Abstract
Halophilic microorganisms are source of potential hydrolytic enzymes to be used in industrial and/or biotechnological processes. In the present study, we have investigated the ability of the moderately halophilic bacterium Halobacillus blutaparonensis (strain M9), a novel species described by our group, to release proteolytic enzymes. This bacterial strain abundantly proliferated in Luria-Bertani broth supplemented with 2.5% NaCl as well as secreted proteases to the extracellular environment. The production of proteases occurred in bacterial cells grown under different concentration of salt, ranging from 0.5% to 10% NaCl, in a similar way. The proteases secreted by H. blutaparonensis presented the following properties: (i) molecular masses ranging from 30 to 80 kDa, (ii) better hydrolytic activities under neutral-alkaline pH range, (iii) expression modulated according to the culture age, (iv) susceptibility to phenylmethylsulphonyl fluoride, classifying them as serine-type proteases, (v) specific cleavage over the chymotrypsin substrate, and (vi) enzymatic stability in the presence of salt (up to 20% NaCl) and organic solvents (e.g., ether, isooctane and cyclohexane). The proteases described herein are promising for industrial practices due to its haloalkaline properties.
Halobacillus blutaparonensis; halophilic bacterium; serine protease
Extracellular proteases of Halobacillus blutaparonensis strain M9, a new moderately halophilic bacterium
Anderson F. SantosI,II; Roberta S. ValleI; Clarissa A. PachecoI,III; Vanessa M. AlvarezI; Lucy SeldinI; André L.S. SantosI,II
IDepartamento de Microbiologia Geral, Instituto de Microbiologia Paulo de Góes, Centro de Ciências da Saúde, Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ, Brazil
IIPrograma de Pós-Graduação em Bioquímica, Instituto de Química, Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ, Brazil
IIIInstituto Federal de Educação, Ciência e Tecnologia do Rio de Janeiro, Rio de Janeiro, RJ, Brazil
Send correspondence to Send correspondence to: A.L.S. Santos Laboratório de Investigação de Peptidases, Departamento de Microbiologia Geral, Instituto de Microbiologia Paulo de Góes, Bloco E-subsolo, Sala 05, Centro de Ciências da Saúde, Universidade Federal do Rio de Janeiro Av. Carlos Chagas Filho 373, Cidade Universitária 21941-902, Rio de Janeiro, RJ, Brazil E-mail: andre@micro.ufrj.br, alsouzasantos@gmail.com
ABSTRACT
Halophilic microorganisms are source of potential hydrolytic enzymes to be used in industrial and/or biotechnological processes. In the present study, we have investigated the ability of the moderately halophilic bacterium Halobacillus blutaparonensis (strain M9), a novel species described by our group, to release proteolytic enzymes. This bacterial strain abundantly proliferated in Luria-Bertani broth supplemented with 2.5% NaCl as well as secreted proteases to the extracellular environment. The production of proteases occurred in bacterial cells grown under different concentration of salt, ranging from 0.5% to 10% NaCl, in a similar way. The proteases secreted by H. blutaparonensis presented the following properties: (i) molecular masses ranging from 30 to 80 kDa, (ii) better hydrolytic activities under neutral-alkaline pH range, (iii) expression modulated according to the culture age, (iv) susceptibility to phenylmethylsulphonyl fluoride, classifying them as serine-type proteases, (v) specific cleavage over the chymotrypsin substrate, and (vi) enzymatic stability in the presence of salt (up to 20% NaCl) and organic solvents (e.g., ether, isooctane and cyclohexane). The proteases described herein are promising for industrial practices due to its haloalkaline properties.
Key words: Halobacillus blutaparonensis, halophilic bacterium, serine proteases.
Introduction
Proteases are omnipresent, being found in a wide diversity of sources such as plants, animals and microorganisms. Microbial proteases are the most important industrial enzymes accounting for around 60% of the total enzyme market and they represent approximately 40% of the total enzyme production in the world (Ng and Kenealy, 1986; Rao et al., 1998; Horikoshi, 1999; Gupta et al., 2002). Proteases can be classified as acid, neutral and alkaline, based on their optimal hydrolytic activity pH range. Among those proteases, the alkaline ones are particularly relevant due to their activity and stability in high pH value (Rao et al., 1998; Gupta et al., 1999, 2002a, 2002b) and, consequently, their wide biotechnological potential for industrial sectors such as laundry detergents, leather tanning, beer, food and pharmaceutical industry (Rao et al., 1998; Schallmey et al., 2004; Fujinami and Fujisawa, 2010). Currently, a great number of commercially available alkaline proteases originate from Bacillus strains (Rao et al., 1998; Gupta et al., 1999, 2002a, 2002b; Schallmey et al., 2004; Fujinami and Fujisawa, 2010).
Over the years, Bacillus species as well as Bacillus-related genera have emerged as promising extracellular protease producers (Schallmey et al., 2004; Fujinami and Fujisawa, 2010). Extracellular proteases from moderately halophilic microorganisms have been thoroughly identified and characterized, including in Bacillus clausii I-52 (Joo et al., 2003), Bacillus subtilis FP-133 (Setyorini et al., 2006), Paenibacillus peoriae NRRL BD-62, Paenibacillus polymyxa SCE2 (Alvarez et al., 2006), Halobacillus sp. SR5-3 (Namwong et al., 2006), Halobacterium sp. SP1(1) (Akolkar et al., 2008), Virgibacillus sp. SK37 (Phrommao et al., 2011) and Geomicrobium sp. EMB2 (Karan and Khare, 2011).
Our study focused on the identification of enzymes produced by a new bacterium species belonging to the Halobacillus genus, which shows the following characteristics: moderately halophilic, Gram-positive, spore-forming, motile, strictly aerobic with rod cells and capability to grow in the absence or in the presence of NaCl. This species was named Halobacillus blutaparonensis since it was isolated from the roots of Blutaparon portulacoides (Barbosa et al., 2006) that is a coastal perennial rhizomatous herb found on the sand strip parallel to the Restinga of Jurubatiba located in the north of Rio de Janeiro State, Brazil, which is an extremely preserved area comprising a strip of sand of 44 km in length. The plant B. portulacoides can endure the salinity stress, high temperatures and tide shift exposure characteristic of the area (Bernardi and Seeliger, 1989). In addition, it is of great medical interest due to the presence of flavonoids, irisone B, sitosterol, vanillic acid, as well as stigmasterol, sitosterol and campesterol steroids (Ferreira and Dias, 2000). Hence, exploring the potential bacteria that are associated to this plant can be a promising source of bioactive compounds. With this task in mind, we have aimed to identify the proteases secreted by the new bacterial species H. blutaparonensis strain M9.
Materials and Methods
Microorganism and culture conditions
The strain M9 of Halobacillus blutaparonensis, originally described by Barbosa et al. (2006), was cultivated in Luria-Bertani (LB) broth that contains in its formulation 0.5% NaCl. Cells were incubated without agitation at 32 ºC for 48 h. Working cultures were kept on LB agar plates at 4 ºC, while long-term storage was followed in LB slants supplemented with 1% CaCO3 (Seldin et al., 1983).
Influence of salt on the bacterial growth
Bacterial cells were incubated in LB broth supplemented with different concentrations of NaCl (0.5%, 2.5%, 5.0% and 10.0%) at 32 ºC up to 96 h. In order to evaluate the growth kinetics, an aliquot of each system was recovered every 24 h, diluted (10-3 to 10-6) and then plated onto LB solid medium containing the respective NaCl concentration. Afterwards, all the plates were incubated for 48 h to count the colony-forming units (CFU).
Influence of salt on the production of proteolytic enzymes
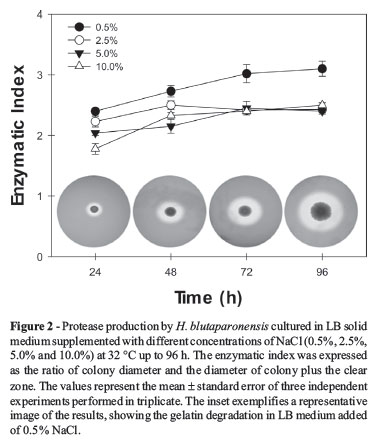
Determination of protease production was primarily performed by using LB agar plates (containing 0.5%, 2.5%, 5.0% and 10.0% NaCl) supplemented with 0.4% gelatin (Sigma-Aldrich Chemical Co., USA). Briefly, ten microliters of the bacterial suspension were placed in the center of agar plates (9-cm diameter Petri dishes) and then incubated at 32 ºC for 24, 48, 72 and 96 h. The gelatin degradation was revealed by flooding the plates with a solution containing 15% (w/v) mercuric chloride in 20% HCl. After 10 min, a clear transparent zone indicated hydrolysis of the gelatin by extracellular proteolytic activity, whereas the rest of the plate became opaque because of the coagulation of gelatin by HgCl2 (Fraziern, 1926). The colony diameter (a) and the diameter of colony plus the clear zone (b) were measured by a digital paquimeter. The enzymatic index (EI) was expressed as follows: EI = b/a. The EI value of three different samples was measured to obtain the average value.
Cell-free culture supernatants
H. blutaparonensis cultures (200 mL) were centrifuged (15.000 g / 20 min / 4 ºC) and the supernatants were filtered through a 0.22-/m membrane (Millipore). The cell-free culture supernatants were concentrated 50-fold using a 10,000 molecular weight cut-off Amicon micropartition system (Stirred Cell Model 8200) (Alvarez et al., 2006). Protein concentration was determined by the method described by Lowry et al. (1951), using bovine serum albumin (BSA) as standard.
Zymography
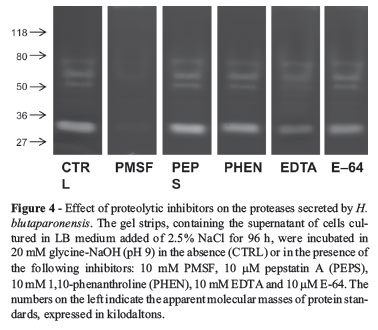
Proteases were assayed and partial characterized by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with 0.1% co-polymerized gelatin as substrate (Heussen and Dowdle, 1980). Gels were loaded with 50 /g of protein per slot. After electrophoresis, at a constant current of 120 V at 4 ºC, SDS was removed by incubation with 10 volumes of 2.5% Triton X-100 for1hat room temperature under constant agitation. In order to promote the proteolysis, the gels were incubated for 48 h at 37 ºC in the following buffer systems: 20 mM sodium phosphate buffer (pH 5.0 and 7.0) and 20 mM glycine-NaOH (pH 9.0) in the absence or in the presence of proteolytic inhibitors (Sigma) (10 mM phenylmethylsulphonyl fluoride (PMSF), 10 mM 1,10-phenanthroline, 10 mM ethylenediaminetetraacetic acid (EDTA), 10 /M trans-epoxysuccinyl L-leucylamido-(4-guanidino) butane (E-64) and 10 /M pepstatin A). The gels were stained for 2 h with 0.2% Coomassie brilliant blue R-250 in methanol:acetic acid:water (50:10:40) and destained overnight in a solution containing methanol:acetic acid:water (5:10:85), to intensify the digestion halos. The molecular masses of the proteases were calculated by comparison with the mobility of low molecular mass standards. The gels were dried, scanned and digitally processed (Alvarez et al., 2006). The quantification of proteolytic halos was performed by densitometrical analyzes using the public domain Image J software.
Cleavage of chromogenic peptide substrates
The crude supernatant fluid of H. blutaparonensis was test to cleave the following chromogenic substrates (Sigma): N-Succinyl-Ala-Ala-Ala-p-nitroanilide (elastase substrate), N-Succinyl-Ala-Ala-Pro-Phe p-nitroanilide (chymotrypsin substrate) and N-Benzoyl-Phe-Val-Arg-pnitroanilide (trypsin substrate). The reactions were started by the addition of each substrate at 1 mM to the supernatant fluid (50 /g) diluted in 20 mM glycine-NaOH, pH 9.0. The reaction mixtures were incubated at 37 ºC up to 120 min and the rate of substrate hydrolyses (the formation of pnitroaniline) were determined by Thermomax Molecular Device microplate reader at 405 nm. A control for each chromogenic substrate, where the substrate was added just after the reactions were stopped, was used as blank. One unit of proteolytic activity was defined as the amount of enzyme that caused an increase of 0.001 in absorbance unit, under standard assay conditions.
Effects of salt and organic solvents on protease activity
The stability of the crude proteases was also investigated in the presence of various organic solvents (at 25% v/v) and salt concentrations (up to 20% NaCl). The reaction mixtures and the rate of substrate hydrolyses were determined as described above.
Statistical analysis
The experiments were performed in triplicate, in three independent experimental sets. The data were analyzed statistically by means of Student's t-test using EPI-INFO 6.04 (Database and Statistics Program for Public Health) computer software. P values of 0.05 or less were considered statistically significant.
Results and Discussion
Figure 1 shows the growth curves of H. blutaparonensis when cultivated at 32 ºC for a period of 1 to 4 days in LB broth supplemented with different salt concentrations. This bacterial species attained the log phase after 24 h of cultivation in LB added with NaCl at either 2.5% or 5.0%, and by the second day in LB added with 0.5% NaCl. Elevated amount of NaCl (10.0%) retarded the bacterial proliferation. Moreover, they differed in growth pattern, which revealed a poor growth of H. blutaparonensis in LB medium containing 0.5%, 5.0% or 10.0% NaCl and an abundant proliferation when LB plus 2.5% NaCl was used (Figure 1, inset).
To adapt to saline conditions, bacteria have developed various strategies to maintain cell structure and function. Studies of such bacteria are of great importance, as they may produce compounds of industrial interest, such as extracellular hydrolytic enzymes that have diverse potential usage in biomedical science and chemical industries (Ventosa and Nieto, 1995; Margesin and Schinner, 2001; Mellado and Ventosa, 2003). Proteases are the largest selling industrial enzymes. Their sale is projected to increase further in the coming years with anticipated applications in protein processing, peptide synthesis and detergent formulations. However, the above-mentioned applications necessitate the proteases to be stable in salts, organic solvents and alkaline pH, respectively (Gupta et al., 1999, 2002a, 2002b). In this context, halotolerant and/or halophilic microorganisms are valuable source of novel enzymes, including proteases, for using in different industrial practices. Taking this finding in mind, the production of proteases by H. blutaparonensis was initially investigated onto LB agar plates supplemented with both NaCl and gelatin during a period of 96 h of growth (Figure 2). The gelatin degradation was observed in bacterial cells grown under different concentration of salt, ranging from 0.5% to 10% NaCl, in a similar way (Figure 2). As it is well-known, the stability and activity of the enzyme in the presence and absence of salt make it quite interesting for wider applications in biotechnological processes (Rao et al., 1998; Gupta et al., 1999, 2002a, 2002b).
After these first results, LB broth supplemented with 2.5% NaCl was chosen to carry out the further experiments. In this context, the secretion of proteolytic enzymes was examined in the culture supernatants of H. blutaparonensis along 96 h of in vitro growth. A simple inspection of the overlay gels showed that the secreted proteases were more active under neutral-alkaline pH range (Figure 3). At least five major proteolytic bands were observed with molecular masses ranging from 80 to 30 kDa. We also observed that different proteolytic expression by H. blutaparonensis was induced depending on culture age: proteases of 80, 70, 55 and 50 kDa were more pronounced in culture supernatant harvested after two days of cultivation (stationary phase) at neutral pH, while the 30 kDa protease had its production enhanced along the 96 h of growth with noticeable activity at alkaline pH (Figure 3). Similarly, maximum protease production in stationary phase is well documented in Pseudoalteromonas sp. strain CP76 (Sanchez-Porro et al., 2003) and Bacillus sphaericus (Singh et al., 2004).
Aiming to characterize these proteases more fully, we tested their activities in gelatin-containing gels in the presence of proteolytic inhibitors. The results showed that the extracellular proteolytic profile of H. blutaparonensis was composed exclusively by serine-type proteases, displaying total sensibility to PMSF (Figure 4). Conversely, 1,10phenanthroline (a zinc metalloprotease inhibitor), E-64 (a cysteine protease inhibitor) and pepstatin A (an aspartic protease inhibitor) did not significantly interfere with the secreted proteolytic activities (Figure 4). Interestingly, EDTA (a calcium metalloprotease inhibitor/chelating agent) partially inhibited the proteases released by H. blutaparonensis (Figure 4). Likewise, Karbalaei-Heidari et al. (2009) purified a 36 kDa alkaline extracellular protease from Halobacillus karajensis strain MA-2 sensible to classical serine protease inhibitors (PMSF and Pefabloc SC) and EDTA, indicating that it probably belongs to the subclass of serine metalloproteases. This inhibition profile is commonly observed in many members of the subtilase superfamily, including subtilisins, which are one of the most valuable industrial enzymes and have been extensively studied in terms of molecular structure, protein engineering and their application (Rao et al., 1998; Gupta et al., 2002a). Gram-positive bacilli are well-known sources for high productivity of subtilisins (Sanchez-Porro et al., 2003).
The degradation of different serine protease substrates was employed using the supernatant fluid of H. blutaparonensis (Figure 5). To this end, we performed kinetic assays using chromogenic peptide substrates specific to elastase, trypsin and chymotrypsin. The serine proteases secreted by H. blutaparonensis showed elevated degradation rate on the chymotrypsin substrate when compared to trypsin or elastase substrates (Figure 5). As expected, PMSF was able to completely abolish the cleavage of the chromogenic chymotrypsin substrate (data not shown).
In industrial applications, use of crude enzyme is preferred over purified preparation. This is to avoid the cost of purification and make the processes commercially viable. The applications of crude proteases have been highlighted in several processes, for instance, the crude protease from the extreme halophilic archaea Halobacterium sp. SP1 (Akolkar et al., 2008) that is stable over a broad pH range and high salt concentration has been effectively used in order to accelerate the fish sauce fermentation process (Akolkar et al., 2010). Joshi et al. (2007) described the production of alkaline serine protease stable in the presence of NaCl, SDS and acetone by a moderately halotolerant strain of Bacillus cereus (designated MTCC 6840) isolated from Lake Nainital, Uttaranchal State, India. Herein, the preliminary characterization of the crude proteases secreted by H. blutaparonensis was undertaken more specifically to assess the novel features of enzyme in terms of its catalytic behavior and stability in distinct organic solvents and different concentrations of salt (Figure 6). The proteases of H. blutaparonensis were highly stable in the presence of NaCl up to 20% along 60 min of experimentation (Figure 6A). Regarding the effects of organic solvents, the proteolytic activity showed high stability in ether, isooctane and cyclohexane (at 25% v/v) after 60 min (Figure 6B); however, chloroform and ethanol reduced the enzymatic activity around 40% and 70%, respectively (Figure 6B).
In conclusion, this is the first study describing the protease production by the moderately halophilic bacterium H. blutaparonensis. Some of the features of the secreted proteases by this new bacterial species, such as production in the absence and presence of different salt concentrations, hydrolytic activity in a wide pH range (especially neutral-alkaline pHs) and stability in salt and organic solvents are credentials to future biochemical studies. So, upcoming works are in progress in our laboratory in order to purify some of the extracellular proteases produced by H. blutaparonensis, aiming to test their application in some industrial/biotechnological processes.
Acknowledgments
This study was supported by grants from the Brazilian Agencies: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa no Estado do Rio de Janeiro (FAPERJ), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho de Ensino e Pesquisa para Graduados da Universidade Federal do Rio de Janeiro (CEPG-UFRJ). André L.S. Santos and Lucy Seldin are supported by CNPq and FAPERJ fellowships. Anderson F. Santos, a postgraduate student of Programa de Pós-Graduação em Bioquímica -Instituto de Química -Centro de Tecnologia -UFRJ, is supported by a CAPES fellowship.
Submitted: May 14, 2012
Approved: April 4, 2013.
All the content of the journal, except where otherwise noted, is licensed under a Creative Commons License CC BY-NC.
- Akolkar AV, Deshpande GM, Raval KN, Durai D, Nerurkar AS, Desai AJ (2008) Organic solvent tolerance of Halobacterium sp. SP1(1) and its extracellular protease. J Basic Microbiol 48:1-5.
- Akolkar AV, Durai D, Desai AJ (2010) Halobacterium sp. SP1(1) as a starter culture for accelerating fish sauce fermentation. J Appl Microbiol 109:44-53.
- Alvarez VM, Von der Weid I, Seldin L, Santos ALS (2006) Influence of growth conditions on the production of extracellular proteolytic enzymes in Paenibacillus peoriae NRRL BD-62 and Paenibacillus polymyxa SCE2. Lett Appl Microbiol 43:625-630.
- Barbosa DC, Von der Weid I, Vaisman N, Seldin L (2006) Halotolerant spore-forming Gram-positive bacterial diversity associated with Blutaparon portulacoides (St. Hill.) Mears, a pioneer species in Brazilian coastal dunes. J Microbiol Biotechnol 16:193-199.
- Bernardi H, Seeliger U (1989) Population biology of Blutaparon portulacoides (St. Hill.) Mears on southern Brazilian backshores. Ciência & Cultura 41:1110-1113.
- Ferreira EO, Dias DAA (2000) Methylenedioxyflavonol from aerial parts of Blutaparon portulacoides Phytochemistry 53:145-147.
- Fraziern C (1926) A method for the detection of changes in gelatin due to bacteria. J Infect Dis 39:302-309.
- Fujinami S, Fujisawa M (2010) Industrial applications of alkaliphiles and their enzymes -Past, present and future. Environ Technol 31:845-856.
- Gupta R, Gupta K, Saxena RK, Khan S (1999) Bleach-stable, alkaline protease from Bacillus sp. Biotechnol Lett 21:135-138.
- Gupta R, Beg QK, Lorenz P (2002) Bacterial alkaline proteases: molecular approaches and industrial applications. Appl Microbiol Biotechnol 59:15-32.
- Gupta R, Beg QK, Khan S, Chauhan B (2002) An overview on fermentation, downstream processing and properties of microbial alkaline proteases. Appl Microbiol Biotechnol 60:381-395.
- Heussen C, Dowdle EB (1980) Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing SDS and copolymerized substrates. Anal Biochem 102:196-202.
- Horikoshi K (1999) Alkaliphiles: some applications of their products for biotechnology.Microbiol Mol Biol Rev 63:735-750.
- Joo HS, Kumar CG, Park GC, Paik SR, Chang CS (2003) Oxidant and SDS-stable alkaline protease from Bacillus clausii I-52: production and some properties. J Appl Microbiol 95:267-272.
- Joshi GK, Kumar S, Sharma V (2007) Production of moderately halotolerant, SDS stable alkaline protease from Bacillus cereus MTCC 6840 isolated from Lake Nainital, Uttaranchal State, India. Braz J Microbiol 38:773-779.
- Karan R, Khare SK (2011) Stability of haloalkaliphilic Geomicrobium sp. protease modulated by salt. Biochemistry (Mosc) 76:686-693.
- Karbalaei-Heidari HR, Amoozegar MA, Hajighasemi M, Ziaee AA, Ventosa A (2009) Production, optimization and purification of a novel extracellular protease from the moderately halophilic bacterium Halobacillus karajensis J Ind Microbiol Biotechnol 36:21-27.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265-275.
- Margesin R, Schinner R (2001) Potential of halotolerant and halophilic microorganisms for biotechnology. Extremophiles 5:73-83.
- Mellado ME, Ventosa A (2003) Biotechnological potential of moderately and extremely halophilic microorganisms. In: Barredo JL (ed) Microorganisms for health care, food and enzyme production. Research Signpost, Kerala, p.233-256.
- Namwong S, Hiraga K, Takada K, Tsunemi M, Tanasupawat S, Oda K (2006) A halophilic serine proteinase from Halobacillus sp. SR5-3 isolated from fish sauce: purification and characterization. Biosci Biotechnol Biochem 70:1395-1401.
- Ng TK, Kenealy WR (1986) Industrial applications of thermostable enzymes. In: Brock, T.D. (Ed.), T.G.M. and Applied Microbiology. John Wiley, p.197-205.
- Phrommao E, Rodtong S, Yongsawatdigul J (2011) Identification of novel halotolerant bacillopeptidase F-like proteinases from a moderately halophilic bacterium, Virgibacillus sp. SK37. J Appl Microbiol 110:191-201.
- Rao MB, Tanksale AM, Ghatge MS, Deshpande VV (1998) Molecular and biotechnological aspects of microbial proteases. Microbiol Mol Biol Rev 62:597-635.
- Rawlings ND, Morton FR, Kok CY, Kong J, Barrett AJ (2008) MEROPS: the peptidase database. Nucleic Acids Res 36:320-325.
- Sanchez-Porro C, Mellado E, Bertoldo C, Antranikian G, Ventosa A (2003) Screening and characterization of the protease CP1 produced by the moderately halophilic bacterium Pseudoalteromonas sp. strain CP76. Extremophiles 7:221-228.
- Schallmey M, Singh A, Ward OP (2004) Developments in the use of Bacillus species for industrial production. Can J Microbiol 50:1-17.
- Seldin L, Van Elsas JD, Penido EGC (1983) Bacillus nitrogen fixers from Brazilian soils. Plant Soil 70:243-255.
- Setyorini E, Takenaka S, Murakami S, Aoki K (2006) Purification and characterization of two novel halotolerant extracellular proteases from Bacillus subtilis strain FP-133. Biosci Biotech Biochem 70:433-440.
- Singh J, Vohra RM, Sahoo DK (2004) Enhanced production of alkaline proteases by Bacillus sphaericus using fed-batch culture. Process Biochem 39:1093-1101.
- Ventosa A, Nieto JJ (1995) Biotechnological applications and potentialities of halophilic microorganisms. World J Microbiol Biotechnol 11:85-94.
Send correspondence to:
Publication Dates
-
Publication in this collection
25 Feb 2014 -
Date of issue
Dec 2013
History
-
Received
14 May 2012 -
Accepted
04 Apr 2013