Abstract
Staphylococcus aureus (S. aureus) is an important human pathogen, which commonly causes the acquired infectious diseases in the hospital and community. Effective and simple antibiotic treatment against S. aureus-related disease becomes increasingly difficult. Developing a safe and effective vaccine against S. aureus has become one of the world's hot spots once again. The key issue of developing the vaccine of S. aureus is how to find an ideal key pathogenic gene of S. aureus. It was previously suggested that EsxA might be a very important factor in S. aureus abscess formation in mice, but clinical experimental evidence was lacking. We therefore expressed EsxA protein through prokaryotic expression system and purified EsxA protein by Ni-affinity chromatography. ELISA was used to detect the anti-EsxA antibodies in sera of 78 patients with S. aureus infection and results showed that the anti-EsxA antibodies were positive in the sera of 19 patients. We further analyzed the EsxA positive antibodies related strains by antimicrobial susceptibility assay and found that all of the corresponding strains were multi-drug resistant. Among those multi-drug resistant strains, 73.7% were resistant to MRSA. The results indicated EsxA is very important in the pathogenesis of S. aureus. We suggested that the EsxA is very valuable as vaccine candidate target antigens for prevention and control of S. aureus infection.
S. aureus; esxA; anti-EsxA antibodies; multi-drug resistant
EsxA might as a virulence factor induce antibodies in patients with Staphylococcus aureus infection
Huiqin ZhouI; Hong DuI; Haifang ZhangII; Haiying ShenI; Ruhong YanI; Yun HeI; Min WangI; Xueming ZhuI
IDepartment of Clinical Laboratory, The Second Affiliated Hospital of Soochow University, Suzhou, Jiangsu, P.R. China
IIDepartment of Biochemistry and Molecular Biology, School of Medical Technology, Jiangsu University, Zhenjiang, Jiangsu, P.R. China
Send correspondence to Send correspondence to: H. Du Department of Clinical Laboratory The Second Affiliated Hospital of Soochow University 1055 Sanxiang Road, 215004 Jiangsu, Suzhou, P.R. China E-mail: hong_du@126.com
ABSTRACT
Staphylococcus aureus (S. aureus) is an important human pathogen, which commonly causes the acquired infectious diseases in the hospital and community. Effective and simple antibiotic treatment against S. aureus-related disease becomes increasingly difficult. Developing a safe and effective vaccine against S. aureus has become one of the world's hot spots once again. The key issue of developing the vaccine of S. aureus is how to find an ideal key pathogenic gene of S. aureus. It was previously suggested that EsxA might be a very important factor in S. aureus abscess formation in mice, but clinical experimental evidence was lacking. We therefore expressed EsxA protein through prokaryotic expression system and purified EsxA protein by Ni-affinity chromatography. ELISA was used to detect the anti-EsxA antibodies in sera of 78 patients with S. aureus infection and results showed that the anti-EsxA antibodies were positive in the sera of 19 patients. We further analyzed the EsxA positive antibodies related strains by antimicrobial susceptibility assay and found that all of the corresponding strains were multi-drug resistant. Among those multi-drug resistant strains, 73.7% were resistant to MRSA. The results indicated EsxA is very important in the pathogenesis of S. aureus. We suggested that the EsxA is very valuable as vaccine candidate target antigens for prevention and control of S. aureus infection.
Key words: S. aureus, esxA, anti-EsxA antibodies, multi-drug resistant.
Introduction
Staphylococcus aureus (S. aureus) is an important human pathogen that causes the acquired infectious diseases in the hospital and community (Lowy, 1998; Talia et al., 2011). The increasing incidence of S. aureus infections through both the healthcare and community settings, are rapidly promoting S. aureus to acquire the antibiotic resistance to both first-line and more novel antibiotics. The number of antibiotic resistance isolates of S. aureus is rapidly increasing (Bal and Gould, 2005; Cunha and Pherez, 2009; Hidron et al., 2008; Kirby et al., 2009; Skiest, 2006). Of which, the methicillin-resistant S. aureus (MRSA) is the most important and the morbidity and mortality of these infectious diseases caused by MRSA is very high. Relying solely on the antibiotic therapy for S. aureus-related disease becomes increasingly difficult. Therefore, to develop effective and safe vaccine of S. aureus has once again become one of the world's hot spots. Thus far there is no very ideal S. aureus vaccine for clinical application. To seek the ideal target genes is the key to develop effective and safe vaccine of S. aureus.
S. aureus pathogenesis in the host relies on the secretion of virulence factor through the secretion system (Abdallah et al., 2007; Jett and Gilmore, 2002; Sibbald et al., 2006). It was reported that the recently named type VII secretion system(T7SS) was present in S. aureus (Abdallah et al., 2007; Jett and Gilmore, 2002; Sibbald et al., 2006).
The T7SS was first found in the Mycobacterium tuberculosis, and the T7SS could secret ESAT-6 (early secreted antigen target 6 kDa) which could trigger cell-mediated immune response in host (Pallen, 2002; Stanley et al., 2003). The T7SS of S. aureus has the ability to secret ESAT-6 like proteins EsxA and EsxB to the extracellular surroundings (Burts et al., 2005). The locus of gene esxA and esxB is arranged with other six genes in the Ess gene cluster. Some genes in this gene cluster such as essA, essB, and essC are necessary for the synthesis and secretion of EsxA and EsxB. It was reported that the secretion of EsxA and EsxB was prevented in the absent of essA, essB, and essC (Burts et al., 2005).
The abscess formation is the most important for the virules of S. aureus (Cheng et al., 2009; Dinges et al., 2000; Novick, 2003). There have been shown that S. aureus strains were reduced obviously capacity of the formation abscesses in the infection process of mice only when sortase mutants defective (Jonsson et al., 2002; Mazmanian et al., 2000). However it was recently reported that the esxA mutant strain show the obvious defect in the formation of abscess in infected mice (Burts et al., 2005), and this suggests that EsxA may play an important role in the process of the pathogenesis for S. aureus. Therefor, EsxA was hopeful to become the candidate antigen for the development of S. aureus vaccine.
In this study, S. aureus was isolated from the clinical specimens of hospitalized patients who came from 10 different ward areas and the antimicrobial susceptibility assay was determined according to 2010 CLSI recommendations (Clinical and Laboratory Standard Institute, 2010). At the same time, the sera of the patients with S. aureus infection were collected. And then the protein EsxA of S. aureus was prepared and used as the antigen to detect the anti-EsxA antibodies in the serum of the patients with S. aureus infection by the indirect ELISA.
Materials and Methods
Collection of strains and serum
The Second Affiliated Hospital of Soochow University (1231 beds) is one of the largest hospital in Suzhou, China. Isolates of S. aureus were obtained from the clinical specimens of hospitalised patients from June 2010 to April 2011. Isolates were confirmed as S. aureus using a Staph SPA agglutination kit, Gram's stain and Phoenix System100 BD Automated Microbiology analyser (BD Diagnostics, USA). At same time, 78 clinical sera were obtained from the coherent patients with S. aureus infection. The S. aureus isolates were mainly associated with lung infection and pyogenic soft-tissue infection and pyogenic post-operative wound surface infections in patients from 10 different ward areas such as the intensive care unit. Every S. aureus strain was isolated from different patients, one strain was corresponding to one patient. Fifty negative control sera were collected from the hospital medical center healthy people.
Antimicrobial susceptibility
Isolates of S. aureus were inoculated onto the Phoenix panel according to the manufacturer's instructions and then the identification and antimicrobial susceptibility of these isolates were determined by Phoenix System-100 BD Automated Microbiology (BD Diagnostics, USA). Results of Minimum Inhibitory Concentrations (MICs) were recorded according to 2010 CLSI criteria (Clinical and Laboratory Standard Institute, 2010). Methicillin-resistant Staphylococcus aureus (MRSA) was confirmed if MIC of oxacillin > 4 µg/mL. S. aureus ATCC 29213 were used as a quality control strain for antimicrobial susceptibility testing.
Preparation of the protein EsxA of S. aureus
The esxA gene was amplified with the following primer pairs: 5'-GCGGATCCATGGCAATGATTAAGA TGAG-3' and 5'-AACTCGAGTTGCAAACCGAAATT ATTAG-3'. The PCR products were cloned into the pGEM-T Easy vector to yield plasmids pGEM-esxA. The gene was cut out from the plasmid with the restriction endonucleases BamH I and Xho I and cloned into the pET-28a vector to generate pET-28a-esxA, verified by sequencing esxA gene. Plasmid pET-28a-esxA was designed for the heterologous protein expression in E. coli Rosetta (Ros) to synthesize the N-terminal histidine-tagged recombinant proteins. E. coli Ros cells carrying pET28a-esxA were grown in LB medium containing ampicillin (100 µg/mL) at 37 ºC for 16 h. An aliquot (1 mL) of an overnight culture was used to inoculate 100 mL of the same medium and incubated at 37 ºC with shaking (250 rpm) until the OD600 of culture reached 0.6. Expression of the recombinant proteins was induced for 3 h at 37 ºC by the addition of IPTG to a final concentration of 100 µM. Cells were harvested by centrifugation at 6,000 g at 4 ºC for 10 min, and resuspended in 10 mL of binding buffer (5 mM imidazole, 0.5 M NaCl, and 5 mM Tris-HCl; pH 7.9). The recombinant proteins EsxAhis6 carring 6 His tag were purified from cell lysate fraction by affinity chromatography with Ni2+-NTA system (QIAGEN, Cologne, Germany) according to the manufacturer's protocol. After extensive washing, the bound proteins were eluted with 5 mM Tris-HCl buffer (pH 7.9) containing 0.2 M imidazole and 0.15 M NaCl. Purified proteins were identified by Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in 12% polyacrylamide gels and stained with Coomassie brilliant blue. Protein concentration was determined by a protein assay reagent (Bio-Rad, California, USA) and with bovine serum albumin as the standard.
Detection of the anti-EsxA antibody in serum by the indirect ELISA
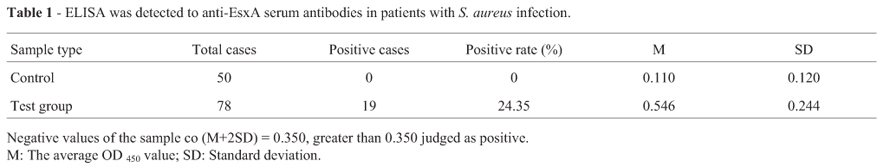
The purified EsxA antigen was diluted to 20 ng/µL. The 96-well ELISA plates were coated with the above diluted EsxA antigen by 10 µL per well, and then put at 4 ºC overnight. The ELISA plates were washed with washing Phosphate Buffered Saline Tween-20 (PBST) three times, each for min dried through pat, and then add 10 µL 10% fetal calf serum into each well. After incubated for1hat room temperature, the ELISA plates were washed as above, and then add 10 µL tested serum into each well. After incubated at 37 ºC for 1 h by the water bath, the ELISA plates were washed as above too. Then, add 10 µL horseradish peroxidase labeled goat anti-human HRP-IgG into each well, and incubate the ELISA plates at 37 ºC for1hbywater bath. At last, wash the ELISA plates with buffer PBST five times, each for min then pat the plates for dry; add substrate buffer A, B the role of 5 µL into each well for 30 min, and then add 2 mol/L sulfuric acid 10 µL into each well to stop the reaction. Absorbance values (OD450) were measured by Microplate Reader Model 680 BIO-RAD Japan). Each experiment was performed three times. The anti-EsxA antibodies in 50 healthy human sera were tested by indirect ELISA assay and calculated the average OD450 value mean (M) and Standard deviation (SD). The value of M+2SD was defined as the threshold value. When the OD450 value of the tested serum was greater than M+2SD, the sample was classified as a positive sample.
Results and Discussion
S. aureus, one of the most important pathogens mainly spread in the community and hospital, can cause superficial infections, osteomyelitis, pneumonia, septic arthritis, endocarditis, meningitis, and even can lead to sepsis or other systemic infection (Lowy, 1998). In recent years, due to the overuse of antibiotics, a variety of new strains of antibiotic resistant have appeared, such as MRSA strain (Coia et al., 2006; Haamann et al., 2011; Muto, 2006; Salgado and Farr, 2006) and new-found vancomycininsensitive S. aureus (Vancomysin-intermediate S. ureus, VISA) strain (Jones et al., 2008; Woodford and Livermore, 2009). Those drug-resistant strains make it more difficult to treat S. aureus infection. There was reported that S. aureus whole-cell vaccine ineffective in clinical, and thus development of effective of vaccine should to find other antigens which could stimulate protective antibodies (Lee, 1998; Watson and Kennedy, 1981), but some clinical study results about S. aureus vaccine developed based on some antigens such as capsular polysaccharide antigen were not satisfactory (Fattom et al., 1996; Rupp et al., 2007; Shinefield, 2006). So far, there was no ideal of S. aureus vaccine.
This study constructed EsxA prokaryotic expression system successfully. E. coli Ros cells harboring pET-28aEsxA was grown in liquid medium at 37 ºC for 3 h in the presence of 0.1 mmol/L IPTG. One predominant band corresponding to the molecular mass of approximately 16 kDa was observed in the crude extract of IPTG-induced E. coli (Figure 1). The recombinant protein in the crude extract was purified by nickel-chelate column chromatography. The purified EsxA was apparently homogenous in the 16 kD protein bands as judged by SDS-PAGE (Figure 1). As shown in Figure 1, EsxA was purified to near homogeneity by Ni2+-NTA, consistent with the experimental design. In order to understand EsxA might be as a virulence factor inducing the production of anti-EsxA antibodies in patients with Staphylococcus aureus infection. We further used the purified EsxA protein antigen to detect the anti-EsxA antibodies in serum of patients with S. aureus infection by indirect ELISA. The antibody level of EsxA in 50 healthy serum samples was tested and the mean OD450 value (M) and standard deviation (SD) were 0.110 and 0.120, respectively. The value of M+2SD was defined as cutoff which was 0.350. It meant that the tested sample was anti-EsxA antibody positive when OD450 value was greater than 0.350. Among the tested 78 clinical samples, 19 samples were positive and the positive rate was 24.35%. The result was shown in Table 1.
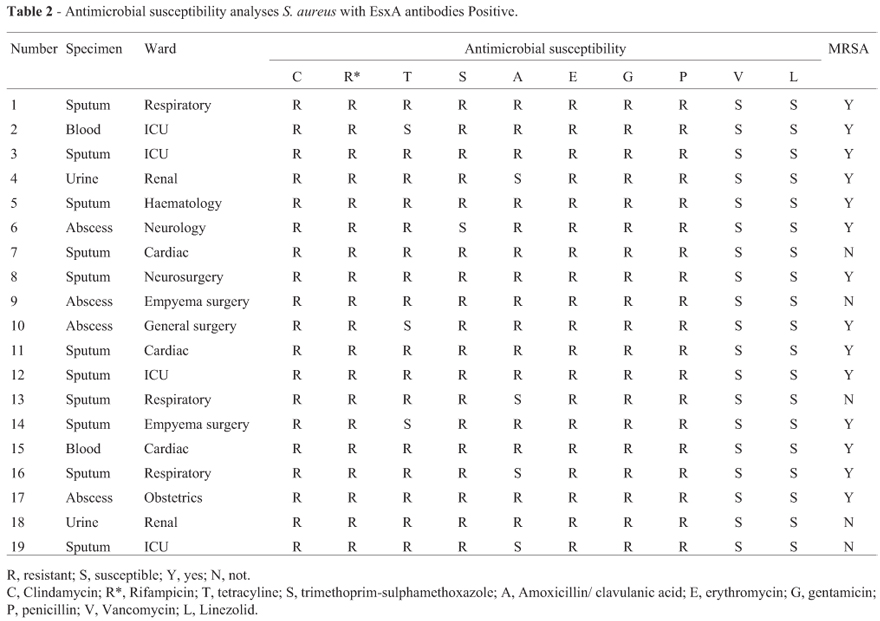
These results indicated that EsxA might be as a virulence factor inducing antibodies in patients with Staphylococcus aureus infection. To our best knowledge, this is the first study to show that EsxA might be a virulence factor and induce the production of anti-EsxA antibodies in patients with S. aureus infection. In addition, we further analyzed antimicrobial susceptibility with the EsxA serum antibodies related strains by Phoenix System-100 BD Automated Microbiology, and found that the corresponding positive strains all were multi-drug resistant, of which MRSA to 73.7% (shown in Table 2).
In summary, we successfully detected the EsxA antibody in the serum of clinical S. aureus infectious patients, and found that EsxA were present in multiple drug-resistant strains, especially MRSA strains. Above all, the results indicated that EsxA might as a virulence factor induce antibodies in patients with Staphylococcus aureus infection and suggested that EsxA maybe is a very valuable candidate target antigens of the new vaccine for prevention and control of S. aureus infection, but it needs further researches to clarify
Acknowledgments
This work was supported by Science Foundation of Jiangsu Province Health Department (H201014).
Submitted: August 28, 2011
Approved: July 2, 2012
All the content of the journal, except where otherwise noted, is licensed under a Creative Commons License CC BY-NC.
- Abdallah AM, Gey van Pittius NC, Champion PA, Cox J, Luirink J, Vandenbroucke-Grauls CM, Appelmelk BJ, Bitter W (2007) Type VII secretion-mycobacteria show the way. Nat Rev Microbiol 5:883-891.
- Bal AM, Gould IM (2005) Antibiotic resistance in Staphylococcus aureus and its relevance in therapy. Expert Opin Pharmacother 6:2257-2269.
- Burts ML, Williams WA, DeBord K, Missiakas DM (2005) EsxA and EsxB are secreted by an ESAT-6-like system that is required for the pathogenesis of Staphylococcus aureus infections. Proc Natl Acad Sci USA 102:1169-1174.
- Cheng AG, Kim HK, Burts ML, Krausz T, Schneewind O, Missiakas DM (2009) Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J 23:3393-3404.
- Clinical and Laboratory Standard Institute (2010) Performance Standards for Antimicrobial Susceptibility Testing; Twenty-First Informational Supplement M100-S20. CLSI, Wayne, pp 60-75.
- Coia JE, Duckworth GJ, Edwards DI, Farrington M, Fry C, Humphreys H, Mallaghan C, Tucker DR (2006) Guidelines for the control and prevention of meticillin-resistant Staphylococcus aureus (MRSA) in healthcare facilities. J Hosp Infect 63:S1-44.
- Cunha BA, Pherez FM (2009) Daptomycin resistance and treatment failure following vancomycin for methicillin-resistant Staphylococcus aureus (MRSA) mitral valve acute bacterial endocarditis (ABE). Eur J Clin Microbiol Infect Dis 28:831-833.
- Dinges MM, Orwin PM, Schlievert PM (2000) Exotoxins of Staphylococcus aureus Clin Microbiol Rev 13:16-34.
- Fattom AI, Sarwar J, Ortiz A, Naso R (1996) Staphylococcus aureus capsular polysaccharide (CP) vaccine and CP-specific antibodies protect mice against bacterial challenge. Infect Immun 64:1659-1665.
- Haamann F, Dulon M, Nienhaus A (2011) MRSA as an occupational disease: A case series. Int Arch Occup Environ Health 84:259-266.
- Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK (2008) NHSN annual update: Antimicrobial-resistant pathogens associated with healthcareassociated infections: Annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect Control Hosp Epidemiol 29:996-1011.
- Jett BD, Gilmore MS (2002) Internalization of Staphylococcus aureus by human corneal epithelial cells: Role of bacterial fibronectin-binding protein and host cell factors. Infect Immun 70:4697-4700.
- Jones RN, Ross JE, Castanheira M, Mendes RE (2008) United States resistance surveillance results for linezolid (LEADER Program for 2007). Diagn Microbiol Infect Dis 62:416 -426.
- Jonsson IM, Mazamanian SK, Schneewind O, Verdrengh M, Bremell T, Tarkowski A (2002) On the role of Staphylococcus aureus sortase and sortase-catalyzed surface protein anchoring in murine septic arthritis. J Infect Dis 185:1417-1424.
- Kirby A, Mohandas K, Broughton C, Neal TJ, Smith GW, Pai P, Nistal DPC (2009) In vivo development of heterogeneous glycopeptide-intermediate Staphylococcus aureus (hGISA), GISA and daptomycin resistance in a patient with methicillin-resistant S. aureus endocarditis. J Med Microbiol 58:376-380.
- Lee JC (1998) An experimental vaccine that targets staphylococcal virulence. Trends Microbiol 6:461-463.
- Lowy FD (1998) Staphylococcus aureus infections. N Engl J Med 339:520-532.
- Mazmanian SK, Liu G, Jensen ER, Lenoy E, Schneewind O (2000) Staphylococcus aureus sortase mutants defective in the display of surface proteins and in the pathogenesis of animal infections. Proc Natl Acad Sci USA 97:5510-5515.
- Muto CA (2006) Methicillin-resistant Staphylococcus aureus control: We didn't start the fire, but it's time to put it out. Infect Control Hosp Epidemiol 27:111-115.
- Novick RP (2003) Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol 48:1429-1449.
- Pallen MJ (2002) The ESAT-6/WXG100 superfamily -And a new Gram-positive secretion system? Trends Microbiol 10:209-212.
- Rupp ME, Holley HP, Lutz J, Dicpinigaitis PV, Woods CW, Levine DP, Veney N, Fowler VGJ (2007) Phase II, randomized, multicenter, double-blind, placebo-controlled trial of a polyclonal anti-Staphylococcus aureus capsular polysaccharide immune globulin in treatment of Staphylococcus aureus Bacteremia. Antimicrob Agents Chemother 51:4249-4254.
- Salgado CD, Farr BM (2006) What proportion of hospital patients colonized with methicillin-resistant Staphylococcus aureus are identified by clinical microbiological cultures? Infect Control Hosp Epidemiol 27:116-121.
- Shinefield HR (2006) Use of a conjugate polysaccharide vaccine in the prevention of invasive staphylococcal disease: Is an additional vaccine needed or possible? Vaccine 24:65-69.
- Sibbald MJ, Ziebandt A, Engelmann S, Hecker M, De Jong A, Harmsen HJ, Raangs GC, Stokroos I, Arends JP, Dubois JY, van DJM (2006) Mapping the pathways to staphylococcal pathogenesis by comparative secretomics. Microbiol Mol Biol Rev 70:755-788.
- Skiest DJ (2006) Treatment failure resulting from resistance of Staphylococcus aureus to daptomycin. J Clin Microbiol 44:655-656.
- Stanley SA, Raghavan S, Hwang WW, Cox JS (2003) Acute infection and macrophage subversion by Mycobacterium tuberculosis require a specialized secretion system. Proc Natl Acad Sci USA 100:13001-13006.
- Talia JM, Debattista NB, Pappano NB (2011) New antimicrobial combinations: Substituted chalcones-oxacillin against methicillin resistant Staphylococcus aureus Braz J Microbiol 42:470-475.
- Watson DL, Kennedy JW (1981) Immunisation against experimental staphylococcal mastitis in sheep-effect of challenge with a heterologous strain of Staphylococcus aureus Aust Vet J 57:309-313.
- Woodford N, Livermore DM (2009) Infections caused by Grampositive bacteria: A review of the global challenge. J Infect 59:S4-16.
Publication Dates
-
Publication in this collection
09 Apr 2013 -
Date of issue
2013
History
-
Received
28 Aug 2011 -
Accepted
02 July 2012