J.R.; Lilburn, T.G.; Parker, C.T.; Saxman, P.R.; Farris, R.J.; Garrity, G.M.; Olsen, G.J.; Sschmidt, T.M.;Tiedje, J.M. (2001) The RDP-II (Ribosomal Database Project). Nucleic Acids Research, 29(1), 173-174.
35. Marroki, A.; Zúñiga, M.; Kihal, M.; Pérez-Martínez, G. (2011) Characterization of Lactobacillus from Algerian goat's milk based on phenotypic, 16S rDNA sequencing and their technological properties. Braz. J. Microbiol. 42 (1), 158-171.
36. Morelli, L.; Cesena, C.; Haën, C.; Gozzini, L. (1998). Taxonomic Lactobacillus composition of feces from human newborns during the first fews days, Microb. Ecol. 35, 205-212 .
37. Mukai, T.; Asasaka, T.; Sato, E.; Mori, K.; Matsumoto, M.; Ohori, H. (2002). Inhibition of binding of Helicobacter pylori to the glycolipid receptors by probiotic Lactobacillus reuteri. FEMS Immunol. Med. Microbiol., 32, 105-110.
38. Narayanan, N.; Roychoudhury, P.; Srivastava, A. (2004). Isolation of adh mutant of Lactobacillus rhamnosus for production of L(+) lactic acid. Electronic J. Biotechnol, 7 (1), 72-84.
39. Nelson, N. (1944). A photometric adaptation of the somogyi method for the determination of glucose. J. Biol.Chem., 153, 375-380.
40. Neysens, P.; Messens, W.; Gevers, D.; Swings, J.; Vuyst, L. (2003). Biphasic kinetics of growth and bacteriocin production with Lactobacillus amylovorus DCE 471 occur under stress conditions. Microbiol., 149, 1073-1082.
41. NCBI - National Center for Biotechnology Information. 2005. Available at: http://www.ncbi.nlm.nih.gov. Acessed 5 May 2005.
42. Orobón, J.L.A. (2003). Tratamientos combinados de bacteriocinas y otros sistemas inhibitorios para la mejora de la seguridad de los productos lácteos. Madrid. 187 p. (Thesis Nutrición y Bromatología III. Universidad Complutense de Madrid).
43. Pancheniak, E.F.R.; Soccol, C.R. (2005). Biochemical characterization and identification of probiotic Lactobacillus for swine. Boletim do Centro de Pesq. Proc. de Alimentos 23(2), 299-310.
44. Pancheniak, E.F.R. (2005). Isolamento, Seleção, Caracterização Bioquímica e Molecular para Produção e Avaliação do Potencial Probiótico de L. reuteri LPB P01-001 em suínos, Curitiba, Brasil, 199p. (PhD Thesis. Tecnologia de Alimentos, UFPR).
45. Parada, J.L.; Caron, C.R.; Medeiros, A.B.P.; Soccol, C.R. (2007). Bacteriocins from lactic acid bacteria: purification, properties and use as biopreservatives. Brazilian Archives of Biology and Technology,50 (3), 521-542.
46. Pirt, S.J. (1965). The maintenance energy of bacteria in growing cultures. Proc R Soc London B, 163, 224231.
47. Portella, A.C.F.; Karp, S.; Scheidt, G.N.; Woiciechwski, A.L.; Parada, J.L.; Soccol, C.R. (2009). Modelling antagonic effect of lactic acid bacteria supernatants on some pathogenic bacteria. Brazilian Archives of Biology and Technology,52, 29-36
48. Reque, E.F.; Pandey, A.; Franco, S. G.; Soccol, C.R. (2000). Isolation, identification and physiological study of Lactobacillus fermentum LPB for use as probiotic in chickens. Braz.J. Microbiol., 31(4), 303-307.
49. Riaz, S.; Nawaz, S. K.; Hasnain, S. (2010) Bacteriocins produced by L. fermentum and L .acidophilus can inhibit cephalosporin resistant E .coli. Braz. J. Microbiol. 41 (3), 643-648.
50. Roberfroid, M.(1993) Dietary fiber, inulin, and oligofructose: A review comparing their physiological effects. Critical Reviews in Food Science and Nutrition. 33 (2),103-148.
51. Rodrigues, L.; Moldes, A.; Teixeira, J.; Oliveira, R. (2006). Kinetic study of fermentative biosurfactant production by Lactobacillus strains. Biochemical Engineering Journal, 28, 109-116.
52. Roos, N. M.; Katan, M. B. (2000). Effects of probiotic bacteria on diarrhea, lipid metabolism, and carcinogenesis: a review of papers published between 1988 and 1998. American Journal of Clinical Nutrition, 71 ( 2), 405-411.
53. Saitou, N.; Nei, M. (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution, 4, 406-425.
54. Salminen, S.; Deighton, M.A.; Benno, Y.; Gorbach, S.L. (1998). Lactic acid bacteria in health and disease. In: Salminen, S., von Wright, A. (Eds.), Lacticacid Bacteria: Microbiology and Functional Aspects, second ed. Marcel Dekker Inc., New York, p. 211253.
55. Schiffrin, E.J., Rochat, F., Link-Amster, H., Aeschlimann, J.M., Donnet-Hughes, A. (1995). Immunomodulation of human blood cells following the ingestion of lactic acid bacteria. J. Dairy Sci. 78 (3), 491-497.
56. Schmidell, W. (2001). Microrganismos e meios de cultura para utilização industrial. In: Biotecnologia industrial. São Paulo: Edgard Blücher, 2, 5-18.
57. Somogyi, M. (1945). A new reagent for the determination of sugars. J. Biol.Chem. 160, 61-68.
58. Tiwari, B.K.;Valdramidis, V.P.; Donnell, C.P.O.; Muthukumarappan, K.; Bourke, P.; Cullen, P.L. (2009). Application of natural antimicrobials for food preservation. J. Agric. Food Chem. 57, 5987-6000.
59. Venkatesh, K.V.; Okos, M.R.; Wankat, P.C. (1993). Kinetic model of growth and lactic acid production from lactose by Lactobacillus bulgaricus. Process Biochemistry, 28, 231-241.
60. Weisburg, W. G.; Barns, S. M.; Pelletier, D.A.; Lane, D.J. (1991).16S ribosomal DNA amplification for phylogenetic study. J of Bacteriol. 173, 697-703.
61. Williams, N.T. (2010). Probiotics. American Journal of Health - System Pharmacy 67(6), 449-458.
62. Xanthopoulos, V.; Litopoulou-Tzanetaki, E.; Tzanetakis, N. Characterization of Lactobacillus isolates from infant faeces as dietary adjuncts. Food Microbiology, London, v. 17, p.205-215, 2000.
63. Young, A.; Blakesley, R. (1991) Sequencing plasmids from single colonies with the dsDNA cycle sequencing system. Focus, 13 (4), 137.
Submitted: July 17, 2010; Returned to authors for corrections: June 22, 2011; Approved: January 16, 2012.
- 1. Bendall, F.; Gaillard-Martinie, B.; Hebraud, M.; Sadoun, D. (2008). Kinetic of production and mode of action of the Lactobacillus paracasei subsp. paracasei anti-listerial bacteriocin, an Algerian isolate. LWT Food Sci. Technol. 41, 1784-1792.
- 2. Bogovic Matijasic, B.; Stojkovic, S.; Salobir, J.; Malovrh, S.;Rogelj, I. (2004). Evaluation of the Lactobacillus gasseri K7 and LF221 strains in weaned piglets for their possible probiotic use and their detection in the faeces. Anim. Res. 53, 3544.
- 3. Bonomi, A.; Schmidell, W. (2001). Modelagem matemática e simulaçăo de processos fermentativos. In: Biotecnologia Industrial Săo Paulo: Edgard Blücher, 2, p.123-178.
-
4Brasil (1981). MinistÚrio da Agricultura. Secretaria Nacional de Defesa Agropecußria, Laborat¾rio Nacional de ReferÛncia Animal. MÚtodos analÝticos oficiais para controle de produtos de origem animal e seus ingredientes. II – mÚtodos fÝsicos e quÝmicos. BrasÝlia.
- 5. Brolazo, E. M.; Leite, D. S.; Tiba, M. R.; Villarroel, M.; Marconi, C.; Simoes, J. A. (2011) Correlation between API 50 CH and multiplex polymerase chain reaction for the identification of vaginal lactobacilli in isolates. Braz. J. Microbiol 42 (1), 225-232.
- 6. Bustos, G.; Moldes, A.B.; Cruz, J.M. Domínguez, J.M. (2004). Formulation of low-cost fermentative media for lactic acid production with Lactobacillus rhamnosus using vinification lees as nutrients. J. Agric. Food. Chem 52, 801-808.
- 7. Carvalho, W.; Silva, D.D.V.; Canilha, L.; Mancilha, I.M. (2005). Aditivos alimentares produzidos por via fermentativa parte I: ácidos orgânicos. Rev. Anal., 18, 70-76.
- 8. Carr, F.J.; Chill, D.; Maida, N. (2002). The lactic acid bacteria: a literature survey. Crit. Rev.Microbiol 28(4), 281-370.
- 9. Chun, J. (1995) Computer-Assisted Classification and Identification of Actinomycetes .UK. (PhD Thesis, University of Newcastle upon Tyne ).
-
10CME - Center for Microbial Ecology. 2005. Available at: 1. http://www.cme.msu.edu/RDP/html/index.htmlá Accessed 5 May 2005.
» link - 11. De Man, J.C.; Rogosa, M.; Sharpe, M.E. (1960). A medium for the cultivation of lactobacilli. J. App. Bacteriol 23, 130-135.
- 12. El-Ziney, M.G.; Arneborg, G.N.; Uyttendaele, M.; Debevere, J.; Jakobsen, M. (1998). Characterization of growth and metabolite production of Lactobacillus reuteri during glucose/glycerol cofermentation in batch and continuous cultures. Biotechnol. Lett. 20 (10), 913-916.
- 13. FAO/WHO, Food and Agriculture Organization of the United Nations / World Health Organization. Guidelines for the evaluation of podsafety/fs_management/en/probiotic_guidelines.pdf, Access in 30 jun 2011.
- 14. Gänzle, M.G. (2004). Reutericyclin: biological activity, mode of action, and potential applications. Appl. Microbiol. Biotechnol., 64, 326-332.
- 15. Germany. DSMZ - Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig. 2005. Available at: http://www.dsmz.de/strains/no020016.htm
- 16. Gil, N.F.; Martinez, R.C.R.; Gomes, B.C.; Nomizo, A.; Martinis, E.C.P. (2010). Vaginal lactobacilli as potencial probiotics against Candida spp. Braz. J. Microbiol. 41(1), 6-14.
- 17. Ginés, S.C.; Maldonado, M.C.; Valdez, G.F. (2000). Purification and characterization of invertase from Lactobacillus reuteri CRL 1100. Current Microbiol. 40, 181-184.
- 18. Giraud, E.; Braumana, A.; Keleke, S.; Lelong, B.; Raimbault, M. (1991). Isolation and physiological study of an amylolytic strain of Lactobacillus plantarum Appl. Microbial Biotechnol, 36, 379-383.
- 19. Giraud, E.; Lelong, B.; Raimbault, M. (1991). Influence of pH and lactate concentration on the growth of Lactobacillus plantarum Appl. Microbiol. Biotechnol., 36, 96-99.
- 20. Guyot, J.P.; Calderon, M.; Morlon-Guyot, J. (2000). Effect of pH control on lactic acid fermentation of starch by Lactobacillus manihotivorans LMG 18010T. J. Applied Microbiol. 88, 176-182.
- 21. Heuer, H.; Krsek, M.; Baker, P.; Smalla, K.; Wellington, E.M.H. (1997). Analysis of actinomycete communities by specific amplification of genes encoding 16S rRNA and gel-electrophoretic separation in denaturing gradients. Applied and Environ. Microbiol., 63, 3233-3241.
- 22. Hijum, S.A.F.T.; Bonting, K.; Maarel, M.J.E.C.; Dijkhuizen, L. (2001). Purification of a novel fructosyltransferase from Lactobacillus reuteri strain 121 and characterization of the levan produced. FEMS Microbiol. Lett 205, 323-328.
- 23. Hiss, H. (2001). Cinética de processos fermentativos. In: Biotecnologia industrial Săo Paulo: Edgard Blücher 2, 93-122.
- 24. Jonsson, H.; Ström, E.; Roos, S. (2001). Addition of mucin to the growth medium triggers mucus-binding activity in different strains of Lactobacillus reuteri in vitro. FEMS Microbiol. Lett. 204, 19-22.
- 25. Kandler, O.; Weiss, N. (1989). Genus Lactobacillus Beijerinck 1901. In: Bergey´s manual of systematic bacteriology. Baltimore: Williams & Wilkins, 2, p.1209-1234.
- 26. Kaplan, Ö.; Bakir, U. (1998). The effect of chemical crosslinking of invertase with dimethyl suberimidate on its pH stability. World J. Microbiol. & Biotechnol. 14, 277-280.
- 27. Kashket, E.R. (1987). Bioenergetics of lactic acid bacteria: cytoplasmic pH and osmotolerance. FEMS Microbiol. Rev. 46, 233-244.
- 28. Kawai, Y.; Ishii, Y.; Uemura, K.; Kitazawa, H.; Saito, T.; Itoh, T. (2001). Lactobacillus reuteri LA6 and Lactobacillus gasseri LA39 isolated from faeces of the same human infant produce identical cyclic bacteriocin. Food Microbiol. 18, 407-415.
- 29. Kimura, M. (1980) A simple model for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Molecular Evol., 16, 111-120.
- 30. Lane, D.J.; Pace, B.; Olsen, G.J.; Stahl, D.A.; Sogin, M.L.; Pace, N.R. (1985) Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proceedings of the National Academy Sciences of the United States of America, 82(20), 6955-6959.
- 31. Lane, D.J. (1991). In: Stackebrandt, E. & Goodfellow, M. Nucleic Acid Techniques in Bacterial Systematics John Wiley & Sons, Chichester, England.
- 32. Le Blanc, J. G.; Garro, M.S.; Savoy de Giori, G. (2004). Effect of pH on Lactobacillus fermentum growth, raffinose removal, α-galactosidase activity and fermentation products. Applied Microb. Cell Physiol 65, 119-123.
- 33. Lee, D.Y.; Seo Y-S.; Rayamajhi N.; Kang, M.L.; Lee, S.I.; Yoo, H.S. (2009). Isolation, characterization, and evaluation of wild isolates of Lactobacillus reuteri from pig feces. The J. of Microbiol., 47(6), 663-672.
- 34. Maidak, B.L.; Cole, J.R.; Lilburn, T.G.; Parker, C.T.; Saxman, P.R.; Farris, R.J.; Garrity, G.M.; Olsen, G.J.; Sschmidt, T.M.;Tiedje, J.M. (2001) The RDP-II (Ribosomal Database Project). Nucleic Acids Research, 29(1), 173-174.
- 35. Marroki, A.; Zúńiga, M.; Kihal, M.; Pérez-Martínez, G. (2011) Characterization of Lactobacillus from Algerian goat's milk based on phenotypic, 16S rDNA sequencing and their technological properties. Braz. J. Microbiol 42 (1), 158-171.
- 36. Morelli, L.; Cesena, C.; Haën, C.; Gozzini, L. (1998). Taxonomic Lactobacillus composition of feces from human newborns during the first fews days, Microb. Ecol 35, 205-212 .
- 37. Mukai, T.; Asasaka, T.; Sato, E.; Mori, K.; Matsumoto, M.; Ohori, H. (2002). Inhibition of binding of Helicobacter pylori to the glycolipid receptors by probiotic Lactobacillus reuteri FEMS Immunol. Med. Microbiol., 32, 105-110.
- 38. Narayanan, N.; Roychoudhury, P.; Srivastava, A. (2004). Isolation of adh mutant of Lactobacillus rhamnosus for production of L(+) lactic acid. Electronic J. Biotechnol, 7 (1), 72-84.
- 39. Nelson, N. (1944). A photometric adaptation of the somogyi method for the determination of glucose. J. Biol.Chem., 153, 375-380.
- 40. Neysens, P.; Messens, W.; Gevers, D.; Swings, J.; Vuyst, L. (2003). Biphasic kinetics of growth and bacteriocin production with Lactobacillus amylovorus DCE 471 occur under stress conditions. Microbiol., 149, 1073-1082.
-
41NCBI - National Center for Biotechnology Information. 2005. Available at: http://www.ncbi.nlm.nih.gov Acessed 5 May 2005.
» link - 42. Orobón, J.L.A. (2003). Tratamientos combinados de bacteriocinas y otros sistemas inhibitorios para la mejora de la seguridad de los productos lácteos Madrid. 187 p. (Thesis Nutrición y Bromatología III. Universidad Complutense de Madrid).
- 43. Pancheniak, E.F.R.; Soccol, C.R. (2005). Biochemical characterization and identification of probiotic Lactobacillus for swine. Boletim do Centro de Pesq. Proc. de Alimentos 23(2), 299-310.
- 44. Pancheniak, E.F.R. (2005). Isolamento, Seleçăo, Caracterizaçăo Bioquímica e Molecular para Produçăo e Avaliaçăo do Potencial Probiótico de L. reuteri LPB P01-001 em suínos, Curitiba, Brasil, 199p. (PhD Thesis. Tecnologia de Alimentos, UFPR).
- 45. Parada, J.L.; Caron, C.R.; Medeiros, A.B.P.; Soccol, C.R. (2007). Bacteriocins from lactic acid bacteria: purification, properties and use as biopreservatives. Brazilian Archives of Biology and Technology,50 (3), 521-542.
- 46. Pirt, S.J. (1965). The maintenance energy of bacteria in growing cultures. Proc R Soc London B, 163, 224231.
- 47. Portella, A.C.F.; Karp, S.; Scheidt, G.N.; Woiciechwski, A.L.; Parada, J.L.; Soccol, C.R. (2009). Modelling antagonic effect of lactic acid bacteria supernatants on some pathogenic bacteria. Brazilian Archives of Biology and Technology,52, 29-36
- 48. Reque, E.F.; Pandey, A.; Franco, S. G.; Soccol, C.R. (2000). Isolation, identification and physiological study of Lactobacillus fermentum LPB for use as probiotic in chickens. Braz.J. Microbiol., 31(4), 303-307.
- 49. Riaz, S.; Nawaz, S. K.; Hasnain, S. (2010) Bacteriocins produced by L. fermentum and L .acidophilus can inhibit cephalosporin resistant E .coli. Braz. J. Microbiol 41 (3), 643-648.
- 50. Roberfroid, M.(1993) Dietary fiber, inulin, and oligofructose: A review comparing their physiological effects. Critical Reviews in Food Science and Nutrition 33 (2),103-148.
- 51. Rodrigues, L.; Moldes, A.; Teixeira, J.; Oliveira, R. (2006). Kinetic study of fermentative biosurfactant production by Lactobacillus strains. Biochemical Engineering Journal, 28, 109-116.
- 52. Roos, N. M.; Katan, M. B. (2000). Effects of probiotic bacteria on diarrhea, lipid metabolism, and carcinogenesis: a review of papers published between 1988 and 1998. American Journal of Clinical Nutrition, 71 ( 2), 405-411.
- 53. Saitou, N.; Nei, M. (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution, 4, 406-425.
- 55. Schiffrin, E.J., Rochat, F., Link-Amster, H., Aeschlimann, J.M., Donnet-Hughes, A. (1995). Immunomodulation of human blood cells following the ingestion of lactic acid bacteria. J. Dairy Sci. 78 (3), 491-497.
- 56. Schmidell, W. (2001). Microrganismos e meios de cultura para utilizaçăo industrial. In: Biotecnologia industrial. Săo Paulo: Edgard Blücher, 2, 5-18.
- 57. Somogyi, M. (1945). A new reagent for the determination of sugars. J. Biol.Chem 160, 61-68.
- 58. Tiwari, B.K.;Valdramidis, V.P.; Donnell, C.P.O.; Muthukumarappan, K.; Bourke, P.; Cullen, P.L. (2009). Application of natural antimicrobials for food preservation. J. Agric. Food Chem. 57, 5987-6000.
- 59. Venkatesh, K.V.; Okos, M.R.; Wankat, P.C. (1993). Kinetic model of growth and lactic acid production from lactose by Lactobacillus bulgaricus Process Biochemistry, 28, 231-241.
- 60. Weisburg, W. G.; Barns, S. M.; Pelletier, D.A.; Lane, D.J. (1991).16S ribosomal DNA amplification for phylogenetic study. J of Bacteriol. 173, 697-703.
- 61. Williams, N.T. (2010). Probiotics. American Journal of Health - System Pharmacy 67(6), 449-458.
- 62. Xanthopoulos, V.; Litopoulou-Tzanetaki, E.; Tzanetakis, N. Characterization of Lactobacillus isolates from infant faeces as dietary adjuncts. Food Microbiology, London, v. 17, p.205-215, 2000.
- 63. Young, A.; Blakesley, R. (1991) Sequencing plasmids from single colonies with the dsDNA cycle sequencing system. Focus, 13 (4), 137.
Publication Dates
-
Publication in this collection
02 May 2012 -
Date of issue
Mar 2012
History
-
Received
17 July 2010 -
Accepted
16 Jan 2012 -
Reviewed
22 June 2011