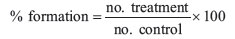Abstract
In this study we report the potential of alcohols as morphogenetic regulators in Candida albicans. All the alcohols tested influenced various modes of growth like planktonic as well as biofilm forms. Viability was affected at high concentrations. Among the alcohols, the response of C. albicans to amyl alcohol (pentanol) was noteworthy. Amyl alcohol at a concentration 0.5% which was not inhibitory to growth and viability specifically inhibited morphogenetic switching from yeast to hyphal forms. It also inhibited normal biofilm development favoring yeast dominated biofilms. Based on this study we hypothesize that alcohols produced under anaerobic conditions may not favor biofilm development and support dissemination of yeast cells. Since anaerobic conditions are not found to favor production of quorum sensing molecules like farnesol, the alcohols may play a role in morphogenetic regulation.
alcohols; signalling; morphogenesis; biofilms; yeast metabolism; Candida albicans
Effect of alcohols on filamentation, growth, viability and biofilm development in Candida albicans
Nitin M Chauhan; Ravikumar B Shinde; S. Mohan Karuppayil
DST-FIST and UGC-SAP Sponsored School of Life Sciences, SRTM University, Nanded, M.S, India
Send correspondence to Send correspondence to: S.M. Karuppayil School of Life Sciences, SRTM University Nanded, 431606 MS, India E-mail: prof.karuppayil@gmail.com
ABSTRACT
In this study we report the potential of alcohols as morphogenetic regulators in Candida albicans. All the alcohols tested influenced various modes of growth like planktonic as well as biofilm forms. Viability was affected at high concentrations. Among the alcohols, the response of C. albicans to amyl alcohol (pentanol) was noteworthy. Amyl alcohol at a concentration 0.5% which was not inhibitory to growth and viability specifically inhibited morphogenetic switching from yeast to hyphal forms. It also inhibited normal biofilm development favoring yeast dominated biofilms. Based on this study we hypothesize that alcohols produced under anaerobic conditions may not favor biofilm development and support dissemination of yeast cells. Since anaerobic conditions are not found to favor production of quorum sensing molecules like farnesol, the alcohols may play a role in morphogenetic regulation.
Key words: alcohols, signalling, morphogenesis, biofilms, yeast metabolism, Candida albicans.
Introduction
Candida albicans is considered as a serious pathogen in immunocompromised patients causing high mortality rate (Fridkin and Irwin, 1983; Jean-Marcel, 1998; Virudes et al., 2003; Parahym et al., 2009). Several studies have shown that in C. albicans, filamentation is a virulence factor (Lo et al., 1997; Saville et al., 2003; Yang, 2003). It also plays a critical role in biofilm formation (Baillie and Douglas, 1999). Thus, inhibition of filamentation may reduce pathogenesis and biofilm formation in C. albicans (Lo et al., 1997; Baillie and Douglas, 1999; Lopez-Ribot, 2005).
Metabolites produced under different environmental conditions are reported as virulence molecules in microbes (Dufour and Rao, 2011). Different alcohols are produced by the yeast Saccharomyces cerevisiae and C. albicans in vitro (Ghosh et al., 2008; Hazelwood et al., 2008). For example in presence of leucine, valine, phenylalanine, tyrosine, and tryptophan as nitrogen sources, Saccharomyces cerevisiae produces isoamyl alcohol, isobutanol, tyrosol, phenylethanol, and tryptophol respectively (Dickinson, 2008; Hazelwood et al., 2008). Alcohols such as ethanol, propanol, isopropanol, 1-butanol, 2-butanol, isoamyl alcohol, and tert-amyl alcohol are reported to induce filamentation in S. cerevisiae (Lorenz et al., 2000). Attachment to tissue culture dishes and polystyrene dishes in Pseudomonas species is affected by methanol, ethanol, propanol, and butanol (Fletcher, 1983). C. albicans in presence ethyl alcohol, dodecanol, isoamyl alcohol, and nerolidol failed to switch from yeast to hyphae under standard induction conditions (Martins et al., 2007; Davis-Hanna et al., 2008; Chauhan et al., 2010). Short chain n-alkanols are reported to affect the morphogenesis in the dimorphic fungus, Aureobasidium pullulans (Moragues et al., 1998). Studies are lacking on the effect of propanol, butanol, isopropanol, pentanol and isobutanol in C. albicans. In this paper we report for the first time the effect of short chain alcohols on induced morphogenesis, growth, and biofilm formation in C. albicans.
Materials and Methods
Organism, media and culture conditions
Candida albicans ATCC 90028 (MTCC 3017) was used throughout the study. The culture was maintained on Yeast-Peptone-Dextrose (YPD) agar slants at 4 ºC. YeastPeptone-Dextrose medium (YPD) was prepared by dissolving individual components (Yeast extract 1%, Peptone 2% and Dextrose 2%) in distilled water. pH was adjusted to 6.5. Solid medium was prepared by adding 2.5% agar powder to YPD broth. Two inducers i.e. 10% horse serum was prepared in deionized distilled water while, RPMI -1640 medium with L-glutamine w/o sodium bicarbonate buffered with 165 mM MOPS (3-[N-morpholine] propane sulphonic acid) was added in sterilized distilled water and pH was adjusted to 7. All the media components and chemicals were from Hi-Media Laboratories Ltd. Mumbai, India. For culture activation, a single colony from the YPD plates was inoculated in 50 mL of YPD broth, in a 250 mL conical flask and incubated at 30 ºC on an orbital shaker at 120 rpm for 24 h. Cells from the activated culture were harvested by centrifugation for 5 min at 2000 g speed and washed three times with PBS (10 mM Phosphate buffer, 2.7 mM Potassium chloride and 137 mM Sodium chloride pH 7.4) and resuspended in PBS.
Filamentation assay
Filament formation was studied using microtiter plate assay in 96 -well microtiter plates as described previously (Chauhan et al., 2010). Briefly, cells from stock were inoculated in various inducer media to get1x106 cells ml-1. Various concentrations of butanol, isobutanol, tertiary-butanol, propanol, isopropanol and pentanol to give final concentrations of 0.06, 0.12, 0.25, 0.5, 1, 2, and 4% (v/v) were added. Wells without alcohol were kept as control. Final volume of assay system in each well was kept 200 µL. The plates were incubated at 37 ºC at 200 rpm on an orbital shaker for 4 to 8 h. After incubation, cells were observed microscopically. Every time, 100 cells were counted and the numbers of yeast and germ tube forms were noted. Percentage of germ tube formation in each well was calculated compared to that of control with the following formula:
where % formation = percentage of germ tube formation, no. treatment = no. of germ tubes in treatment and no. control = no. of germ tubes in control.
Growth and viability assay
Effect of alcohols on growth of C. albicans yeast phase cells was determined by standard CLSI M27-A microbroth dilution method (NCCLS, 1997). Various concentrations of butanol, isobutanol, tertiary-butanol, propanol, isopropanol, and pentanol (amyl alcohol) ranging from 0.025 to 4% v/v were added to RPMI 1640 medium in 96-well microtiter plates. Each well was inoculated with 1 x 103 cells mL-1. Final volume in each well was kept as 200 µL. Plates were incubated at 35 ºC on an orbital shaker for 24 h. Optical density was measured at 620 nm wavelength using a 96-well microplate reader (Multiscan Ex-Thermo Electron Corp. USA) and absorbance of alcohols treated wells were compared with that of control. Plate count was done to see the effect on viability (Chauhan et al., 2010). Cells from the respective wells were diluted to get 300 to 400 colonies and an aliquot of the sample was spread on YPD agar plates. The plates were incubated at 30 ºC for 24 h and colony count was done. Percentage of viability of treated cells was calculated by comparing with the colony count of control wells.
Biofilm formation and MTT assay
To see the effect of various alcohols on biofilm development, Candida biofilms were developed on polystyrene surface of 96-well plates(Chauhan et al., 2010). A cell suspension of1x107 cells mL-1 was prepared in PBS and 100 µL was inoculated in each well. The plates were incubated at 37 ºC at 50 rpm for 90 min for adhesion of cells on the surfaces. Non-adhered cells were removed by washing the wells, 2-3 times with PBS after 90 min. 200 µL of RPMI-1640 medium along with various concentrations of butanol, isobutanol, tertiary-butanol, propanol, isopropanol, and pentanol ranging from 0.025 to 4% v/v and one control without alcohols was added to each well. The plates were incubated at 37 ºC for 24 h to allow biofilm formation. After incubation, wells were washed to remove any planktonic cells and biofilms were observed under an inverted light microscope (Metzer, India). Measurement of metabolic activity of biofilm was done by MTT assay with little modification (Hawser and Douglas, 1997). The tetrazolium salt 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide (MTT, Hi-media, Mumbai, India) was used in an assay. 50 µL of MTT solution (stock solution containing 5 mg MTT-ml of PBS, diluted 1:5 in prewarmed 0.15 M PBS prior to addition) was added in each well. The plate was in cubated for 5 h at 37 ºC. Dimethyl sulfoxide (200 µL) was added to each well to solubilise MTT formazan product and optical density was measured at 450 nm using a 96-well microplate reader (Multiscan Ex-Thermo Electron Corp. USA). Percentage of metabolic activity in biofilm formation was calculated compared to that of control (without alcohol).
Statistical analysis
All the experiments were done in triplicates and standard deviation from the mean was calculated. Effect of alcohols on biofilm formation was analyzed using Two-way Anova (Bonferroni posttests) by GraphPad Prism 5 software. P < 0.05 was considered statistically significant.
Results
Effect of various alcohols on induced morphogenesis
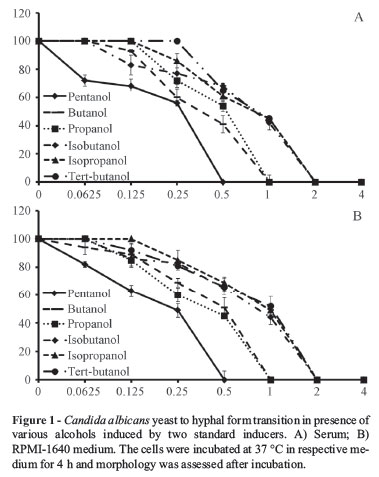
All the alcohols inhibited filamentation in a concentration dependent manner induced by two standard inducers (Figure 1). Responses of C. albicans to alcohols were found to be similar for both the inducers at all time points studied. Pentanol at a concentration of 0.5% showed complete inhibition of filamentation, while 1% of butanol and propanol completely blocked hyphae. Isobutanol, isopropanol and tertiary-butanol at 2% completely halted yeast to hyphal form transitions (Figure 3). The efficacy of alcohols towards induced morphogenesis is as follows:
Pentanol < Butanol, Propanol < Isobutanol, Isopropanol, Tertiary-Butanol
Growth and viability of C. albicans cells in presence of alcohols
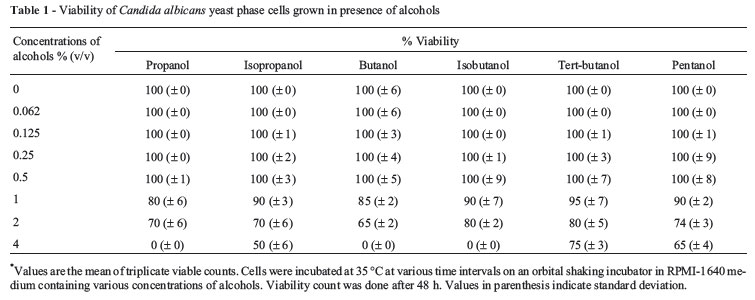
Alcohols upto 0.5% of concentration did not cause any effect on viability. Addition of 4% of various alcohols inhibited growth by 60-80%. 2% of butanol, isobutanol, tertiary-butanol, and pentanol reduced growth by 15-20%, whereas propanol and isopropanol at the same concentration inhibited growth by 20-25%. 4% of butanol, isobutanol, and propanol were found to be toxic to Candida cells, while isopropanol, tertiary-butanol, and pentanol caused 35-50% reduction in viability at 4% (Table 1). The sensitivity of Candida cells for alcohols is as follows:
Butanol < Propanol < Isobutanol < Isopropanol < Pentanol < Tertiary-butanol
Candida biofilms in presence of various alcohols
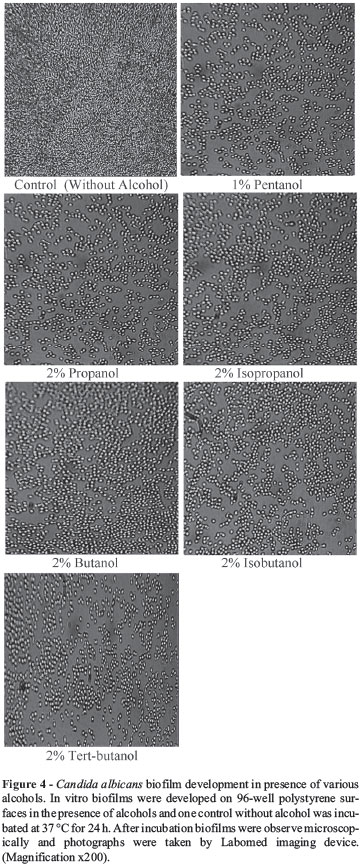
All the alcohols studied significantly (p = 0.0005) inhibited biofilm formation in a concentration dependent manner. Treatment with 4% of alcohols caused 50-60% reduction in metabolic activity in Candida biofilm (Figure 2). Addition of 2% of butanol, isobutanol, tertiary-butanol, propanol, and isopropanol after adhesion phase inhibited biofilm development and patches of few adhered yeast cells were observed, whereas similar effect was seen at 1% of pentanol (Figure 4). 1% of butanol, isobutanol, tertiarybutanol, propanol, and isopropanol inhibited filamentation in biofilms and favoured `yeast only' biofilm. Pentanol caused considerable inhibition of biofilm at 0.5% concentration leading to yeast only biofilm formation.
Discussion
The behavior and production of metabolites by Candida albicans in vivo may greatly vary depending on the niches it colonizes in the human body like gastrointestinal tract, vaginal, or oral cavities (Ghosh et al., 2008). In deep seated mycosis or in biofilms on prosthetic devices it may live in a polymicrobial environment and may be subject to anaerobic conditions or exposed to the metabolites its own or that of other organisms (Dumitru et al., 2004; Dufour and Rao, 2011). In gastrointestinal tract, deep seated mycosis, and biofilms it may produce considerable amount of alcohols, because of the anaerobic conditions. The quorum sensing molecule, farnesol is undetectable under anaerobic conditions of growth, suggesting signalling roles for other metabolites (Hornby et al., 2011). Alcohols are very common metabolites produced by yeast under anaerobic conditions. Interestingly, the concentrations of alcohols produced under anaerobic conditions are more in comparison to yeast cells produced under aerobic growth (Ghosh et al., 2008). Most of the alcohols tested by us inhibited yeast to hyphal form switching.
We have earlier reported the morphogenetic regulatory properties of ethyl alcohol (Chauhan et al., 2010) and its oxidative product, acetaldehyde in C. albicans (Chauhan et al., 2011). In this paper we report for the first time the effect of pentanol, butanol, propanol, isobutanol, isopropanol, and tertiary-butanol on morphogenetic switching, biofilm formation, growth, and viability in C. albicans. Among the various alcohols studied, pentanol (amyl alcohol) was found to be very effective. 0.5% of pentanol completely blocked induced morphogenesis without affecting viability of C. albicans. The hyphal inhibitory concentration of pentanol did not alter growth and viability of Candida cells. The same concentration of pentanol showed around 50% reduction in metabolic activity in biofilm development (Figure 2). While for other alcohols the hyphal inhibitory concentration showed 10-30% reduction in viability and 35-45% decreased in metabolic activity was observed in Candida biofilms compared to that of control (Table 2). Based on our results, we have proposed a model for the role of alcohols in C. albicans biofilms (Figure 5).
Deeper regions of mature biofilms may produce anaerobic conditions (Dumitru et al., 2004). Anaerobic conditions also persist in deep seated mycosis. The type of alcohol produced may vary depending on the amino acid availability. For example, availability of leucine, isoleucine, valine, and threonine may favor the production of amyl alcohol, isoamyl alcohol, butanol, and propanol respectively (Dickinson et al., 1998; Lorenz et al., 2000; Hazelwood et al., 2008). The alcohols may exert effects depending on its concentrations. At lower concentrations like 0.5% in case of amyl alcohol it may inhibit morphogenetic switching and support yeast phase growth. While at high concentrations it may slow down the growth rate. Most of the alcohols may exert similar effect like ethyl alcohol, amyl alcohol, isoamyl alcohol, etc. Favoring of yeast phase growth may support disseminative mode of growth and also aid to escape from the alcohol poisoned environment. It may also offer ecological advantages for C. albicans over other competing organisms which are more susceptible to alcohols than Candida under mixed species of populations.
Acknowledgments
The authors thank Prof. S. B. Nimse, Honourable Vice Chancellor, SRTM University, Nanded (M.S), India, for encouragement and motivation.
Submitted: August 2, 2012
Approved: April 4, 2013.
All the content of the journal, except where otherwise noted, is licensed under a Creative Commons License CC BY-NC.
- Baillie GS, Douglas LJ (1999) Role of dimorphism in the development of Candida albicans biofilms. J Med Microbiol 48:671-679.
- Chauhan NM, Raut JS, Karuppayil SM (2010) A morphogenetic regulatory role for ethyl alcohol in Candida albicans Mycoses 54:e697-703.
- Chauhan NM, Raut JS, Karuppayil SM (2011) Acetaldehyde inhibits the yeast-to-hypha conversion and biofilm formation in Candida albicans Mycoscience 52:356-360.
- Davis-Hanna A, Piispanen AE, Staeva II, Hogan DA (2008) Farnesol and dodecanol effects on the Candida albicans RascAMP signaling pathway, and the regulation of morphogenesis. Mol Biol 67:47-62.
- Dickinson JR (2008) Filament formation in Saccharomyces cerevisiae-a Review. Folia Microbiol 53:03-14.
- Dickinson JR, Harrison SJ, Hewlins JE (1998) An investigation of the metabolism of valine to isobutyl alcohol in Saccharomyces cerevisiae The J Biol Chem 273:25751-25756.
- Dufour N, Rao RP (2011) Secondary metabolites and other small molecules as intercellular pathogenic signals. FEMS Microbiol Letters 314:10-17.
- Dumitru R, Hornby JN, Nickerson KW (2004) Defined anaerobic growth medium for studying Candida albicans basic biology and resistance to eight antifungal drugs. Antimicrob Agents Chemother 48:2350-2354.
- Fletcher M (1983) Effects of methanol, ethanol, propanol, and butanol on bacterial attachment to surfaces. J Gen Microbiol 129:633-641.
- Fridkin SK, Irwin MY (1983) Epidemiology of nosocomial fungal infections. Clin Microbiol Reviews 09:499-511.
- Ghosh S, Kebaara BW, Atkin AA, Nickerson KW (2008) Regulation of Aromatic alcohol production in Candida albicans Appl Environ Microbiol. 74:7211-7218.
- Hawser SP, Douglas LJ (1994) Biofilm formation by Candida species on the surface of catheter materials in vitro Infect Immun 62:915-921.
- Hazelwood LA, Daran JM, Maris JA, Pronk JT, Dickinson JR (2008) The Ehrlich pathway for fusel alcohol production: a century of research on Saccharomyces cerevisiae Appl Environ Microbiol 74:2259-2266.
- Hornby JB, Jensen EC, Lisec AD, Tasto JJ, Jahnke B, Shoemaker R, Dussualt P, Nickerson KW (2001) Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl Environ Microbiol. 67:2982-2992.
- Jean-Marcel S (1998) Candida adherence phenomena, from commensalism to pathogenecity. Int Microbiol 1:117-122.
- Lo HJ, Kohler JR, DiDomencia B, Loebenberg D, Fink GR (1997) Nonfilamentous Candida albicans are avirulent. Cell 90:939-949.
- Lopez-Ribot JL (2005) Candida albicans Biofilms: More Than Filamentation. Current Biol 15:R453-R455.
- Lorenz MC, Cutler NS, Heitman J (2000) Characterization of alcohol-induced filamentous growth in Saccharomyces cerevisiae Mol Biol Cell 11:183-199.
- Martins M, Henriques M, Azeredo J, Rocha SM, Coimbra MA, Oliveira R (2007) Morphogenesis control in Candida albicans and Candida dubliniensis through signaling molecules produced by planktonic and biofilm cells. Eukaryo Cell 6:2429-2436.
- Moragues MD, Asturias JA, Sevilla M (1998) Morphogenetic potency of short-chain n-alkanols related to Aureobasidium pullulans Current Microbiol 16:243-245.
- National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeast (1997) Approved standard NCCLS M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- Parahym AMR, Melo LRB, Morais VLL, Neves RP (2009) Candidiasis in pediatric patients with cancer interned in university hospital. Braz J Microbiol 40:321-324.
- Saville SP, Lazzell AL, Monteagudo C, Lopez-Ribot JL (2003) Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukary Cell 02:1053-1060.
- Virudes A, Peman J, Canton E, Ubeda P, Lopez-Ribot JL, Goberando L (2003) Candidemia at a tertiary care hospital: epidemiology, treatment, clinical outcome, and risk factors for death. Eur J Clin Microbiol Infect Dis 01:767-774.
- Yang Y (2003) Virulence factors of Candida species. J Microbiol Immunol Infect 36:223-228.
Send correspondence to:
Publication Dates
-
Publication in this collection
25 Feb 2014 -
Date of issue
Dec 2013
History
-
Received
02 Aug 2012 -
Accepted
04 Apr 2013