Abstract
Biosynthesis of active secondary metabolites by fungi occurs as a specific response to the different growing environments. Changes in this environment alter the chemical and biological profiles leading to metabolites diversification and consequently to novel pharmacological applications. In this work, it was studied the influence of three parameters (fermentation length, medium composition and aeration) in the biosyntheses of antimicrobial metabolites by the fungus Aspergillus parasiticus in 10 distinct fermentation periods. Metabolism modulation in two culturing media, CYA and YES was evaluated by a 2² full factorial planning (ANOVA) and on a 2³ factorial planning, role of aeration, medium composition and carbohydrate concentration were also evaluated. In overall, 120 different extracts were prepared, their HPLC profiles were obtained and the antimicrobial activity against A. flavus, C. albicans, E. coli and S. aureus of all extracts was evaluated by microdilution bioassay. Yield of kojic acid, a fine chemical produced by the fungus A. parasiticus was determined in all extracts. Statistical analyses pointed thirteen conditions able to modulate the production of bioactive metabolites by A. parasiticus. Effect of carbon source in metabolites diversification was significant as shown by the changes in the HPLC profiles of the extracts. Most of the extracts presented inhibition rates higher than that of kojic acid as for the extract obtained after 6 days of fermentation in YES medium under stirring. Kojic acid was not the only metabolite responsible for the activity since some highly active extracts showed to possess low amounts of this compound, as determined by HPLC.
Aspergillus parasiticus; factorial planning; metabolites diversification; kojic acid; antimicrobial activity
MEDICAL MICROBIOLOGY
Modulation of antimicrobial metabolites production by the fungus Aspergillus parasiticus
Adriana A.P. Bracarense; Jacqueline A. Takahashi
Departamento de Química, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brazil
Send correspondence to Send correspondence to: J.A. Takahashi Departamento de Química, Universidade Federal de Minas Gerais Av. Antonio Carlos 6627 31270-901 Belo Horizonte, MG, Brazil E-mail: jat@qui.ufmg.br
ABSTRACT
Biosynthesis of active secondary metabolites by fungi occurs as a specific response to the different growing environments. Changes in this environment alter the chemical and biological profiles leading to metabolites diversification and consequently to novel pharmacological applications. In this work, it was studied the influence of three parameters (fermentation length, medium composition and aeration) in the biosyntheses of antimicrobial metabolites by the fungus Aspergillus parasiticus in 10 distinct fermentation periods. Metabolism modulation in two culturing media, CYA and YES was evaluated by a 22 full factorial planning (ANOVA) and on a 23 factorial planning, role of aeration, medium composition and carbohydrate concentration were also evaluated. In overall, 120 different extracts were prepared, their HPLC profiles were obtained and the antimicrobial activity against A. flavus, C. albicans, E. coli and S. aureus of all extracts was evaluated by microdilution bioassay. Yield of kojic acid, a fine chemical produced by the fungus A. parasiticus was determined in all extracts. Statistical analyses pointed thirteen conditions able to modulate the production of bioactive metabolites by A. parasiticus. Effect of carbon source in metabolites diversification was significant as shown by the changes in the HPLC profiles of the extracts. Most of the extracts presented inhibition rates higher than that of kojic acid as for the extract obtained after 6 days of fermentation in YES medium under stirring. Kojic acid was not the only metabolite responsible for the activity since some highly active extracts showed to possess low amounts of this compound, as determined by HPLC.
Key words: Aspergillus parasiticus, factorial planning, metabolites diversification, kojic acid, antimicrobial activity.
Introduction
Throughout the centuries, many active metabolites were discovered from varied natural sources, such as superior plants, animals, insects and microorganisms. Amongst these sources, the microorganisms are probably the group that generates metabolites possessing the most readily industrial applications because they are chemo-organotrophic, present a high growing rate on a short cycle of life and generate large amounts of biomass in short time. Therefore, industrial production of fungal metabolites demands less complex operational control processes (Demain, 2000). By the other hand, the great number of existing microorganisms can make the initial chemo-biological prospection of extracts from natural sources very time-consuming, increasing the length of research in this area (Harvey, 2007). Therefore, the use of interactive chemobiological tools to screen suitable conditions for bioactive metabolites production is very welcome (Lam, 2007). Natural products already isolated from fungi possess numerous pharmacological uses, in special as antibacterial and antifungal agents (Takahashi and Lucas, 2008a; Takahashi et al., 2008b; Tanseer and Anjum, 2011; Newman and Cragg, 2012). Research in this area has been neglected in face of the profit perspective of some more fashioned diseases. However, the scope of antimicrobial activity has become increasingly important since fungal and bacterial infections have grown enormously in recent years, especially associated to individuals with immune system deficiency like those with acquired immunodeficiency syndrome (AIDS). Life expectance increase, with the raise of a great number of elderly in the world population, the chronic use of corticosteroids and the boost of invasive medical procedures also contributed to the increase in the number of reported infections (Perfect, 2012). This new framework claims for the search of antimicrobial agents more effective and with less adverse effects. Production of antimicrobial metabolites by fungi is natural, since their survival in the natural habitats depends on their efficiency to stop the growth of other co-habitant microorganisms.
Fungi as A. flavus and C. albicans are related to important human infections that require close attention. A. flavus is responsible for huge losses in agricultural sanitary and nutritional quality in grains, through the production of secondary metabolites called aflatoxins, responsible for poisoning several species of animals, as well as being carcinogenic (Luo et al., 2009; Roze et al., 2011; Sajid et al., 2011). This fungus is also responsible for causing pulmonary aspergillosis, an opportunistic disease that affects mostly people with an already weakened immune system (Mahmoud et al., 2011; Mellon et al., 2011). C. albicans can be normally found in the human body, but under certain conditions, this microorganism can cause infections known as candidiasis, which affect mainly immune deficient patients. Local C. albicans infections can quickly develop into serious systemic infections (Seneviratne et al., 2008).
Several fungi from Aspergillus genus are also known for their ability to produce various bioactive metabolites for the pharmaceutical industry, such as terrecyclic acid A, metabolite with anticancer and antibacterial activity (Turbyville et al., 2005; Bok et al., 2006) and lovastatin, a very interesting metabolite used for cholesterol reduction (Bizukojc et al., 2012), both produced by the fungus Aspergillus terreus. Other active compounds produced by Aspergillus species are asperlicin, metabolite of Aspergillus alliaceus employed to treat neurological disorders (Butler, 2008), echinocandin B produced by A. nidulans, with antifungal activity (Aly et al., 2011) and fumagillin, metabolite of A. fumigatus, an angiogenesis inhibitor that has also antiparasitic activity (Sanchez, 2012).
A. parasiticus produces an interesting metabolite called kojic acid, a compound currently used in the pharmaceutical industry since it aggregates important biological activities, such as anti-inflammatory, antifungal, antibacterial, antitumor and insecticidal (Novontny et al., 1999). Recently kojic acid also started to be employed in treatments for reduction of expression marks around the eyes area (Hyde et al., 2010), broadening the scope of its industrial applications. In the present work, A. parasiticus was cultivated under 120 distinct conditions, and the changes in metabolites production were monitored by High Pressure Liquid Chromatography coupled to a diiodoarray detector (DAD-HPLC). The extracts obtained were submitted to a biological screening to monitor their antimicrobial activity against Gram-positive (Staphylococcus aureus) and Gram-negative bacteria (Escherichia coli), a yeast of relevant global clinical importance (Candida albicans) and a filamentous fungus (Aspergillus flavus) that causes not only human diseases but also agricultural losses. Kojic acid was quantified in all extracts by DAD-HPLC through the elaboration of a calibration curve using external standard methodology (Hu et al., 2011) in order to understand kojic acid role in the extracts activity.
Materials and Methods
Microorganism and culturing conditions
The fungus A. parasiticus ATCC 15517 was obtained from the Microorganisms' Collection hold by Fundação Oswaldo Cruz (FIOCRUZ - RJ, Brazil). The fungus was stored on test tubes containing potato dextrose agar (PDA, Himedia, India) at the temperature of 11 ºC. In the fermentations, A. parasiticus was grown in liquid media with the following compositions (in distilled water): CYA medium (35.0 g/L Czapek broth; 5.0 g/L yeast extract; 30.0 g/L sucrose), YES medium (150.0 g/L sucrose; 20.0 g/L malt extract; 0.5 g/L MgSO4.7H2O), LCG20 medium (20.0 g/L D-Glucose; 5.0 g/L bacteriological peptone; 1.0 g/L K2HPO4; 0.5 g/L MgSO4.7H2O; 5.0 g/L NaCl); LCG150 medium (150.0 g/L D-Glucose; 5.0 g/L bacteriological peptone; 1.0 g/L K2HPO4; 0.5 g/L MgSO4.7H2O; 5.0 g/L NaCl); medium LCS20 (20.0 g/L sucrose; 5.0 g/L bacteriological peptone; 1.0 g/L K2HPO4; 0.5 g/L MgSO4.7H2O; 5.0 g/L NaCl) and medium LCS150 (150.0 g/L sucrose; 5.0 g/L bacteriological peptone; 1.0 g/L K2HPO4; 0.5 g/L MgSO4.7H2O; 5.0 g/L NaCl).
Experimental planning
In order to optimize the production of active metabolites, three parameters (fermentation length, medium composition and aeration) were varied during the fermentation in 10 distinct fermentation periods (3, 6, 9, 12, 15, 18, 21, 24, 27 and 30 days). For each period, the parameters were evaluated by a 22 full factorial planning (ANOVA), to screen two culturing media, CYA and YES. In this experiment, there were also evaluated the aeration and the medium composition. On a 23 factorial planning, there were studied the influence of the liquid media LCG and LCS and the role of the variables aeration, medium composition and carbohydrate concentration. Details on the experimental conditions can be found on Tables 1 and 2.
For the preparation of the spore suspension used in the experiments, A. parasiticus was incubated for 7 days at 28 ºC in PDA. After this period, the spores were recovered, counted on a Neubauer Chamber and the appropriate dilution was prepared to achieve the final concentration of 5 x 106 spores/mL. The experiments took place upon the inoculation of a spore suspension in 500 mL Erlenmeyer flasks, containing 100 mL of culturing medium. There were used a total of 240 flasks, in which 120 different growing conditions were evaluated in duplicate.
Extracts preparation
The work up was carried on by vacuum filtration to separate the mycelium from the broth followed by extraction of the broth with ethyl acetate (3 x 50 mL/100 mL of broth) on a separating funnel. The organic layers were combined and the solvent was vacuum removed on a rota evaporator. The resulting extracts were transferred to clean vials and weighted.
High performance liquid chromatography analyses
Aliquots (10 mg) of the homogenised extracts were filtered with PTFE organic solvents modified membranes (MILLEX LCR 0.45 µm -Millipore) and analysed by High Performance Liquid Chromatography on a SHIMADZU LC10AD series Liquid Chromatograph coupled to an automatic sampler SIL-10AF SHIMADZU Auto Injector and a Diiodo Array Detector SPDM10avp. The separations were accomplished using a reversed phase Supelcosil TM LC18 column (250 x 4.6 mm; 5 /m). The volume of injected samples was 20 /L, at the concentration of 1 mg/mL. The system was operated by the CLASS LC10 Software. The mobile phase was constituted by a mixture of water containing 0.05% of formic acid and methanol. The linear elution gradient used was H20-Methanol 70:30 to 0:100 in 30 min, maintaining for additional 10 min in the last concentration. The gradient was brought back to the initial value in 5 min and maintained at this point for additional 5 min, to equilibrate the system, prior to the injection of the next sample. The eluent flux used was 0.5 mL/min.
External standardization of Kojic acid
To determine the amount of kojic acid produced in the experiments using different culture media, external standard methodology was used to build a productivity curve for this compound. A methanol solution of kojic acid (Acros Organics, Geel, Belgium) at the concentration of 1 mg/mL was prepared and, from it, 10 serial dilutions were prepared. Methanol solutions with known amounts of kojic acid were injected on the chromatograph. The calibration curve was generated by regression analysis (linear model) interposing the nominal concentrations of kojic acid solutions with the area of the peak obtained in the chromatogram. Statistical analysis of the data and return calculations were achieved using Microsoft Office Excel 2007. This experiment was carried out in quadruplicate.
Antimicrobial assays
Antibacterial and antifungal assays were carried out with the bacteria S. aureus ATCC 25923, E. coli ATCC 25723, the yeast C. albicans ATCC 18804 and the filamentous fungus A. flavus CCT 4952. The assays were conducted in microplates prepared with fixed concentrations of each extract (1000 /g/mL) and with kojic acid (250 /g/mL) in DMSO. Microorganisms were inoculated as suspensions. For the preparation of the spore suspension, A. flavus was incubated for 7 days at 28 ºC in PDA. After this period, the spores were recovered, counted on a Neubauer Chamber and the appropriate dilution was prepared for the final concentration of5x103 spores/mL. To prepare bacterial and C. albicans inocula, the respective strains were incubated for 24 h at 37 ºC in Broth Heart Infusion medium (BHI). Turbidity was adjusted with aid of a spectrophotometer model SP-22, BIOSPECTRO, by adding sterile water to the cells until reaching 75-76% of transmittance at the fixed wavelength of 530 nm. Microorganisms growth was measured on a ELISA reader (Thermoplate, TP-READER), at 492 nm, after 24 and 48 h (antibacterial tests) and after 48 h for C. albicans and A. flavus. The assay was carried in duplicate.
Results
The HPLC profiles of the extracts prepared (120 in total) were obtained and the extracts were assayed to monitor the modulation of the metabolites antimicrobial profiles upon the growing conditions alterations. Afterwards, the results of the biological screening were analysed using a statistical tool to determine which interactions among the variables in study influenced the biological activity (Table 3). There were considered significant the variables that presented p : 0.05, i.e., a confidence interval of 95%.
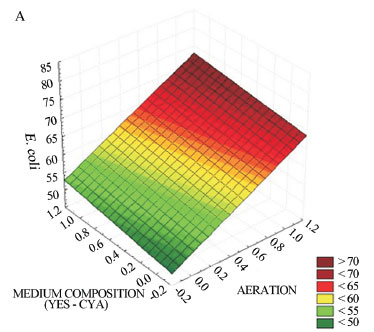
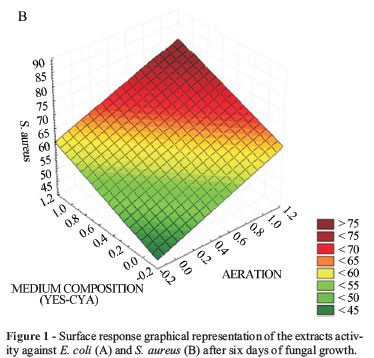
Graphics referring to the response surface from the 22 (evaluation of CYA and YES media) and 23 (evaluation of factorial LCG and LCS media) complete factorial planning (ANOVA) were prepared in order to analyse the effect of the growing conditions on the production of bioactive metabolites by A. parasiticus. The analyses led into consideration the conditions in which the variables presented significant results. Figure 1 illustrates the 22 response graphic for antimicrobial activity against E. coli (Figure 1A) and S. aureus (Figure 1B) of extracts prepared after 6 days of A. parasiticus cultivation. It can be noticed that the interactions among the type of culturing medium employed in the fermentation and presence of stirring showed significant results. The optimal conditions found at this stage of the fermentative process comprised the use of both superior levels, i.e., YES as the culture medium under stirring of 150 rpm (condition C01, Table 3).
Figure 2 shows a graphic for the response surface for the 23 planning for antimicrobial activity against C. albicans of extracts prepared after 24 days of A. parasiticus cultivation. It can be noticed that the interactions among the carbohydrate concentration, type of culturing medium employed in the fermentation and presence of stirring showed significant results. The optimal conditions found refer to the lower levels in relation to the type of carbohydrate and carbohydrate concentration and to the higher levels for stirring. Therefore, best condition, in this case, was found for LCG culture medium, with 20 g/L of glucose and under stirring of 150 rpm (condition C11, Table 3).
The yield of kojic acid in the extracts was determined using high-performance liquid chromatography with external calibration. Methanol solutions of kojic acid at known concentrations were injected in the HPLC chromatograph individually in order to trace a calibration curve. Kojic acid peak presented a wave length with maximum absorption in 270 nm and 100% of chromatographic pureness. The calibration curve, constructed through the intersection of the area of the chromatographic peaks and the nominal concentration, can be visualized in Figure 3. The procedure for adjustment of the straight line was carried through by the method of linear regression. Linear regression equation was y = 139419507. 07x - 2992550.64 and the coefficient of correlation r 2 = 0.99. Kojic acid was screened by antimicrobial assays against A. flavus, C. albicans, E. coli and S. aureus and its biological activity was compared to the activities detected for the crude extracts obtained from A. parasiticus in the diversified growing conditions utilized in this study. Figure 4 shows kojic acid antimicrobial profile as determined by the biological screening performed in this study.
Discussion
In this study, manipulation of growing conditions focused six different culture media: CYA, YES, LCG20, LCG150, LCS20 and LCS150. The CYA, YES and LCS possess sucrose as carbon source, varying only the composition of this component: CYA (30 g/L), LCS20 (20 g/L), YES and LCS150 (150 g/L). Culture medium LCG20 and LCG 150 present glucose as carbon source at concentrations of 20 and 150 g/L, respectively. As the nitrogen sources, LCG and LCS media have bacteriological peptone; medium YES has, in its composition, malt extract, and medium CYA, contains yeast extract. The production of metabolites by A. parasiticus was evaluated after 3, 6, 9, 12, 15, 18, 21, 24, 27 and 30 days and in presence or absence of stirring. The variation of these parameters has allowed to achieve a wide scope of biosynthetic responses, since, at the end, there were evaluated 120 different fermentation conditions. Many secondary metabolites of great economic importance are produced in low yields or even are not produced by some fungal species due to the use of unfavourable cultivation conditions (Bills et al., 2008). By the other hand, production of bioactive metabolites as a result of manipulation of growing conditions has been shown to be important in the discovery of novel major bioactive metabolites, as exemplified by Bode et al. (2002), which isolated more than 20 different metabolites from the same organism, varying only the cultivation conditions.
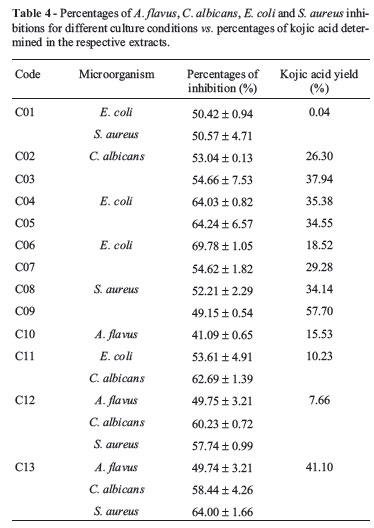
Since A. parasiticus is known for producing large amounts of kojic acid, it was necessary to evaluate the influence of this compound in the results. Therefore, kojic acid activity was determined against all microorganisms targeted by this study and quantified in all extracts. Kojic acid presented moderate activity against all the tested microorganisms under the conditions utilized in this study, ranging from 45.63 ± 0.09% against C. albicans and up to 63.73 ± 3.81% against S. aureus. Therefore, if the extracts activity were only related to the presence of kojic acid, extracts presenting greater percent production of kojic acid should also present higher percentage of microbial inhibition when compared to extracts containing lower amounts of kojic acid. However, the bioassays with the crude extracts evidenced that most of them presented inhibition rates higher than that of kojic acid, as shown in Table 4, for some selected conditions.
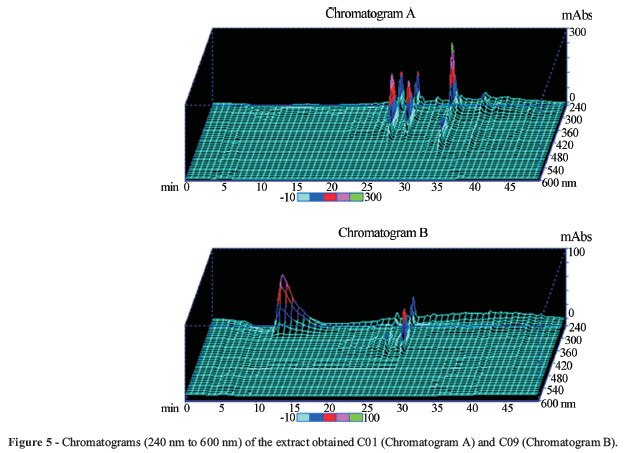
This observation can be exemplified by the extract codified as C01, that showed activities against the microorganisms E.coli and S. aureus (50.42 ± 0.94% and 50.57 ± 4.71% respectively) at levels not consistent with their respective kojic acid contents (0.04%), evidencing the presence of other antimicrobial metabolites in the extracts. The presence of such novel bioactive compounds in the extracts was also observed in their HPLC-DAD profiles. Figure 5 (Chromatogram A) shows the chromatogram of extract C01.
Production of kojic acid by the extract obtained from the condition C09 reached 57.70%, where the carbon source used was sucrose, also used to prepare extract C01. In this case, nitrogen source, the culturing time (21 days) and the absence of agitation led to a different metabolites profile, as can be seen in Figure 5 (chromatograms A and B). In the chromatogram B (Figure 5) the peak for kojic acid can be visualized with the retention time of 10.38 min.
The same occurred for fungal extracts produced under conditions C11 and C12 that, despite producing low amounts of kojic acid (10.23 and 7.66% respectively) presented high inhibition of the tested microorganisms (Table 4).When comparing the HPLC chromatogram of kojic acid with the chromatograms of the extract produced with conditions C10 and C11 (without and with stirring respectively), represented in Figure 6, the presence of metabolites with retention times different of kojic acid was detected.
It is known that the use of glucose by microorganisms during the biosynthesis of kojic acid is essential (Bentley, 2006; El-Aasar, 2006), therefore higher yields ok kojic acid were observed in the culture media LCG that contained higher amounts of glucose (150 g/L). In the media LCS, where glucose was replaced by sucrose, production of kojic acid was also predominant since, in this case, glucose can be readily obtained upon sucrose hydrolysis. Interestingly, kojic acid production did not reach high yields when A. parasiticus was grown in the culture medium YES, which has also a high concentration of sucrose (150 g/L). In this case, other factors like cultivation time (6 days) and stirring (150 rpm) overcame glucose availability in the biosynthesis, leading to lower yield of kojic acid.
The diversity of metabolites produced by A. parasiticus in the different growing media utilized was accessed by HPLC-DAD, according to the number of peaks detected in the chromatograms with different retention indexes (RI) (Abreu et al., 2012). The fermentation carried out employing condition C01, in addition to presenting the lowest production of kojic acid, offered the largest production of other secondary metabolites. As previously discussed, fungal growing using YES medium in such specific condition did not favour kojic acid biosynthesis. It is possible that the metabolites diversification, in this case, arose from an alternative biosynthetic route using fructose, since sucrose enzymatic conversion to glucose also generates fructose, on an enzymatic reaction catalyzed by invertase.
Literature reports that alteration of the carbon source in the medium produces biological activity variation. For instance, extracts obtained using sucrose as a carbon source presented higher biological activity against microorganisms such as E. coli and Fusarium oxysporium when compared to extracts from culture media containing only glucose or fructose in their composition (El-Banna, 2006). Endophyte fungi Apiospora montagnei and Arthrinium state also produced extracts with high biological activity when cultured in media containing sucrose as carbon source (Ramos and Said, 2011). These data corroborate the results obtained, indicating that hydrolysis of sucrose into glucose and fructose allows the development of distinct biosynthetic routes for production of bioactive metabolites.
The biological tests results were very encouraging, especially those obtained for A. parasiticus extract produced in under culturing condition C01 that, in addition to present relevant activity against the bacteria E. coli and S. aureus, produced the highest metabolic diversity among all 120 different cultivation conditions evaluated in the biological and chromatographic screening. Use of medium LCG20 without stirring showed to be the most suitable condition (69.78 ± 1.05% inhibition) to produce metabolites active against E. coli. Among fungal conditions evaluated for production of bioactive metabolites, extract produced under condition C10 showed selectivity towards the fungus A. flavus (41.09 ± 0.65% activity). Conditions C02 C03 led to extracts selective towards the fungus C. albicans (53.04 ± 0.13 and 54.66 ± 7.53% activity respectively). These results demonstrate the great potential for production of bioactive metabolites under these specific conditions. Since the activity results are reported for crude extracts, antimicrobial activity in the range of 50% are extremely exciting, since major compounds in fungal extracts usually do not exceed 1% of the total extract.
Acknowledgments
The authors thank Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for finantial help.
Submitted: November 23, 2012
Approved: September 9, 2013.
All the content of the journal, except where otherwise noted, is licensed under a Creative Commons License CC BY-NC.
- Abreu LM, Costa SS, Pfenning LH, Takahashi JA, Larsen TO, Andersen B (2012) Chemical and molecular characterization of Phomopsis and Cytospora-like endophytes from different host plants in Brazil. Fungal Biol 116:249-260.
- Aly AH, Debbab A, Proksch P (2011) Fifty years of drug discovery from fungi. Fungal Divers 50:3-19.
- Bentley R (2006) From miso, sake and shoyu to cosmetics: a century of science for kojic acid. Nat Prod Rep 23:1046-1062.
- Bills GF, Platas G, Fillola A, Jimenez MR, Collado J, Vicente F, Martin J, Gonzalez A, Bur-Zimmermann J, Tormo JR, Pelaez F (2008) Enhancement of antibiotic and secondary metabolite detection from filamentous fungi by growth on nutritional arrays. J Appl Microbiol 104:1644-1658.
- Bizukojc M, Pawlak M, Boruta T, Gonciarz J (2012) Effect of pH on biosynthesis of lovastatin and other secondary metabolites by Aspergillus terreus ATCC 20542. J Biotechnol 162:253-261.
- Bode HB, Bethe B, Hofs R (2002) Zeeck Axel Big effects from small changes: possible ways to explore nature's chemical diversity. Chembiochem 3:619-27.
- Bok JW, Hoffmeister D, Maggio-Hall SA, Muillo R, Glasner JD, Keller NP (2006) Genomic Mining for Aspergillus Natural Products. Chem Biol 13:31-37.
- Butler MS (2008) Natural products to drugs: natural productderived compounds in clinical trials. Nat Prod Rep 25:475-516.
- Demain AL (2000) Microbial biotechnology. Trends Biotechnol 18:26-31.
- El-Aasar SA (2006) Cultural Conditions Studies on Kojic Acid Production by Aspergillus parasiticus Int J Agri Biol 8:468-473.
- El-Banna NM (2006) Effect of carbon source on the antimicrobial activity of Coryne-bacterium kutscheri and Corynebacterium xerosis Afr J Biotechnol 5:833-835.
- Harvey AL (2007) Natural products as a screening resource. Curr Opin Chem Biol 11:480-484.
- Hyde KD, Bahkali AH, Moslem MA (2010) Fungi - An unusual source for cosmetics. Fungal Divers 43:1-9.
- Hu M, Cai Y, Liao X, Hao Z, Liu J (2011) Development of an HPLC method to analyze and prepare elsinochrome C and hypocrellin A in the submerged fermentation broth of Shiria sp. SUPER - H168. Biomed Chromatogr 26:737-742.
- Lam KS (2007) New aspects of natural products in drug discovery. Trends Microbiol 15:279-289.
- Luo Y, Gao W, Doster M, Michailides TJ (2009) Quantification of conidial density of Aspergillus flavus and A. parasiticus in soil from almond orchards using real-time PCR. J Appl Microbiol 106:1649-1660.
- Mahmoud DA, Hassanein NM, Youssef KA, Zeid MAA (2011) Antifungal activity of different neem leaf extracts and the nimonol against some important human pathogens. Braz J Microbiol 42:1007-1016.
- Mellon JE, Zelaya CA, Dowd MK (2011) Inhibitory effects of gossypol-related compounds on growth of Aspergillus flavus Lett Appl Microbiol 52:406-412.
- Newman DJ, Cragg GM (2012) Natural Products as Sources of New Drugs over the 30 Years from 1981 to 2010. J Nat Prod 75:311-335.
- Novontny L, Rauko P, Abdel-Hamid M, Váchalková A (1999) Kojic acid - A new leading molecule for a preparation of compounds with an anti-neoplasic potential. Neoplasma 46:89-92.
- Perfect JR (2012) The Impact of the Host on Fungal Infections. Am J Medicine 125:S39-S51.
- Ramos HP, Said S (2011) Modulation of biological activities produced by an endophytic fungus under different culture conditions. Adv Biosci Biotechnol 2:443-449.
- Roze LV, Kptina AV, Laivenieks M, Beaudry RM, Jones DA, Kanarsky AV, Linz JE (2011) Volatiles influence growth, development, and secondary metabolism in Aspergillus parasiticus Appl Microbiol Biotechnol 92:359-370.
- Sajid I, Shaaban KA, Hasnain S (2011) Identification, Isolation and Optimization of antifungal metabolites from the Streptomyces malachitofuscus CTF9. Braz J Microbiol 42:592-604.
- Sanchez DG (2012) Fumagillin and Structrually Related Molecules as Source of New Drugs. Org Chem 9:126-142.
- Seneviratne CJ, Jin L, Samaranayake LP (2008) Biofilm lifestyle of Candida: a mini review. Oral Dis 14:582-90.
- Tanseer S, Anjum T (2011) Modification of C and N source for enhanced production of Cyclosporin `A' by Aspergillus terreus Braz J Microbiol 42:1374-1383.
- Takahashi JA, Lucas EMF (2008) Ocorrência e diversidade estrutural de metabólitos fúngicos com atividade antibiótica. Quím Nova 31:1807-1813.
- Takahashi JA, Castro MCM, Souza GG, Lucas EMF, Bracarense AAP, Abreu LM, Marriel IE, Oliveira MS, Floreano MB, Oliveira TS (2008) Isolation and screening of fungal species isolated from Brazilian cerrado soil for antibacterial activity against Escherichia coli, Staphylococcus aureus, Salmonella typhimurium, Streptococcus pyogenes and Listeria monocytogenes J Mycol Méd 18:198-204.
- Turbyville TJ, Wijeratne EMK, Whitesell L, Gunatilaka AAL (2005) The anticancer activity of the fungal metabolite terrecyclic acid A is associated with modulation of multiple cellular stress response pathways. Mol Cancer Ther 4:1569-1576.
Send correspondence to:
Publication Dates
-
Publication in this collection
29 May 2014 -
Date of issue
2014
History
-
Received
23 Nov 2012 -
Accepted
09 Sept 2013