Abstract
Two compost formulations based on oat straw (Avena sativa) and brachiaria (Brachiaria sp.) were tested for the cultivation of three Agaricus bisporus strains (ABI-07/06, ABI-05/03, and PB-1). The experimental design was a 2 x 3 factorial scheme (composts x strains) with 6 treatments and 8 repetitions (boxes containing 12 kg of compost). The chemical characterization of the compost (humidity, organic matter, carbon, nitrogen, pH, raw protein, ethereal extract, fibers, ash, cellulose, hemicellulose, and lignin) before and after the cultivation of A. bisporus and the production (basidiomata mass, productivity, and biological efficiency) were evaluated. Data were submitted to variance analysis, and averages were compared by means of the Tukey's test. According to the results obtained, the chemical and production characteristics showed that the best performances for the cultivation of A. bisporus were presented by the compost based on oat and the strain ABI-07/06.
Agaricus bisporus; productivity; strains; compost; nutritional value
RESEARCH PAPER
Dynamics of the chemical composition and productivity of composts for the cultivation of Agaricus bisporus strains
Meire Cristina Nogueira de AndradeI; João Paulo Furlan de JesusII; Fabrício Rocha VieiraII; Sthefany Rodrigues Fernandes VianaII; Marta Helena Fillet SpotoIII; Marli Teixeira de Almeida MinhoniII
ICentro de Ciências Exatas e Sociais Aplicadas, Universidade do Sagrado Coração, Bauru, SP, Brazil
IIDepartamento de Produção Vegetal/Defesa Fitossanitária, Módulo de Cogumelos, Faculdade de Ciências Agronômicas,Universidade Estadual Paulista "Júlio de Mesquita Filho", Botucatu, SP, Brazil
IIIDepartamento de Agroindústria, Alimentos e Nutrição, Escola Superior de Agricultura "Luiz de Queiroz", Universidade de São Paulo, Piracicaba, SP, Brazil
Correspondence Correspondence: M.C.N. Andrade Centro de Ciências Exatas e Sociais Aplicadas Universidade do Sagrado Coração Rua Irmã Arminda 10-50, Jardim Brasil 17011-160 Bauru, SP, Brazil E-mail: mcnandrade@hotmail.com
ABSTRACT
Two compost formulations based on oat straw (Avena sativa) and brachiaria (Brachiaria sp.) were tested for the cultivation of three Agaricus bisporus strains (ABI-07/06, ABI-05/03, and PB-1). The experimental design was a 2 x 3 factorial scheme (composts x strains) with 6 treatments and 8 repetitions (boxes containing 12 kg of compost). The chemical characterization of the compost (humidity, organic matter, carbon, nitrogen, pH, raw protein, ethereal extract, fibers, ash, cellulose, hemicellulose, and lignin) before and after the cultivation of A. bisporus and the production (basidiomata mass, productivity, and biological efficiency) were evaluated. Data were submitted to variance analysis, and averages were compared by means of the Tukey's test. According to the results obtained, the chemical and production characteristics showed that the best performances for the cultivation of A. bisporus were presented by the compost based on oat and the strain ABI-07/06.
Key words:Agaricus bisporus, productivity, strains, compost, nutritional value.
Introduction
Composting is one of the crucial phases of the cultivation cycle of Agaricus sp. It is important for an appropriate balance of nutrients, especially carbon and nitrogen (Andrade et al., 2008; Minhoni et al., 2005).
Several compost formulations might be used for the production of A. bisporus. According to Miller and Macauley (1989), the components for the formulation of cultivation composts of mushrooms are arranged into three categories: complex structure and hard decomposition materials, plenty of cellulose, hemicellulose, and lignin (straws, sugarcane bagasse, and animal litter); compost activator materials, plenty of proteins, fat, and carbohydrates (urea, soy bran, cottonseeds, etc.); and inorganic conditioners (gypsum and lime).
In Brazil, the compost known as "synthetic" has been well used, mainly because it makes the addiction of manure or animal litter unnecessary, once those materials are not cheap and/or easily found (Kopytowski Filho, 2006). Thus, straw such as wheat, rice, brachiaria oat, coast-cross, and tifton associated with sugarcane bagasse and supplemented with cereal bran (soy, wheat, rice, etc.) has been successfully used in the formulations for Agaricus sp. cultivation (Andrade et al., 2008; Zied et al., 2011).
Besides the quality of the compost, the choice of the fungus strain also influences productivity. However, a few scientific studies have related the influence of fungi strains in the chemical composition of composts.
Therefore, the objective of this work was to evaluate production (basidiomata mass, productivity, and biological efficiency) and chemical characterization [organic matter, carbon, nitrogen, pH, ash, raw fiber, ethereal extract, raw protein, neutral detergent fiber (NDF), acid-detergent fiber (ADF), hemicellulose, lignin, and cellulose] of composts based on brachiaria and oat straw, during the cultivation of A. bisporus strains (ABI-07/06, ABI-05/03, and PB-1).
Materials and Methods
The experimental design was a 2 x 3 factorial scheme, containing 2 types of composts (brachiaria and oat) and 3 Agaricus bisporus strains (ABI-07/06, ABI-05/03, and PB-1), with 10 repetitions each. The experimental unit corresponded to a box containing 12 kg of wet compost. Data were submitted to variance analysis, and averages were compared by means of the Tukey's test, by using SISVAR 4.2 statistical program, developed by the Department of Mathematical Sciences from the Federal University of Lavras, Minas Gerais, Brazil (UFLA).
Spawn was prepared using the strains ABI-07/06, ABI-05/03, and PB-1. Strain ABI-07/06 came from the city of Piedade-SP, ABI-05/03 from Cabreúva-SP, and PB-1 from the Brasmicel Company (Suzano-SP). These strains were stored in CA (compost-agar) culture medium, immersed in sterilized mineral oil, and preserved at 8 °C.
The spawn used for the production of the tertiary matrix and spawn was based on sorghum grains, plaster, and lime and the ingredients used in the formulation of the composts were previously analyzed (Table 1).
Phase 1 was carried out in a sheltered room with cement floor, open sides, and natural ventilation. Before piles mounting, the grass was moistened and revolved every 2 days, totalizing 7 days of grass moistening (Table 2).
Piles mounting began with a grass layer (20 cm high), followed by a crushed sugar cane layer (20 cm high), and so on until the formation of piles that were approximately 1.8 m high and 1.8 m wide. A mixture of soy bran and urea was separately prepared and equally added between the piles layers. The irrigation was manually performed with a hose to maintain humidity around 70%. The composts were revolved every 2 days, and water was added whenever needed. In total, the piles were revolved 5 times during the 12 days of phase 1 (Table 2).
In phase 2, the composts were transferred to 56.5 x 46.5 x 28.5 cm (length, width, and height) polypropylene lattice boxes, which were randomly arranged inside the Dalsem chamber for pasteurization and the conditioning of composts (Table 2). Pasteurization was performed under 61 ± 1 °C for 6 hours and conditioning, for 11 days under 48 ± 1 °C.
The compost was transferred to smaller, polyethylene boxes, and spawned by adding 1.5 of the spawn of A. bisporus for every kilo of wet compost (12 kg of compost/box). At the end, each box was covered internally with transparent polyethylene plastic film, and the boxes were randomly disposed inside a climatic chamber (Dalsem Mushroom) at 25 ± 1 °C.
Soil red dystrophic, latossolic A moderato of medium texture from the Lageado farm (FCA/UNESP) was used as casing layer. First, pH was corrected to 7.0 by adding calcitic lime in the rate of 30 kg/ m3 of soil. Next, the soil was moistened to 60% and kept resting for 20 days. Then, 30% of charcoal was added, with a thickness of 1 to 2 cm, and the soil was submitted to pasteurization under 61 °C during 6 hours in a Dalsem Mushrooms chamber. The casing layer was manually spread over the compost. Each box had 15 kg of casing layer, resulting in a thickness of approximately 4 cm. The compost was covered with plastic and incubated for 15 days in the Dalsem chamber, at a temperature of 25 ± 1 °C.
After the colonization of the casing layer, the boxes were disposed at random on shelves inside the Dalsem Mushrooms chamber, with an average temperature maintained at 25 ± 1 °C and a relative air humidity between 75 and 85%. The irrigation of the boxes was manually performed by using a canvas hose, as well as mushrooms harvest.
Three samples of each compost were collected at the end of phases I and II of composting and at the end of the cultivation cycle (phase III), which were dehydrated in stove at 65 °C for 48 hours and ground in a knife mill, 30-mesh sieve, for chemical analyses. The Weende method, described by Silva and Queiroz (2002), was used to evaluate raw protein, ethereal extract, raw fiber, and ash. ADF (acid-detergent fiber) and NDF (neutral detergent fiber), hemicellulose, cellulose, and lignin (Klason) measurements were carried out by the Van-Soest method, described by Silva and Queiroz (2002).
The productivity (P) was expressed as the fresh weight of mushrooms (FWM) / the fresh weight of compost (FWC) x 100, and the biological efficiency (BE%) was expressed as the fresh weight of mushrooms (FWM) / the dry weight of compost (DWC) x 100 (2). FWM was determined at the end of harvesting, and FWC was determined at the end of phase II.
Results and Discussion
The average humidity content of the compost exhibited progressive reduction with time, with the highest average being observed at the end of phase I (75.3%), followed by phases II and II (69.3 and 63.7%, respectively) (Figure 1). This reduction occurred due to the different management phases during the preparation of the compost (Table 2), when straws are initially much more irrigated in pre-composting than in the subsequent phases. These results are satisfactory within a standard composting, where the humidity content of approximately 70% is recommended at the end of phase II of composting (Andrade et al., 2008).
By comparing the composts for organic matter and carbon, the oat-based compost obtained the highest averages (Figures 2 and 4 ). The compost at the end of phase II also presented higher averages of organic matter and carbon, regardless of the type of compost (Figures 3 and 5 ).
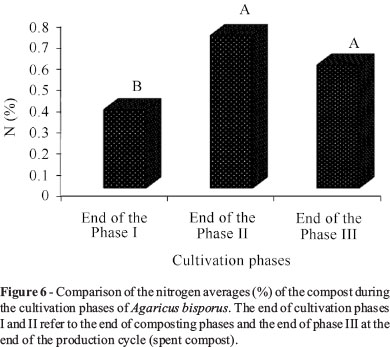
The final phases I and II of the cultivation refer to the final of the composting phases and the final of phase III at the end of the production cycle (spent compost).
Phases II and III obtained the highest averages (0.7 and 0.6%, respectively) for nitrogen contents in relation to phase I (0.4%) (Figure 6). Akinyele and Akinyosoye (2005) assign the increase of nitrogen in the compost to the extracellular enzymes produced by the fungus during the growing phase, and Sales-Campos et al. (2010) assign it to the increase of fungal mycelium in the residual substrate. Still other authors assign such an increase either to the fixation ability of N or to the presence of nitrogen-fixing bacteria (Kurtzman and Zadrazil, 1982; Bisaria et al., 1990). However, the carbohydrates consumption in general and the biomass loss in the form of CO2 are the main responsible for the elevation of the concentration of nitrogen is. Similar to this, in spite of there being a loss of nitrogen in the form of ammonia, most ends are converted into microbial protein.
The pH averages obtained in the oat-based compost did not exhibit significant differences in the cultivation phases (Figure 7). There was a significant pH reduction for the compost based on brachiaria at the end of phase II in relation to phases I and III. According to Chang and Miles (1989), that reduction possibly occurs due to the production of metabolites.
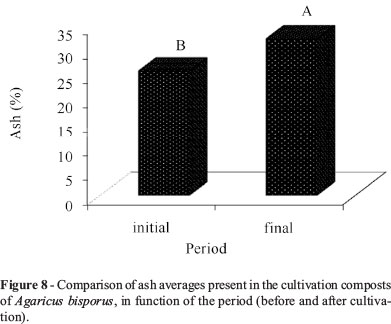
The ash content of the composts in the final period of cultivation was higher than that in the initial phase (Figure 8). Rajarathnam et al. (1992) assign that increase to a constant utilization of the organic matter by the fungus during the whole cultivation cycle and a consequent liberation of minerals to the final (residual) substrate.
There is difference between averages followed by different letters are statistically different among each other (Tukey, 5%).
The raw fiber content was higher in the initial phase than in the final phase of cultivation (Figure 9). According to Rajarathnam et al. (1992), the reduction in the fiber fraction of the substrate occurs, because the fungi responsible for the white rottenness have an affinity with residues with high lignin and cellulose contents. They use their enzymatic complex to degrade that raw material, thus allowing the nutrients present in the residue to become more absorbable by their cells.
We also found that the composts in the initial phase obtained the highest raw protein averages (Figure 10) due to their consumption by A. bisporus during its development and formation of mushrooms, thus causing a decrease in the nutritional facts of the composts as the time of cultivation increases.
We verified a reduction in NDF and ADF contents of the final compost in relation to the initial compost during the cultivation period (Figures 11 and 12). According to Castro (2003), reductions in NDF and ADF contents in the final (residual) substrate are expected, because fungi have an enzymatic ability to degrade components of the cellular wall that are present in the raw material of vegetable origin. These results agree with several other works that also reported reductions in NDF and ADF fractions in substrates treated by fungi (Belewu and Belewu, 2005; Gonçalves et al., 2010).
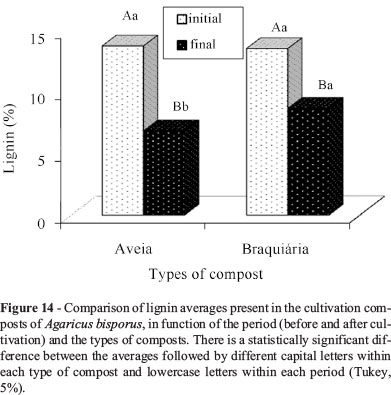
Hemicellulose, lignin, and cellulose contents of the composts were reduced along the period (Figures 13, 14 and 15). According to Andrade et al. (2010), both holocellulose (hemicellulose and cellulose set) and lignin contain carbon, hydrogen, and oxygen and serve as an energy source for fungal growth, a fact that explains their reduction along the cultivation cycle. Gonçalves et al. (2010) report that the significant reduction in lignin is related to the efficiency of the fungi to degrade that structure. In addition, sometimes, they degrade lignin rather than cellulose and hemicellulose.
In the final phase of cultivation, the compost inoculated with strain PB-1 of A. bisporus obtained the highest hemicellulose average (Figure 13). This result possibly shows that strains ABI-07/06 and ABI-05/03 exhibited a slightly more efficient degrading action of the compost at the end of the cultivation cycle.
The compost based on brachiaria presented a higher lignin average (Figure 14) during the final phase. No significant difference was observed in the composts with regard to the cellulose content at the end of the cultivation cycle (Figure 15).
Strain ABI-07/06 and the compost based on oat obtained the best production performances of A. bisporus (Tables 3 and 4).
The influence of the strain and/or compost on the production of Agaricus sp. has already been reported by several authors (Miller and Maculey, 1989; Kopytowski Filho, 2006; Baysal et al., 2007; Andrade et al., 2008; Siqueira et al., 2011). Baysal et al. (2007) evaluated the production of A. bisporus in different formulations of composts and casing layers and found that the highest mushroom productivity was obtained by wheat straw mixed with pigeon manure with peat of Caykara and perlite mixture serving as casing material.
Averages followed by different letters in the columns are statistically different among each other (Tukey, 5%).
Conclusions
According to the chemical and production characteristics, the compost based on oat and strain ABI-07/06 presented the best performances for the cultivation of A. bisporus.
Submitted: July 20, 2012;
Approved: April 4, 2013.
All the content of the journal, except where otherwise noted, is licensed under a Creative Commons License CC BY-NC.
- Akinyele BJ, Akinyosoye FA (2005) Effect of Volvariella volvaceae cultivation on the chemical composition of agrowastes. African J of Biotech 4:979-983.
- Andrade MCN, Zied DC, Minhoni MTA, Kopytowski Filho J (2008) Productivity of four Agaricus bisporus strains in three compost formulations and chemical composition analyses of the mushrooms. Braz J Microbiol 39:593-598.
- Andrade MCN, Zied DC, Minhoni MTA, Sansigolo CA (2010) Análise química da madeira e casca de diferentes tipos de eucalipto antes e durante o cultivo de shiitake em toras. Árvore 34:165-175.
- Baysal E, Yigitbasil ON, Colak M, Toker H, Simsek H, Yilmaz F (2007) Cultivation of Agaricus bisporus on some compost formulas and locally available casing materials. Part I: Wheat straw based compost formulas and locally available casing materials. African J of Biotech 6:2225-2230.
- Belewu MA, Belewu KY (2005) Cultivation of mushroom (Volvariella volvaceae) on banana leaves. African J of Biotech 4:1401-1403.
- Bisaria R, Vasudevan P, Bisaria VS (1990) Utilization of spent agro-residues from mushroom cultivation for biogas production. Appl Microb Biotech 33:606-609.
- Castro ALA (2003) Resíduo de lixadeira do algodão: Produção de cogumelo, ensilagem e alterações da composição bromatológica e degradabilidade. 69 p. (M.Sc. Dissertation. Universidade Federal de Lavras. UFLA).
- Chang ST, Miles PG (1989) Edible mushrooms and their cultivation CRC Press Inc., Boca Raton, Florida.
- Gonçalves CCM, Paiva PCA, Dias ES, Siqueira FG (2010) Avaliação do cultivo de Pleurotus sajor-caju (fries) sing. sobre o resíduo de algodão da industria têxtil para a produção de cogumelos e para alimentação animal. Ciênc Agrotec 34:220-225.
- Kopytowski Filho J (2006) Produtividade e eficiência biológica de Agaricus blazei (Murrill) Heinemann, em diferentes condições de cultivo 134 p. (Ph.D. Thesis. Faculdade de Ciências Agronômicas. Universidade Estadual Paulista. FCA/UNESP).
- Kurtzman RH, Zadrazil F (1982) Physiological and taxonomic considerations for cultivation of Pleurotus mushrooms. In: Chang ST Quimio TH (Eds.) Tropical mushrooms: Biological nature and cultivation methods, The Chinese University Press, Hong Kong, p. 299-348.
- Miller FC, Maculey BJ (1989) Substrate usage and odours in mushrooms composting. Aust J Exp Agric 29:119-124.
- Minhoni MTA, Kopytowski Filho J, Andrade MCN (2005) Cultivo de Agaricus blazei Murrill ss. Heinemann Fundação de Estudos e Pesquisas Agrícolas e Florestais, Botucatu, 141 p.
- Rajarathnam S, Shashireka MN, Bano Z (1992) Biopotentialities of basidiomacromycetes. Eur J Appl Microbiol Biotechnol 37:233-361.
- Sales-Campos C, Araujo LM, Minhoni MTA, Andrade MCN (2010) Análise físico-química e composição nutricional da matéria prima e de substratos pré e pós cultivo de Pleurotus ostreatus Interc 35:70-76.
- Silva DJ, Queiroz AC (2002) Análise de alimentos: métodos químicos e biológicos. UFV, Viçosa.
- Siqueira FG, Matos ET, Silva EG, Silva R, Dias E (2011) Biological efficiency of Agaricus brasiliensis cultivated in compost with nitrogen concentrations. Hortic Bras 29:157-161.
- Zied DC, Minhoni MTA, Kopytowski Filho J, Barbosa L, Andrade MCN (2011) Medicinal mushroom growth as affected by non-axenic casing soil. Pedosphere. 21:146-153.
Publication Dates
-
Publication in this collection
27 Mar 2014 -
Date of issue
Dec 2013
History
-
Received
20 July 2012 -
Accepted
04 Apr 2013