Abstract
We here identified for the first time the presence of Mycobacterium avium paratuberculosis (MAP) sheep (S) strain in Argentina. IS900 polymerase chain reaction (PCR) was positive. The S strain was compared with MAP cattle (C) strains by using IS1311 PCR-restriction endonuclease analysis (PCR-REA), multiplex PCR and restriction fragment length polymorphism (RFLP) analysis.
paratuberculosis; sheep; typing; PCR; PCR-REA
First identification of Mycobacterium avium paratuberculosis sheep strain in Argentina
G.E. TraveríaI; M. ZumarragaII; I. EtchechouryII; M.I. RomanoII; A. CataldiII; M.F. Alvarado PinedoI; I. PavlikIII; R. PribylovaIII; J.R. RomeroI
IVeterinary Center of Diagnostic and Research, Faculty of Veterinary Science, University of La Plata, Chascomús, Buenos Aires, Argentina
IIBiotechnology Institute, National Institute of Agricultural Technology, Castelar, Argentina
IIIVeterinary Research Institute, Hudcova, Brno, Czech Republic
Correspondence Correspondence: G.E. Travería Veterinary Center of Diagnostic and Research, Faculty of Veterinary Science, University of La Plata 7130 Chascomús Buenos Aires, Argentina E-mail: traveria@fcv.unlp.edu.ar
ABSTRACT
We here identified for the first time the presence of Mycobacterium avium paratuberculosis (MAP) sheep (S) strain in Argentina. IS900 polymerase chain reaction (PCR) was positive. The S strain was compared with MAP cattle (C) strains by using IS1311 PCR-restriction endonuclease analysis (PCR-REA), multiplex PCR and restriction fragment length polymorphism (RFLP) analysis.
Key words: paratuberculosis, sheep, typing, PCR, PCR-REA.
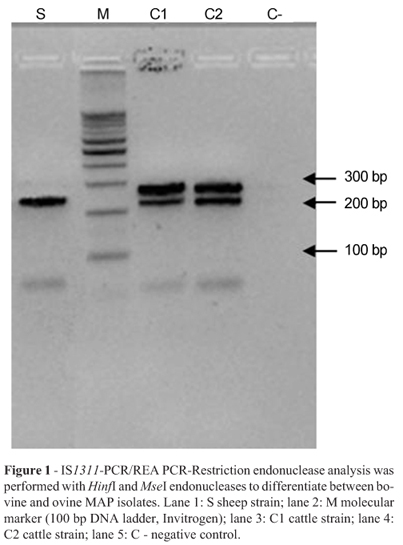
Paratuberculosis is a chronic proliferative enteritis of domestic and wild ruminants caused by Mycobacterium avium paratuberculosis (MAP). In small ruminants, progressive loss of weight is the most common clinical sign of the disease, but unlike cattle, clinically affected sheep not always present diarrhoea. Animals are usually infected through the faecal-oral route and it can take years before the onset of the clinical disease occurs (Sweeney et al., 1996). Based on restriction fragment length polymorphisms (RFLP) analysis, MAP strains have been classified into cattle (C), sheep (S) and intermediate (I) types (Collins et al., 1990; De Lisle et al., 1992; Pavlik et al., 1995, 1999). Sheep tend to be infected by the S strains (Whittington et al., 2000), which are characterised by culture requirements different from those of the C strains. By pulsed field gel electrophoresis (PFGE), MAP strains can also be divided into type I (sheep strain), type II (cattle strain) and type III (a sub-type of type I also known as intermediate strain) (De Juan et al., 2005; Stevenson et al., 2002). PCR-based methods, such as IS1311-PCR/REA targeting IS1311 after digestion using HinfI restriction nuclease (Marsh et al., 1999) or multiplex DMC-PCR assay, can also distinguish between C (type II) and S (type I/III) isolates (Collins et al., 2002). Since the first description of a sheep with paratuberculosis in Argentina (Ault, 1942), there have been no mentions on differences between isolates of MAP from sheep and cattle (Jorge et al., 2000). In the present work, blood was collected by jugular venipuncture from one sheep prior to euthanasia by intravenous injection of a sodium pentobarbital overdose (Euthanyle, 400 mg/mL, 30 mL i.v., Brower, Buenos Aires, Argentina), and then full necropsy was performed. Samples taken for analysis included faeces, ileocaecal lymph node and fragments of ileum. Faecal samples from two bovines infected with MAP were obtained from a herd with endemic paratuberculosis. After proper decontamination, five drops of faecal samples were inoculated onto each of four Löwenstein Jensen (LJ; home made) slant media supplemented with 2 mg/L of mycobactin J (Allied Monitor, Inc., Fayette, Missouri, USA), without sodium pyruvate, and onto another four Herrold egg-yolk media (HEYM) with 2 mg/L of mycobactin J and 0.4% of sodium pyruvate (Whipple et al., 1992). Tubes were incubated at 37 ºC for 52 weeks and the growth was monitored by naked eye, stereomicroscope and microscopic examination (De Juan et al., 2006; Whittington et al., 1999). Putative MAP colonies grown on solid medium from bovine and ovine isolates were subjected to PCR as described by Collins et al. (1993). IS1311 PCR/REA: amplification of the IS1311 sequence (primers in Table 1) digested with HinfI and MseI endonucleases was carried out as described by Marsh et al. (1999). Multiplex PCR: DNA from ovine and bovine samples was extracted according to Van Soolingen et al. (1991), and the PCR assay was performed as described by Collins et al. (2002); primer DMC529 had a concentration two times higher than primers DMC531 and DMC533 (primers in Table 1). The products were resolved by electrophoresis on a 2% agarose gel with 5% ethidium bromide and visualized in a Gel Doc XR documentation system (Bio-Rad, Hercules, CA, USA). RFLP: DNA was extracted as described previously (Van Soolingen et al., 1991). RFLP analysis was performed as described by Van Embden et al. (1993). RFLP profiles were analysed as described by Pavlik et al. (1999) and Green et al. (1989). At histopathology, large numbers of macrophages were seen throughout the mucosa and lamina propria with numerous acid-fast bacilli (AFB), and lymphocytes were scattered throughout the lamina propria and mucosa, as previously reported (Perez et al., 1996). A valid test was confirmed by the presence of more than one precipitin line indicating a strong positive reaction. Ovine samples were culture-positive on the LJ media with mycobactin J after six months of incubation. Colonies of the ovine strain were very small and with no pigmentation. No growth was observed in HEYM with mycobactin J. Positive culture for the bovine strains was observed after three months of incubation on Herrold's egg yolk slants. Colonies from cultures of ovine ileum and cattle faeces suspected to be MAP based on their morphology, slow growth rate and staining of acid-fast rods were confirmed by PCR on the base of the presence of IS900. Restriction endonuclease BstEII analysis showed two different profiles representing the S and C strains (Figure 1). These profiles match with those seen in earlier studies (Collins et al., 1990; Pavlik et al., 1999). Both IS1311-PCR/REA and DMCPCR classified the ovine strain as S type and the bovine isolates as C type. Examination of ileal Ziehl-Neelsen (ZN) smears effectively demonstrated abundant AFB. Histopathological findings showed diffuse inflammatory infiltrate with large numbers of macrophages. This was also reflected in the ZN sections with high numbers of AFB located intracellulary, typical of the multibacillary (lepromatous) form of paratuberculosis. These observations cor-relate with clinical signs and the presence of a high mycobacterial load in intestinal lesions, Juste et al. (1991) and Whittington et al. (1999) showed that MAP culture from sheep is possible in the case of appropriate media usage. In order to improve culture of the sheep strain isolate, LJ medium supplemented with mycobactin J and without sodium pyruvate and HEYM with sodium pyruvate and mycobactin J were used. LJ was found to support the growth of the ovine strain after approximately six months of incubation, with very small colonies observed. In HEYM, which is frequently used to isolate MAP from cattle, no growth was observed for the sheep strain after one year of incubation. The growth requirements observed in this study are in agreement with reports (De Juan et al., 2006) demonstrating different phenotypic characteristics of the S and C strains of MAP. MAP was confirmed by PCR amplification of the IS900 element. Using RFLP analysis, IS1311 PCR/REA and a multiplex DMC-PCR, the ovine strain was demonstrated to belong to the sheep S group and bovine strains to the cattle C group, confirming for the first time that both of these MAP strains are present in Argentina.
Acknowledgments
We would like to thank Dr Douglas Begg (from the Faculty of Veterinary Science, University of Sydney, Australia) for his critical reading of the manuscript and helpful suggestions and Petra Svastova (Veterinary Research Institute, Brno, Czech Republic) for technical support. The work was supported by the Ministry of Agriculture (No. MZe0002716202, QH81065) and by the Ministry of Education, Youth and Sports (AdmireVet, CZ 1.05/2.1.00/01.0006; ED0006/01/01.) of the Czech Republic.
Submitted: May 15, 2011;
Approved: September 10, 2012.
All the content of the journal, except where otherwise noted, is licensed under a Creative Commons License CC BY-NC.
- Ault CN (1942) A case of Johnes disease in sheep (in Spanish). Rev Med Vet 14:401-405.
- Collins DM, Gabric DM, De Lisle GW (1990) Identification of two groups of Mycobacterium paratuberculosis strains by restriction endonuclease analysis and DNA hybridization. J Clin Microbiol 28:1591-1596.
- Collins DM, Stephens D, De Lisle GW (1993) Comparison of polymerase chain reaction tests and faecal culture for detecting Mycobacterium paratuberculosis in bovine faeces. Vet Microbiol 36:289-299.
- Collins DM, De Zoete M, Cavaignac SM (2002) Mycobacterium avium subsp. paratuberculosis strains from cattle and sheep can be distinguished by a PCR test based on a novel DNA sequence difference. J Clin Microbiol 40:4760-4762.
- De Lisle GW, Collins DM, Huchzermeyer HF (1992) Characterization of ovine strains of Mycobacterium paratuberculosis by restriction endonuclease analysis and DNA hybridization. Onderstepoort J Vet Res 59:163-5.
- De Juan L, Alvarez J, Romero B, Bezos J, Castellanos E, Aranaz A, Mateos A, Dominguez L (2006) Comparison of four different culture media for isolation and growth of type II and type I/III Mycobacterium avium subsp. paratuberculosis strains isolated from cattle and goats. Appl Environ Microbiol 72:5927-5932.
- De Juan L, Mateos A, Domínguez L, Sharp JM, Stevenson K (2005) Genetic diversity of Mycobacterium avium subspecies paratuberculosis isolates from goats detected by pulsed-field gel electrophoresis. Vet Microbiol 106:249-57.
- Green EP, Tizard ML, Moss MT, Thompson J, Winterbourne DJ, McFadden JJ, Hermon-Taylor J (1989) Sequence and characteristics of IS900, and insertion element identified in a human Crohn's disease isolated of Mycobacterium paratuberculosis Nucleic Acids Res 17:9063-9073.
- Jorge MC, Schettino DM, Torres P, Bernardelli A (2000) First description of concomitant infection with tuberculosis and paratuberculosis in dairy sheep in Argentina. Rev Sci Tech 19:800-809.
- Juste RA, Marco JC, de Ocariz CS, Aduriz JJ (1991) Comparison of different media for the isolation of small ruminant strains of Mycobacterium paratuberculosis Vet Microbiol 28:385-390.
- Marsh I, Whittington R, Cousins D (1999) PCR-restriction endonuclease analysis for identification and strain typing of Mycobacterium avium subsp. paratuberculosis and Mycobacterium avium subsp. avium based on polymorphisms in IS1311 Mol Cell Probes 13:115-126.
- Pavlik I, Bejckova L, Pavlas M, Rozsypalova Z, Koskova S (1995) Characterization by restriction endonuclease analysis and DNA hybridization using IS900 of bovine, ovine, caprine and human dependent strains of Mycobacterium paratuberculosis isolated in various localities. Vet Microbiol 45:311-318.
- Pavlik I, Horvathova A, Dvorska L, Bartl J, Svastova P, Dumaine R, Rychlik I (1999) Standardisation of restriction fragment length polymorphism for Mycobacterium avium subspecies paratuberculosis J Microbiol Methods 38:155-167.
- Perez V, Garcia Marin JF, Badiola JJ (1996) Description and classification of different types of lesion associated with natural paratuberculosis infection in sheep. J Comp Pathol 114:107-122.
- Stevenson K, Hughes VM, de Juan L, Inglis NF, Wright F, Sharp JM (2002) Molecular characterization of pigmented and nonpigmented isolates of Mycobacterium avium subsp. paratuberculosis J Clin Microbiol 40:1798-804.
- Sweeney RW (1996) Transmission of paratuberculosis. Vet Clin North Am Food Anim Pract 12:305-12.
- Van Soolingen D, Herman PWM, De Haas PEW, Soll DR, Van Embden JDA (1991) The occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains evaluations of IS-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J Clin Microbiol 29:2578-2586.
- Van Embden JDA, Cave MD, Crawford JT, Dale JW, Eisenach KD, Gicquel B, Hermans PWM, Martin C, Mc Adam R, Shinnick TM, Small P (1993) Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for standardized methodology. J Clin Microbiol 31:406-409.
- Whittington RJ, Hope AF, Marshall DJ, Taragel CA, Marsh I (2000) Molecular epidemiology of Mycobacterium avium subsp. paratuberculosis:IS900 restriction fragment length polymorphism and IS1311 polymorphism analyses of isolates from animals and a human in Australia. J Clin Microbiol 38:3240-3248.
- Whittington RJ, Marsh I, McAllister S, Turner MJ, Marshall DJ, Fraser CA (1999) Evaluation of modified BACTEC 12B radiometric medium and solid media for culture of Mycobacterium avium subsp. paratuberculosis from sheep. J Clin Microbiol 37:1077-1083.
- Whipple DL, Kapke PA, Andersen PR (1992) Comparison of a commercial DNA probe test and three cultivation procedures for detection of Mycobacterium paratuberculosis in bovine feces. J Vet Diag Invest 4:23-27.
Correspondence:
Publication Dates
-
Publication in this collection
10 Jan 2014 -
Date of issue
Sept 2013
History
-
Received
15 May 2011 -
Accepted
10 Sept 2012