Abstract
Saccharomyces cerevisiae S1, which is a locally isolated and improved strain showed viability at 40, 45 and 50ºC and produced ethanol at 40, 43 and 45ºC. When the cells were given heat shock at 45ºC for 30min and grown at 40ºC, 100% viability was observed for 60h, and addition of 200gl-1 ethanol has led to complete cell death at 30h. Heat shock given at 45ºC (for 30min) has improved the tolerance to temperature induced ethanol shock leading to 37% viability at 30h. when the cells were subjected to ethanol (200gl-1 for 30 min) and osmotic shock (sorbitol 300gl-1), trehalose contents in the cells were increased. The heat shocked cells showed better viability in presence of added ethanol. Soy flour supplementation has improved the viability of S. cerevisiae S1 to 80% in presence of 100gl-1 added ethanol and to 60% in presence of 300gl-1 sorbitol. In presence of sorbitol (200gl-1) and ethanol (50gl-1) at 40ºC, 46% viability was retained by S. cerevisiae S1 at 48h and it was improved to 80% by soy flour supplementation.
Thermo-tolerance; ethanol-tolerance; osmo-tolerance; viability Saccharomyces cerevisiae
INDUSTRIAL MICROBIOLOGY
Osmo-, thermo- and ethanol- tolerances of Saccharomyces cerevisiae S1
Sandrasegarampillai Balakumar; Vasanthy Arasaratnam* * Corresponding Author. Mailing address: Department of Biochemistry, Faculty of Medicine, University of Jaffna, Kokuvil, Sri Lanka.; Tel.: 0094 21 222 6514.; E-mail: arva26arva@yahoo.com
Department of Biochemistry, Faculty of Medicine, University of Jaffna, Kokuvil, Sri Lanka
ABSTRACT
Saccharomyces cerevisiae S1, which is a locally isolated and improved strain showed viability at 40, 45 and 50ºC and produced ethanol at 40, 43 and 45ºC. When the cells were given heat shock at 45ºC for 30min and grown at 40ºC, 100% viability was observed for 60h, and addition of 200gl-1 ethanol has led to complete cell death at 30h. Heat shock given at 45ºC (for 30min) has improved the tolerance to temperature induced ethanol shock leading to 37% viability at 30h. when the cells were subjected to ethanol (200gl-1 for 30 min) and osmotic shock (sorbitol 300gl-1), trehalose contents in the cells were increased. The heat shocked cells showed better viability in presence of added ethanol. Soy flour supplementation has improved the viability of S. cerevisiae S1 to 80% in presence of 100gl-1 added ethanol and to 60% in presence of 300gl-1 sorbitol. In presence of sorbitol (200gl-1) and ethanol (50gl-1) at 40ºC, 46% viability was retained by S. cerevisiae S1 at 48h and it was improved to 80% by soy flour supplementation.
Key words: Thermo-tolerance, ethanol-tolerance, osmo-tolerance, viability Saccharomyces cerevisiae
INTRODUCTION
The tolerance of yeast to its substrate (osmo-tolerance), fermentation product (ethanol- tolerance) and temperature (thermo-tolerance) has great potential to be used in industrial scale fermentation. At the beginning of fermentation, cells are subjected to high substrate concentration and as the ethanol level increases, both the substrate and product causes stress to the organism (14). During fermentation, heat is liberated due to exothermic reactions and if the environmental temperature is already high, the fermenter temperature tends to increase (1). Therefore the yeast should have temperature-, osmotic pressure- and ethanol- tolerating capacities to perform efficiently in industrial scale. In tropical countries like Sri Lanka, maintaining the operating temperature at or around the optimum fermentation temperature requires cooling, which is expensive. Significant cost savings become apparent if the fermenter can be kept at or above 40ºC. In addition, ethanol recovery cost shall also be low if the process is carried out at higher temperatures. This however would require a yeast strain that could produce high titre of ethanol at higher temperatures.
Good yeast strain should have osmo-, ethanol- and combined ethanol and osmo-tolerances along with thermo- tolerance properties. Therefore this study was undertaken to evaluate the tolerance levels and fermentative capacities of a locally isolated and improved organism, Saccharomyces cerevisiae S1 (2) up to 45ºC for its application in local distilleries.
MATERIALS AND METHODS
Materials
Soy bean from local market was powdered and dried at 80ºC. All the other materials were purchased from standard suppliers: culture media Oxoid limited USA, and other chemicals are from Sigma-Aldrich, USA.
Saccharomyces cerevisiae S1
Saccharomyces cerevisiae S1 is a locally isolated and improved thermotolerant strain (2); maintained in peptone, yeast extract and nutrient (PYN) agar (2.5gl-1) slants.
Analytical methods
Glucose (23), trehalose (TCA soluble anthrone positive carbohydrate) (36), ethanol (39) and viable cell count (30) were determined by standard methods.
Peptone, yeast extract and nutrient (PYN) medium
The medium contained (gl-1) peptone, 3.5, yeast extract, 3.0, MgSO4.7H2O, 1.0, KH2PO4, 2.0; and (NH4)2SO4, 1.0 at pH 5.0. Under different experimental conditions, different amounts of glucose were added to the medium and represented as glucose (amount in gl-1) PYN medium (2).
Inoculum of S. cerevisiae S1
Glucose (50gl-1) PYN medium (100ml) was inoculated with 2 loops full of Saccharomyces cerevisiae S1 and incubated at 36ºC for 18h with shaking at 150rpm.
Thermo- tolerance and ethanol production
S. cerevisiae S1 grown at 36ºC in glucose (50gl-1) PYN medium for 18h was incubated at 40, 45, 50 and 55ºC separately in triplicates and viability was monitored. All the following treatments were done in triplicates. For the ethanol production studies, inocua (10%, v/v, 18h) were added to the glucose (100gl-1) PYN medium and incubated at 40, 43 and 45ºC separately with shaking (150rpm).
Temperature shift cultivation on ethanol tolerance
Culture of S. cerevisiae S1 prepared at 36ºC in glucose (50gl-1) PYN medium was given different treatments as shown in Table 1 and the viable cell count was monitored.
Heat shock on trehalase content and thermo-tolerance
To 18h old S. cerevisiae S1 grown at 36ºC in glucose (50gl-1) PYN medium, heat shock was given by incubating at 45ºC for 30min. Control did not have heat treatment. Then 1ml aliquots of the test and control cultures were mixed with 1ml normal saline (pre-equilibrated at 58ºC) and incubated at 58ºC for 5min. The viability was determined. Trehalose was extracted (37) and estimated (36). Yeast cells without heat shock were used as control. Weight of the dry cells was measured.
Ethanol shock on trehalose content
Ethanol content in the S. cerevisiae S1 culture grown for 18h at 36ºC in glucose (50gl-1) PYN medium was measured and ethanol was added to make up the total concentration to 200gl-1. After 30min, cells subjected to ethanol shock were harvested by centrifugation (7 x 103 rpm) and trehalose content and dry cell weight were measured. To the control, no ethanol shock was given.
Growth temperature on thermo-tolerance
S. cerevisiae S1 inocula prepared at 28, 32 and 36ºC were incubated at 58ºC and viability was monitored. In another set-up 18 old culture grown at 28ºC was incubated at 36ºC for 90 min and then incubated at 58ºC and the viability was monitored.
Soy flour supplementation on thermo-tolerance
Viability of S. cerevisiae S1 grown at 40ºC in glucose (100gl-1) PYN medium supplemented with 20gl-1 soy flour was monitored while the control medium did not have soy flour.
Soy flour supplementation on osmo-tolerance
Sorbitol (0-400gl-1) was added to glucose (100gl-1) PYN. Soy flour (40 gl-1) was added to the test while the control did not have soy flour. Glucose (200gl-1) PYN medium and glucose (300gl-1) PYN medium with and without soy flour supplementation were also taken. Viable cell count and ethanol were determined at 48h of incubation.
Soy flour supplementation on ethanol tolerance
to glucose (100gl-1) PYN medium with and without soy flour (40gl-1), ethanol (0-200g l-1) was added and incubated at 40ºC. Viable cell count and ethanol were measured at 48h.
Combined effects of osmo- and ethanol stresses
Sorbitol (200gl-1) was added separately into different medium prepared with and without soy flour and, viable cell count and ethanol were measured at 48h.
RESULTS AND DISCUSSION
Thermo-tolerance and ethanol production
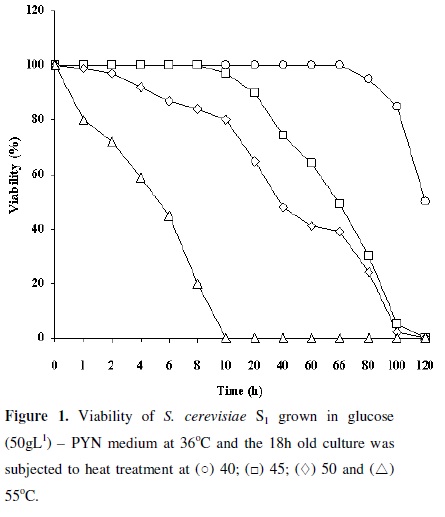
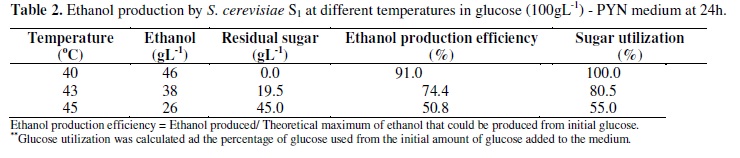
When 18h old cultures of S. cerevisiae S1 grown in glucose (50gl-1) PYN at 36ºC were incubated at different temperatures, the organism retained 100% viability up to 72, 10.0, 2.0 and 0.5h respectively at 40, 45, 50 and 55ºC and the organism lost 50% of its viability at 120, 66, 39 and 5h at the respective temperatures (Figure 1). Since the organism showed considerable viability at higher temperatures, its ethanol producing ability at 40, 43 and 45ºC were studied. Temperatures above 45ºC were not considered, as the fermentation processes do not exceed 42ºC in large industrial level operations (1). The ethanol production at 40, 43 and 45ºC in glucose (100gl-1) PYN medium was 46, 38 and 26gl-1 respectively (Table 2). D'Amore et al., (7) also have shown that the increase in the temperature from 40 to 45ºC resulted in a decrease in the rate and extent of glucose utilization and ethanol production. Since S. cerevisiae S1 was able to survive and produce ethanol from 40 to 45ºC, the effect of heat stress on its ethanol tolerance was studied.
Temperature shift cultivation on ethanol tolerance
When 18h old S. cerevisiae S1 inoculum was given different treatments (Table 1) and grown at either 36 or 40ºC, the viability of control cultures which had no treatments (heat shock or added ethanol) at either 36 or 40ºC remained almost 100% (Figure 2). The heat shocked cells (at 45ºC for 30 min) showed 100% viability when they were grown at 40ºC without added ethanol. When 200gl-1 ethanol was added to the culture (18h) grown at 36ºC, complete death of cells was observed at 60h, whereas at 40ºC in presence of ethanol complete cell death occurred at 30h. Therefore the toxic effect of ethanol was aggravated by the increase in incubation temperature. However the temperature induced ethanol toxicity was nullified by a brief heat shock (30min) at 45ºC. Heat shocked culture showed 37% viability at 30 h at 40ºC in the presence of ethanol as against complete cell death of the cells, which were not given heat shock.
It was reported that ethanol had specific and non-specific effects directly on membranes and proteins (16). Heat shock leads to changes in the fatty acid composition of the membranes (22, 25), synthesis of heat shock proteins (5, 12, 17, 22, 40) and accumulation of trehalose (19, 38, 4, 11, 15, 26, 28, 29, 31, 32, 33, 42). To evaluate whether the heat shock induced ethanol tolerance in Saccharomyces cerevisiae S1 was due to the accumulation of trehalose (TCA soluble anthrone positive carbohydrate), the changes in trehalose content along with heat shock and thermo- tolerance were studied.
Heat shock on trehalose content and thermo-tolerance
Trehalose content was increased by 90.3% in heat shocked cells; while 28% viability was observed at 30h for heat shocked cells as against complete cell death for culture not undergone heat shock. Trehalose content was increased from 62 (±2.0) to 118 (±5.0) (mmole glc / g dry cell weight) after heat shock treatment. Therefore the increase in trehalose content could be linked to enhanced thermo- tolerance of S. cerevisiae S1 cells as for other yeast (19, 28, 34) strains. Accumulation of enormous amounts of trehalose (28) could be due to increased levels of trehalose metabolism (42). Trehalose at first regarded only as an energy reserve (13), but latter studies have shown the correlation between the trehalose content of yeast cells and their resistance to temperature extremes (24, 28, 41), high osmotic pressure and high ethanol concentrations (18). Trehalose acts as a protecting agent to the cell membranes and proteins under conditions that deplete intracellular water (6, 9). Since the relationship between trehalose content and thermo-tolerance was observed in this study, the effect of ethanol stress on the trehalose content of the cells was also studied.
Ethanol shock on trehalose content
Trehalose content was 66 (±1.0) and 95 (±4.0) µmoles glucose equivalent/dry cell weight respectively in control and test samples. A 44% increase in trehalose content was induced by ethanol shock. Ethanol causes water stress by lowering water activity (aw) and thereby interferes with hydrogen bonding within and between hydrated cell components leading to ultimate disruption of enzymes and membrane structure and function (8). Yeast cells exposed to ethanol, synthesize compatible solutes such as glycerol and trehalose as protectants (14). Ethanol induced leakage was inhibited by the accumulated trehalose (20, 26, 27). Under water stress, cells synthesize compounds that protect the structure and function of hydrated cell components, the compatible solutes and the trehalose formed show protective effects on membranes (28). When the ethanol shock as well as heat shock was studies individually, both studies have shown the induced accumulation of trehalose. In the mean time heat shocked cells showed better ethanol tolerance. The stress conditions have been circumvented by the production of trehalose. The accumulation of trehalose in the heat shocked cells was more than that in the ethanol stressed cells. Since the heat shock at 45ºC for 30min has improved the viability of S. cerevisiae S1, the effect of growth temperature on the thermo-tolerance of the organism was studied.
Growth temperature on thermo-tolerance
The cultures grown at different temperatures showed vast difference in thermo-tolerance at 58ºC (Figure 3). In addition to growth temperature, heat shock prior to high temperature exposure had significant effect on thermo-tolerance. When the culture grown at 28ºC were incubated at 36ºC and was subjected to 58ºC, 20% viability was observed, while the cells directly transferred from 36 to 58ºC showed 2% viability at 10h.
The increase in the thermo-tolerance due to the rapid shift in growth temperature from 28 to 36ºC resulted in protection from death at 58ºC. When the growth temperatures were 23, 30 and 36ºC, the survival fraction (%) of the S. cerevisiae at 52ºC at 5min were 90, 10 and 0.8 respectively (7). In our experiment the cultures grown at 28, 32 and 36ºC showed 1, 12 and 28% viability at 58ºC after 5h. The shift in growth temperature from 28 to 36ºC has resulted in significant increase in viability (50%). Viability of the cells at 58ºC reached zero at 10 and 20 h with cultures grown at 28 and 32ºC respectively. At 30min, 0.1 and 1.0% viability was observed with cultures grown at 36ºC and heat shocked (28 to 36ºC) cultures respectively. Shifting yeast culture from 23 to 36ºC for a brief period would result in improved viability (22).
Soy flour supplementation on thermo-tolerance
Soy flour supplementation has improved the thermo-tolerance of S. cerevisiae S1 at high temperature (Table 3). At 40ºC, when soy flour was supplemented, the duration of 100% viability was extended from 72 to 86h. At 45ºC the same was increased from 10 to 16h by soy flour supplementation. The soy flour supplement was not effective at 50 and 55ºC. The increase in the viability at 40 and 45ºC by soy flour supplementation suggests that S. cerevisiae S1 cells required additional nutrients to withstand high temperature. Addition of 1% yeast extract and 1µmol oleic acid (ml medium-1) has eliminated the initial death phase of S. cerevisiae at 40ºC (42). Soybean flour contains 38% proteins and 21% lipids (44), and of the lipids appreciable amount (26%) of oleic acid is present (3). Therefore the effect of soy flour could be linked to its oleic acid content. Beneficial effects of soybean on viability of cells in high gravity molasses at high temperature was reported (10). One effect of heat is to induce nutritional requirements for lipids (44). Therefore improvement in thermo-tolerance by soy flour supplementation could be due to its unsaturated fatty acid content. Since soy flour has improved the thermo-tolerance of S. cerevisiae S1, its effect on osmo- tolerance was determined.
Soy flour supplementation on osmo-tolerance
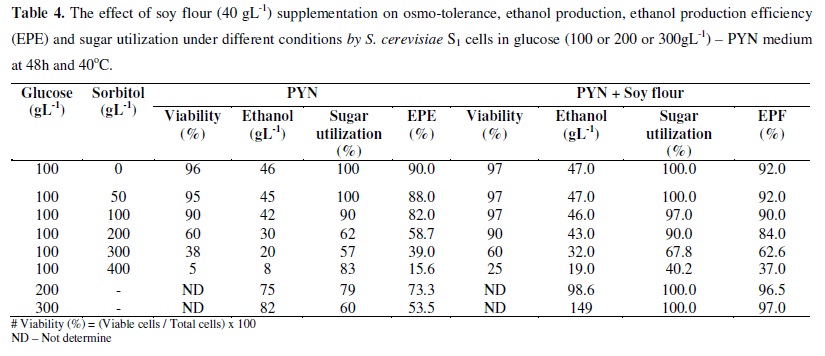
The osmo-tolerance of S. cerevisiae S1 in presence of different concentrations of (0-400gl-1) of non-metabolizable sugar sorbitol (44) was determined.
The viability and ethanol producing ability of S. cerevisiae S1 were significantly not affected by sorbitol up to 100gl-1. With 200gl-1 sorbitol (containing 100gl-1 glucose), the viability and the ethanol production were reduced to 60% and 30gl-1 respectively (Table 4). Further increase in sorbitol exerted severe effect on both viability and ethanol production. Therefore the results suggest that high sugar levels caused the death of the cells along with decreasing ethanol producing ability, i.e. both the aerobic and anaerobic metabolisms of the yeast were affected. Increase in solute concentration results in increased osmotic pressure and decreased water activity (14, 44). It has been previously reported that the increase in the osmotic pressure of the medium can decrease the viability and fermentative ability, in addition to the intracellular ethanol (44).
Supplementation of soy flour increased the viability and fermentative ability of S. cerevisiae S1 at high sugar levels (Table 4). Therefore the additional nutrients support S. cerevisiae S1 to combat the osmotic pressure and / or water stress.
The sugar utilization and ethanol production efficiency of S. cerevisiae S1 are higher in sorbitol (100gl-1 )- glucose (100gl-1) PYN medium than glucose (200gl-1) PYN (without sorbitol) (Table 5). However, when these media were supplemented with soy flour, increased sugar utilization and ethanol production efficiency were observed with glucose (200gl-1) PYN (without sorbitol) medium. The same is true when either 200gl-1 sorbitol or 200gl-1 glucose was added to glucose (100gl-1) PYN medium (Table 4). From the results it can be concluded that the inhibitory effect given by metabolizable sugar was more than that of non-metabolizable sugar and this effect could be rectified by adding soy flour (40.0gl-1) to PYN medium. The studies showed that both viability and ethanol fermentative ability of S. cerevisiae S1were affected by increasing the osmotic pressure and these effects were reduced by the soy flour supplementation. A study was carried out to find the tolerance property of the organism to both osmotic and ethanol stresses in presence of soy flour.
Soy flour supplementation on ethanol-tolerance
Ethanol tolerance of S. cerevisiae S1 was determined with different concentrations (0-200gl-1) of added ethanol in glucose (100gl-1) PYN medium supplementation. The effect of soy flour supplementation on ethanol tolerance was also studied. The viability was reduced with the increased added ethanol concentration. Complete cell death was observed at 48h with 150gl-1 added ethanol (Table 5). With 100gl-1 added ethanol, the viability was improved from 70 to 80% with soy flour supplementation at 48h. With 150 and 200gl-1 added ethanol, complete cell death was observed in un-supplemented media and in soy flour supplemented media 20.0 and 0.0% viability was observed respectively. The results suggested that soy flour supplementation was ineffective at and above 200gl-1 added ethanol.
Since the soy flour seems to improve the osmo- and ethanol tolerances individually another experiment was performed to see its effect on combined osmo- and ethanol tolerances of S. cerevisiae S1.
Combined effects of osmo- and ethanol stresses
Combined effect of different concentrations (0-200gl-1) of added ethanol and sorbitol (200gl-1) on S. cerevisiae S1 was studied in glucose (100gl-1) PYN and soy flour supplemented glucose (100gl-1) PYN media separately. With the increase in added ethanol amount to the glucose (100gl-1) PYN medium having 200gl-1 sorbitol, complete cell death was observed at 100gl-1 added ethanol, while 10% viability was recorded with the addition of soy flour (Table 6). In the glucose (100gl-1) PYN medium with 200gl-1 sorbitol, ethanol produced was 28 and 12gl-1 with 0 and 50gl-1 added ethanol respectively (Table 6). Hence the addition of ethanol has reduced ethanol production by 57%. However with soy flour supplementation, the ethanol produced was 42 (without added ethanol) and 30gl-1 (with 50gl-1 added ethanol). This is only a 30% decrease in ethanol production. Thus soy flour has increased the fermentative ability of the S. cerevisiae S1 under combined osmotic- and ethanol stresses. The viability also has been increased from 60 to 90 and 46 to 80 with soy flour supplementation at 0 and 50gl-1 added ethanol respectively. At higher ethanol levels (100 - 200gl-1) with sorbitol (200gl-1), even the soy flour supplementation could not enhance the viability and fermentative capacity of yeast significantly. The combined effects of osmotic- and ethanol stresses were more pronounced than their individual effects (Table 6). The results suggest that there are additional complex nutritional requirements for the survival of S. cerevisiae S1 under combined stressed conditions. During high gravity fermentation, initially high osmotic stress is experienced by the strain and as the fermentation proceeds the sugar depletes and the alcohol concentration increases. Hence as the fermentation proceeds, the strain undergoes ethanol stress. Therefore a strain with both ethanol- and osmo- tolerance is necessary to comply with the high gravity fermentation successfully.
CONCLUSION
S. cerevisiae S1 has shown viability and ethanol producing ability between 40 to 50ºC and 40 to 45ºC respectively. When the cells were given heat shock prior to inoculation, they were able to perform better and tolerate heat, ethanol and osmotic stresses by producing trehalose. The cells were able to tolerate thermo-, ethanol and osmotic stresses better in presence of soy flour than in the absence of soy flour. Therefore more detailed study has to be to be made to find the effect of soy flour on S. cerevisiae S1.
ACKNOWLEDGEMENTS
The authors thank the Sida / SAREC and IPICs, Sweden for financial assistance.
Submitted: August 16, 2010; Returned to authors for corrections: April 27, 2011; Approved: January 16, 2012.
- 1. Attfield, P.V.; Raman, A.; Northcott, C.J. (1992). Construction of Saccharomyces cerevisiae strains that accumulate relatively low concentrations of trehalose, and their application in testing the contribution of the disaccharide to stress tolerance. FEMS Microbiol. Lett 94, 271-276.
- 2. Balakumar, S.; Arasaratnam, V. (2009). Comparison of industrial scale ethanol production from palmyrah-based carbon source by commercial yeast and a mixed culture from palmyrah toddy. J. Inst. Brew 115(2), 105-109.
- 3. Balakumar, S.; Arasaratnam, V.; Balasubramaniam, K. (2001). Isolation and improvement of thermotolerant Saccharomyces cerevisiae strain. World J. Microbiol. Biotechnol. 17(7), 739-746.
- 4. Budavari, S.; O* Neil, M.J.; Simith, A.; Heckelman, P.E.; Kinneary, J.F. (1996). The Merk Index 12th Edn, Merk. Research Laboratories. Merk & Co., Inc. p.1492.
- 5. Canamas, T.P.; Vinas, I.; Usall, J.; Magan, N.; Solsona, C.; Teixido, N. (2008). Impact of mild heat treatments on induction of thermotolerance in the biocontrol yeast Candida sake CPA-1 and viability after spray-drying. J. Appl. Microbiol 104, 767-775.
- 6. Cavicchioli, R.; Watson, K. (1986). Loss of heat-shock acquisition of thermotolerance in yeast is not correlated with loss of heat-shock proteins, FEBS Lett. 207(1), 149-152.
- 7. Crowe, H.J.; Crowe, M.L.; Cahpmann, D. (1984). Preservation of membranes in anhydrobiotic organisms. Science 223, 701-703.
- 8. D'Amore, T.; Celotto, G.; Russell, I.; Stewart, G.G. (1989). Selection and optimization of yeast suitable for ethanol production at 40şC. Enzyme Microbial Technol. 11, 411-416.
- 9. D'Amore, T.; Stewart, G.G. (1987). Ethanol tolerance of yeast. Enzyme Microbial. Technol. 9, 322-330.
- 10. Dijck, P.V.; Colavizza. D.; Smet, P.; Thevelein, J.M. (1995) Differential importance of trehalose in stress resistance in fermenting and non-fermenting Saccharomyces cerevisiae cells. Appl. Environ. Microbiol. 6(1), 109 115.
- 11. Ezeogu, L.I.; Okolo, B.N. (1994). Effect of molasses concentration and medium supplementation on the adaptability of a high level ethanol tolerance palm wine Saccharomyces isolate. Biotechnol. Lett. 16 (1), 95-100.
- 12. Gancedo, C.; Flores, C-L (2004). The importance of a functional trehalose biosynthetic pathway for the life of yeasts and fungi. FEMS Yeast Res 4, 351-359.
- 13. Gancedo, C.; Serrano, R. (1989). Energy yielding metabolism. In: Rose, A.H., Harrison, J.S. (eds). The yeasts Vol. 3. Harcourt Brace Jovanovich Publishers, London, p.205-259.
- 14. Guyot, S.; Ferret, E.; Gervais, P. (2005). Responses of Saccharomyces cerevisiae to thermal stress. Biotechnol. Bioeng 92(4), 403-409.
- 15. Hallsworth, E.T. (1998). Ethanol induced water stress in yeast. J. Ferment. Bioengineer. 85 (2), 125-137.
- 16. Hottiger, T.; Boller, T.; Wiemken, A. (1987). Rapid changes of heat and desiccation tolerance correlated with changes of trehalose content in Saccharomyces cerevisiae cells subjected to temperature shifts. Federation of European Biochemical Society. 220 (1), 113-115.
- 17. Jones, R.P.; Greenfield, P.F. (1987). Ethanol and the fluidity of the yeast plasma membrane. Yeast. 3, 223-232.
- 18. Lee, Y-R.J.; Nagao, R.T.; Key, J.L. (1994). A soybean 101-kD heat shock protein complements a yeast HSP104 deletion mutant in acquiring thermotolerance. The Plant Cell 6(12), 1889-1897.
- 19. Liu, H.J.; Liu, D.H.; Zhong, J.J. (2005). Interesting physiological response of the osmophilic yeast Candida Krusei to heat shock. Enzyme Microbial. Technol. 36 (4), 409-416.
- 20. Mansure, J.J.; Souza, R.C.; Panek, A.D. (1997). Trehalose metabolism in Saccharomyces cerevisiae during alcoholic fermentation. Biotechnol. Lett. 19 (12), 1201 1203.
- 21. Mansure, J.J.C.; Panek, A.D.; Crowe, L.M.; Crowe, J.H. (1994). Trehalose inhibits ethanol effects on intact yeast cells and liposomes. Biochemica Biophysica Acta. 1191, 309-316.
- 22. Maranon, I.M.; Chaudanson, N.; Joly, N.; Gervais, P. (1999). Slow heat rate increases in yeast thermotolerance by maintaining plasma membrane integrity. Biotechnol. Bioeng 65(2), 176-181.
- 23. McAlister, L.; Finkelstein, D.B. (1980). Heat shock proteins and thermal resistance in yeast. Biochem. Biophys. Res. Commun. 93(3), 819-824.
- 24. Miller G.L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 81, 426-428.
- 25. Odemeru, J,D.; Amore, T.; Russel, I.; Steward, G.G. (1993). Alterations in fatty acid composition and trehalose concentration of Saccharomyces brewing strains in response to heat and ethanol shock. J. Ind. Microbiol. 11, 113-199.
- 26. Ogawa, Y.; Nitta, A.; Uchiyama, H.; Imamura, T.; Shimoi, H.; Ito, K. (2000). Tolerance mechanism of the ethanol-tolerant mutant of sake yeast. J. Bioscience and Bioengineering. 90(3), 313- 320.
- 27. Parsell, D.A.; Taulien, J.; Lindquist, S. (1993). The role of heat-shock proteins in thermotolerance. J. Biol. Sc. 339(1289), 279-286.
- 28. Peres, M.F.S.; Laluce, C. (1998). Ethanol tolerance of thermotolerant yeasts cultivated on mixtures of sucrose and ethanol. J. Ferment. Bioengineer. 85(4), 388-397.
- 29. Piper, P.W. (1993). Molecular events associated with acquisition of heat tolerance by the Saccharomyces cerevisiae FEMS Microbiol. Rev. 11, 339-356.
- 30. Ribeiro, M.J.S.; Reinders, A.; Boller, T.; Wiemken, A.; Virgilio, C.D. (1997). Trehalose synthesis is important for the acquisition of termotolerance in Schizosaccharomyces pombe Mol. Microbiol 25(3), 571-581.
- 31. Riberio, M.J.S., Leao, L.S.C., Morais, P.B., Rosa, C.A. and Panek, A.D. (1999). Trehalose accumulation by tropical yeast strains submitted to stress conditions. Antonie van Leeuwenhoek. 75, 245-251.
- 32. Sami, M.; Ikeda, M.; Yabuuchi, S. (1994). Evaluation of the alkaline methylene blue staining method for yeast activity determination. J. Ferment. Bioengineer. 78(3), 212 216.
- 33. Sharma, S.C. (1997). A possible role of trehalose in osmotolerance and ethanol tolerance in Saccharomyces cerevisiae FEMS Microbiol. Lett 152, 11-15.
- 34. Simola, M.; Hanninen, A.L.; Stranius, A-M.; Makarow, M. (2000). Trehalose is required for conformational repair of heat-denatured proteins in the yeast endoplasmic reticulum but for maintenance of membrane traffic functions after severe heat stress. Mol. Microbiol 37(1), 42-53.
- 35. Singer, M.A.; Lindquist, S. (1998). Thermotolerance in Saccharomyces cerevisiae: the Yin and Yang of trehalose. Trends Biotechnol. 16, 460-468.
- 36. Smith, B.J.; Yaffe, M.P. (1991). Uncoupling thermotolerance from the induction of heat shock proteins. Proc. Natl. Acad. Sci., USA. 88(24), 11091-11094.
- 37. Spiro, R.G. (1966). Analysis of sugars found in glycoprotein. Complex carbohydrates. In: Neufeld, E.F.; Ginsburg, V (Eds). Methods in Enzymology Vol. 8 Academic Press, New York. p. 3-5.
- 38. Thermotolerance and trehalose accumulation induced by heat shock in yeast cells of Candida albicans FEMS Microbiol. Lett 146:65-71.
- 39. Trevalelyan, W.E.; Harrison, J.S. (1956). The trehalose content of Baker's yeast during anaerobic fermentation. Biochem. J 62, 177-182.
- 40. Varly, H.A.; Gowenlocki, H.; Bell, M. (1980). Practical Clinical Biochemistry. Vol. 2, 5th Edn. William Heiemann Medical Books Ltd, London. p. 312 313.
- 41. Virgilio, C.D.; Piper, P.; Boller, T.; Wiemken, A. (1991). Acquisition of thermotolerance in Saccharomyces cerevisiae without heat shock protein hsp 104 and in the presence of protein synthesis. FEBS Lett. 288(1, 2), 86-90.
- 42. Virgilio, C.D.; Simmen, U.; Hottiger, T.; Boller, T.; Wiemken, A. (1990). Heat shock induces enzymes of trehalose metabolism, trehalose accumulation and thermotolerance in Schizosaccharomyces pombe, even in the presence of cycloheximide. FEBS Lett 273(1, 2), 107-110.
- 43. Walton, E.F.; Pringle, J.R. (1980). Effect of growth temperature upon heat sensitivity in Saccharomyces cerevisiae. Arch. Microbiol. 124, 285-287.
- 44. Watson. K. (1987). Temperature relations. The Yeasts, Vol. 3. Rose, A.H.; Harrision, J.S. (eds). Harcout Brace Jovanovich Publishers, London. p. 41-71.
Publication Dates
-
Publication in this collection
02 May 2012 -
Date of issue
Mar 2012
History
-
Received
16 Aug 2010 -
Accepted
16 Jan 2012 -
Reviewed
27 Apr 2011