Abstract
The aim of the study was to analyze epidemiological and microbiological aspects of oral colonization by methicillin-resistant Staphylococcus of health care workers in a cancer hospital. Interview and saliva sampling were performed with 149 health care workers. Antimicrobial resistance was determined by disk diffusion and minimum inhibitory concentration. Polymerase Chain Reaction, Internal Transcribed Spacer-Polymerase Chain Reaction and Pulsed Field Gel Electrophoresis were performed for genotypic characterization of methicillin-resistant Staphylococcus. Risk factors were determined by logistic regression. Methicillin-resistant Staphylococcus colonization prevalence was 19.5%, denture wearing (p = 0.03), habit of nail biting (p = 0.04) and preparation and administration of antimicrobial (p = 0.04) were risk factors identified. All methicillin-resistant Staphylococcus were S. epidermidis, 94.4% of them had mecA gene. Closely related and indistinguishable methicillin-resistant S. epidermidis were detected. These results highlight that HCWs which have contact with patient at high risk for developing infections were identified as colonized by MRSE in the oral cavity, reinforcing this cavity as a reservoir of these bacteria and the risk to themselves and patients safety, because these microorganisms may be spread by coughing and talking.
health personnel; occupational health; Staphylococcus; methicillin resistance; oncology service; hospital
RESEARCH PAPER
Methicillin-resistant Staphylococcus sp. colonizing health care workers of a cancer hospital
Dayane de Melo CostaI; André KipnisII; Lara Stefânia Netto de Oliveira Leão-VasconcelosII; Larissa Oliveira Rocha-VilefortIII; Sheila Araújo TellesIV; Maria Cláudia Dantas Porfírio Borges AndréII; Anaclara Ferreira Veiga TippleIV; Ana Beatriz Mori LimaIII; Nádia Ferreira Gonçalves RibeiroIII; Mayara Regina PereiraI; Marinésia Aparecida Prado-PalosIV
INúcleo de Estudos e Pesquisa de Enfermagem em Prevenção e Controle de Infecções Relacionadas à Assistência à Saúde, Faculdade de Enfermagem, Universidade Federal de Goiás, Goiânia, GO, Brazil
IIInstituto de Patologia Tropical e Saúde Pública, Universidade Federal de Goiás, Goiânia, GO, Brazil
IIISecretaria Municipal de Saúde de Goiânia, Goiânia, GO, Brazil
IVFaculdade de Enfermagem, Universidade Federal de Goiás, Goiânia, GO, Brazil
Correspondence Correspondence: D.M. Costa Núcleo de Estudos e Pesquisa de Enfermagem em Prevenção e Controle de Infecções Relacionadas à Assistência à Saúde Faculdade de Enfermagem Universidade Federal de Goiás Rua 60, Qd 107, Lt 01, nº 19, Setor Central 74055-160 Goiânia, GO, Brazil E-mail: daynesaga@yahoo.com.br
ABSTRACT
The aim of the study was to analyze epidemiological and microbiological aspects of oral colonization by methicillin-resistant Staphylococcus of health care workers in a cancer hospital. Interview and saliva sampling were performed with 149 health care workers. Antimicrobial resistance was determined by disk diffusion and minimum inhibitory concentration. Polymerase Chain Reaction, Internal Transcribed Spacer-Polymerase Chain Reaction and Pulsed Field Gel Electrophoresis were performed for genotypic characterization of methicillin-resistant Staphylococcus. Risk factors were determined by logistic regression. Methicillin-resistant Staphylococcus colonization prevalence was 19.5%, denture wearing (p = 0.03), habit of nail biting (p = 0.04) and preparation and administration of antimicrobial (p = 0.04) were risk factors identified. All methicillin-resistant Staphylococcus were S. epidermidis, 94.4% of them had mecA gene. Closely related and indistinguishable methicillin-resistant S. epidermidis were detected. These results highlight that HCWs which have contact with patient at high risk for developing infections were identified as colonized by MRSE in the oral cavity, reinforcing this cavity as a reservoir of these bacteria and the risk to themselves and patients safety, because these microorganisms may be spread by coughing and talking.
Key words: health personnel, occupational health, Staphylococcus, methicillin resistance, oncology service, hospital.
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) is recognized as an important pathogen in the context of health care-associated infections (HAIs). Over recent years, coagulase-negative Staphylococcus (CoNS), has also distinguished itself in HAIs, concurrently with advances in medical practice including increased use of implanted devices, and an increased number of immunocompromised patients (Casey et al., 2007).
Health care workers (HCWs) colonized with methicillin-resistant Staphylococcus (MRS) may serve as reservoirs and potential spreaders of these bacteria in health care and community settings (Eveillard et al., 2004; Albrich and Harbarth, 2008; Liakopoulos et al., 2008; Lis et al., 2009; Nubel et al., 2013). In addition, the HCWs themselves may suffer to this condition, getting infection due to colonization (Albrich and Harbarth, 2008; Haamann et al., 2011). In most studies, nasal colonization is investigated (Albrich and Harbarth, 2008). However, the oral cavity (saliva) has been identified as a potential reservoir for these microorganisms (Ohara-Nemoto et al., 2008; Rosa et al., 2009; Prado-Palos et al., 2010), which may be spread by coughing and talking. Further, upper respiratory tract infections are among the most frequent in HCWs colonized by MRSA (Albrich and Harbarth, 2008; Haamann et al., 2011).
Due to severity of infections by Staphylococcus and its multidrug resistance, HCWs colonized by these bacteria who treat of patients considered at high risk for infections (such as cancer patients) require particular attention. These patients are especially susceptible to infections due to, among other factors, a suppressed immune system caused by underlying malignancy and its treatment (Kamboj and Sepkowitz, 2009).
In this context, we aimed to evaluate epidemiological and microbiological aspects of the oral colonization by MRS of HCWs in a cancer hospital.
Material and Methods
Design, local and population
This cross-sectional study was carried out from May 2009 to November 2010 in a hospital with 212 beds, referral for cancer treatment in Central Brazil, located in Goiânia, Goiás. Study population was composed by HCWs who worked in the wards (WD) (WD1A, WD1B, WD1C, WD2A, WD3A e WDBC), surgical center (SC), service of hospital infection control (SHIC), dressing sector (DS), endoscopy sector (EDS), emergency sector (ES), chemotherapy sector (adult and child) (QTS), rehabilitation and physiotherapy sector (RFS), radiotherapy sector (RTS), intensive care unit (ICU) and bone marrow transplantation (BMT), and administrative secretaries of the ICU. Those who exercised administrative function, except in the ICU, and those who were using antimicrobial agents at the time or within the last seven days before data collection were excluded.
The study was approved by the Ethical Committee of the Associação de Combate ao Câncer em Goiás (ACCG) (Protocol: CEP-ACCG/040/08), in agreement with the Declaration of Helsinki. Interview and saliva sampling were performed after signing of the Informed Consent Term by the participants.
Data and saliva sampling
Data were collected through interviews, and saliva collection (1.0 mL), without stimulation (Nauntoffe et al., 2005) in sterilized polyethylene bottles, which were transferred to the laboratory, stored to 2 to 8 °C and processed within 24 h of collection. Data and saliva sampling were collected by researchers and trained undergraduate nursing students from the Universidade Federal de Goiás.
Microbiological procedures
The saliva collected (20 µL) were streaked on Trypticase Soy Agar (TSA), Trypticase Soy Broth (TSB) with 4% NaCl and 6 µg/mL of oxacillin, and Mannitol Salt Agar, incubated at 35 °C for 48 h. Characteristic colonies of Staphylococcus were submitted to Gram's staining and standard identification tests (Koneman et al., 2008). S. aureus and CoNS were tested for antimicrobial susceptibility by disk diffusion (Clinical and Laboratory Standards Institute, 2007; 2009) to: cefoxitin (30 µg), ciprofloxacin (5 µg), clindamycin (2 µg), dalfopristin-quinupristin (15 µg), erythromycin (15 µg), gentamicin (10 µg), linezolid (30 µg), mupirocin (5 µg), oxacillin (1 µg), rifampicin (5 µg), sulfamethoxazole-trimethoprim (25 µg) and tetracycline (30 µg). The S. aureus and CoNS that were resistant to oxacillin and/or cefoxitin on the disk diffusion test were tested in the minimum inhibitory concentration (MIC) to oxacillin by Etest®. S. aureus with MIC ≥ 4 µg/mL and CoNS with MIC ≥ 0.5 µg/mL were considered meticillin-resistant (Clinical and Laboratory Standards Institute, 2009). ATCC 25923 S. aureus was used as quality control.
Molecular typing of MRS
The mecA gene in MRS was verified through the Polymerase Chain Reaction (PCR) (Murakami et al., 1991). The species of MR-CoNS were identified by Internal Transcribed Spacer-PCR (ITS-PCR), with minor modifications (Couto et al., 2001). Pulsed Field Gel Electrophoresis (PFGE) with SmaI was performed as described previously (Chung et al., 2000).
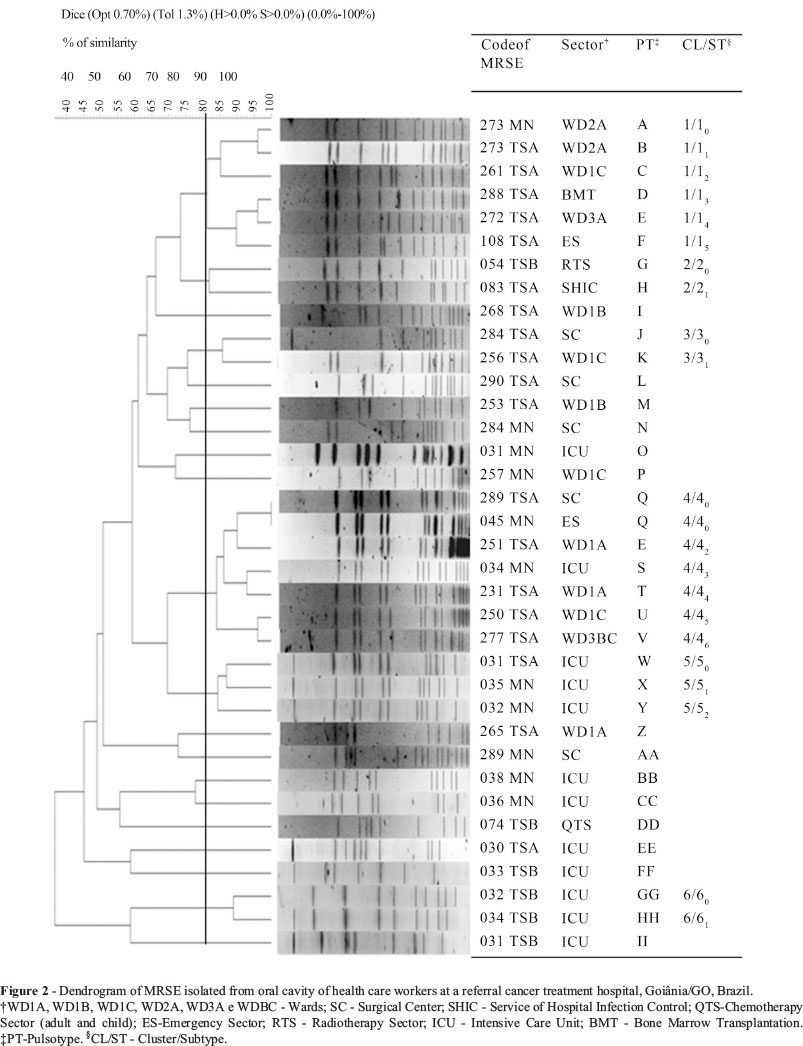
The PFGE were interpreted according to the Tenover et al. (1995) criteria and by computed analysis through the program BioNumerics (version 5.0; Applied Maths, Ghent, Belgium). The dendrogram was constructed based on the position and presence of bands and unweighted pair group method for arithmetic averages and using Dice coefficient of similarity with optimization and tolerance parameters of 0.7% and 1.3%, respectively. The pulsotype (PT) was defined as a single electrophoretic profile identified with one or two capital letters. Cluster (CL) was defined as a group of profiles (n >2) with a coefficient ≥ 80% of similarity and identified with Arabic numeral. The subtypes within the same cluster were identified with the cluster number added as a subscript Arabic numeral.
Statistical analysis
Statistical evaluations were performed using Statistical Package for the Social Sciences, version 18.0 (SPSS Inc., Chicago, IL). Prevalence of colonization by Staphylococcus spp. and MRS were calculated to a confidence interval of 95% (95% CI). Univariate analysis was performed to estimate the odds ratio of colonization by MRS associated with the prediction variables investigated: age, sex, level of education, occupation, work shift, work contract,length of professional activity, working at another health care setting, preparation and administration of antimicrobial, use of personal protective equipment, frequency of hand hygiene, use of ring, watch/bracelet, long earrings and necklace, nails size, habit of nail-biting, eating outside the feeding area, use of mouthwash, denture wearing, self-medication (antimicrobial) and frequency ofupper respiratory tract infection.Those that had p values < 0.10 were submitted to multivariate analysis using the logistic regression model. Proportion differences were compared using the chi-square test (χ2) or Fisher's exact test, when appropriate. P values < 0.05 were considered statistically significant.
Results
Participated of the study 53.6% (149/278) of the total population, the most were female (129/149; 86.6%), nursing technician (112/149; 75.2%) and worked at WD (42/149; 28.2%) (Table 1).
The prevalence of HCWs colonized by CoNS was 25.5% (38/149), by S. aureus (21/149) was 14.1% and by both was 6.7% (10/149). MR-CoNS were isolated from 19.5% (29/149) (95% CI: 13.7-26.4) of them. MRSA was not detected.
Among the variables investigated, denture wearing, habit of nail biting, as well as preparation and administration of antimicrobial were independently associated with colonization by MRS (Table 2).
Sixty-one (66.3%) CoNS and 31 (33.7%) S. aureus were isolated. According to the MIC to oxacillin, 59.0% (36/61) of CoNS were methicillin-resistant. The resistence to other antimicrobials tested was more prevalent among MR-CoNS than those methicillin-sensitive (Figure 1). All MR-CoNS isolates were identified as S. epidermidis, and 94.4% of them were mecA gene positive.
The prevalence of antimicrobial susceptibility profile of S. aureus isolates was: 83.9% to erythromycin, 87.1% to tetracycline, 90.3% to clindamycin, 90.3% to rifampicin, 93.6% to gentamicin, 96.8% to mupirocin and trimethoprim-sulfamethoxazole, and all isolates (100%) were sensitive to ciprofloxacin, dalfopristin/quinupristin and linezolid.
The dendrogram constructed (Figure 2) from the restriction patterns of chromosomal DNA generated by PFGE showed: 35 pulsotypes (A to II), six clusters (1-6) and 21 subtypes. The two biggest clusters, 1 and 4, were composed by methicillin-resistant S. epidermidis (MRSE) isolates from five and seven HCWs, respectively, of distinct sectors. Two MRSE of cluster 4 (subtype 40), isolated from HCWs of the SC (289 TSA) and ES (045 MN), were indistinguishable.
Discussion
In this study, S. aureus and CoNS, including MRSE, were isolated from the oral cavity of HCWs from a referral cancer treatment hospital. The presence of MRSE (Rosa et al., 2009) and MRSA (Prado-Palos et al., 2010) was identified in the oral cavity of HCWs from public hospitals in Brazil, reinforcing this cavity as a reservoir of these bacteria and the risk to HCWs' health. Additionally, there is the potential exchange of Staphylococcus spp. between this cavity and the nasal cavity (Ohara-Nemoto et al., 2008).
Poor hygiene of dentures may influence the presence of MRSE in the saliva of the denture-wearing population. Moreover, association between the isolation of Candida from the oral cavity and hands/fingers prosthesis users was reported (Darwazeh et al., 2001). Therefore, adequate denture and hand washing, especially to HCWs, are emphasized as simple and safe measures to prevent colonization by these microorganisms.
It is known that subungual areas harbor high concentrations of bacteria, predominantly CoNS (McGinley et al., 1988). Thus, the habit of nail-biting contributes to the transport of microorganisms to the oral cavity and is characterized as a bad hygiene habit and a risk behavior, particularly to HCWs. This also highlights the importance of keeping nails natural, clean, and short, with no artificial fingernails (World Health Organization, 2009).
We suggest a possible explanation for the association between preparation and administration of antimicrobial agents and colonization by methicillin-resistant isolates may be related to inadequate attitudes of HCWs during this procedure, when handling them without gloves, improper disposal of materials/waste used, and no hand washing, providing the selective pressure of bacteria on the hands and environment of drug compounding. However, no evidence was found about this issue, indicating the needed investigation about this relationship.
MRSE was more resistant to other antibiotics tested when compared to methicillin-susceptibility CoNS (Figure 1), emphasizing that methicillin-resistant isolates are frequently resistant to antimicrobial non-beta lactams indicated for the treatment of staphylococcal infections. For example, 47.4% of MRSE were resistant to mupirocin which is recommended for nasal decolonization by MRSA. It is interesting to emphasize that S. epidermidis might be a possible reservoir of the mupA gene, which confers resistance to mupirocin, for MRSA (Hurdle et al., 2005). However, all S. aureus, CoNS and MRSE were sensitive to dalfopristin/quinupristin and linezolid, indicating that these antimicrobials are effective to treat infections by them.
Closely related isolates (Clusters 1-6) were identified among HCWs in different sectors and MRSE isolated from two HCWs, one in SC and one in ES, were genetically indistinguishable (Cluster 4/subtype 40). The close relation between isolates from HCWs may be due to nosocomial exposure of the workers by a dominant group of MRSE.
Significant reduction in the incidence of MRSA in a trauma ICU was observed after identification and decolonization of colonized HCWs (Ben-David et al., 2008), emphasizing that unrecognized MRSA-colonized HCWs may serve as significant reservoirs, and thus could impair other infection control measures (Ben-David et al., 2008; Nubel et al., 2013). We agree with this view and extend it to HCWs colonized by MR-CoNS. We also believe that knowledge of MRS colonization may contribute to changing HCWs attitudes in relation to prevention and control measures (Eveillard et al., 2004). With regard to occupational safety, colonization or infection of HCWs with MRSA should be considered an occupational hazard and injury according to local legislation (Albrich and Harbarth, 2008; Dulon et al., 2013), and we emphasize that colonization by MR-CoNS also represents a risk to HCWs' health.
In conclusion, HCWs which have contact with patient at high risk for developing infections were identified as colonized by MRSE in the oral cavity, highlighting a risk to themselves and patients safety, and other individuals in the hospital and community/home. Furthermore, it reinforces the role of the oral cavity (saliva) as a reservoir of these bacteria and the need to re-think policies for occupational health combined with the control of multidrug-resistant organisms and, consequently, to contribute to the successful control of HAIs.
This study has limitations regarding the method to data collection, this was based only on subjectivity of the report of the participants and the size of studied population, this was relatively small, so the generalization of the risk factors for colonization by MRSE, to other health care settings may be limited. Therefore, further studies on this theme are needed.
Acknowledgments
We thank PhD Fabiana Cristina Pimenta, PhD Milca Severino Pereira, Núcleo de Estudos e Pesquisa de Enfermagem em Prevenção e Controle de Infecções Relacionadas à Assistência à Saúde, Laboratório de Bacteriologia Médica and the Laboratório de Bacteriologia Molecular of the Instituito de Patologia Tropical e Saúde Pública of the UFG.
Submitted: February 11, 2013
Approved: December 13, 2013
All the content of the journal, except where otherwise noted, is licensed under a Creative Commons License CC BY-NC.
- Albrich WC, Harbarth S (2008) Health care workers: source, vector or victim of MRSA? Lancet Infect Dis 8:289-301.
- Ben-David D, Mermel LA, Parenteau S (2008) Methicillin-resistant Staphylococcus aureus transmission: the possible importance of unrecognized health care worker carriage. Am J Infect Control 36(2):93-7.
- Casey AL, Lambert PA, Elliott TS (2007) Staphylococci. Int J Antimicrob Agents 29(Suppl 3):S23-32.
- Chung M, Lencastre H, Matthews P, Tomasz A, Adamsson I, Aires de Sousa M et al. (2000) Molecular typing of methicillin-resistant Staphylococcus aureus by pulsed-field gel electrophoresis: comparison of results obtained in a multilaboratory effort using identical protocols and MRSA strains. Microb Drug Resist 6:189-198.
- Clinical and Laboratory Standards Institute-CLSI (2007) Performance standards for antimicrobial disk diffusion susceptibility testing; 17th informational supplement. Approved standard M100-17. Clinical and Laboratory Standards Institute. Wayne, Pennsylvania.
- Clinical And Laboratory Standards Institute-CLSI (2009) Performance Standards for Antimicrobial Susceptibility Testing.In: CLSI. Abstract of the 19th Informational Supplement Document M100-S19, Wayne, Pennsylvania.
- Couto I, Pereira S, Miragaia M, Sanches IS, Lencastre H (2001) Identification of clinical Staphylococcal isolates from humans by internal transcribed spacer PCR. J ClinMicrobiol 39:3099-3103.
- Darwazeh AMG, Al-Refai S, Al-Mojaiwel S (2001) Isolation of Candida species from the oral cavity and fingertips of complete denture wearers. J Prosthet Dent 86:420-423.
- Dulon M, Haamann F, Nienhaus A (2013) Involvement of occupational physicians in the management of MRSA-colonised healthcare workers in Germany-a survey. J Occup Med Toxico l8:16.
- Eveillard M, Martin Y, Hidri N, Boussougant Y, Joly-Guillou M (2004) Carriage of Methicillin-Resistant Staphylococcus aureus Among Hospital Employees: Prevalence, Duration, and Transmission to Households. Infect Control Hosp Epidemiol 25(2):114-120.
- Haamann F, Dulon M, Nienhaus A (2011) MRSA as an occupational disease: a case series. Int Arch Occup Environ Health 84:259-266.
- Hurdle JG, O'Neill AJ, Mody L, Chopra I, Bradley SF (2005) In vivo transfer of high-level mupirocin resistance from Staphylococcus epidermidis to methicillin-resistant Staphylococcus aureus associated with failure of mupirocin prophylaxis. J Antimicrob Chemother 56(6):1166-1168.
- Kamboj M, Sepkowitz KA (2009) Nosocomial infections in patients with cancer. Lancet Oncol 10:589-597.
- Koneman EW, Allen SD, Janda WM, Schreckenberger PC, Winn WCJ (2008) Diagnóstico Microbiológico: Texto e Atlas Colorido, 6th ed. Guanabara Koogan, Rio de Janeiro.
- Liakopoulos V, Petinaki E, Efthimiadi G, Klapsa D, Giannopoulou M, Dovas S et al. (2008) Clonal relatedness of methicillin-resistant coagulase-negative Staphylococci in the haemodialysis unit of a single university centre in Greece. Nephrol. Dial. Transplant 23(8):2599-2603.
- Lis DO, Pacha JZ, Idzik D (2009) Methicillin resistance of airborne coagulase-negative Staphylococci in homes of persons having contact with a hospital environment. Am J Infect Control 37:177-182.
- McGinley KJ, Larson EL, Leyden JJ (1988) Composition and Density of Microflora in the Subungual Space of the Hand. J Clin Microbiol 26(5):950-953.
- Murakami K, Minamide W, Wada K, Nakamura E, Teraoka H, Watanabe S (1991) Identification of methicillin-resistant strains of Staphylococci by polymerase chain reaction. J Clin Microbiol 29(10):2240-2244.
- Nauntoffe B, Tenovuo J, Lagerlof F (2005) Secreção e composição da saliva. In: Fejerskov O, Kidd E. (eds) Cárie Dentária: A Doença e seu Tratamento Clínico. 1st ed. Livraria Santos, São Paulo, p 7-48.
- Nubel U, Nachtnebel M, Falkenhorst G, Benzler J, Hecht J, Kube M, et al. (2013) MRSA transmission on a neonatal intensive care unit: Epidemiological and genome- based phylogenetic analyses. PloS One8(1):e54898.
- Ohara-Nemoto Y, Haraga H, Kimura S, Nemoto TK (2008) Occurrence of Staphylococci in the oral cavities of healthy adults and nasal-oral trafficking of the bacteria. J Med Microbiol 57:95-99.
- Prado-Palos MA, Costa DM, Gir E, Suzuki K, Pimenta FC (2010) Atuação de enfermagem em Unidades de Terapia Intensiva: implicações para disseminação de micro-organismo multirresistente. Rev Panam Infectol 12(1):37-42.
- Rosa JO, Moura JP, Palos MAP, Gir E, Reis C, Kipnis A (2009) Detecção do gene mecA em estafilococos coagulase negativa resistentes à oxacilina isolados da saliva de profissionais da enfermagem. Rev Soc Bras Med Trop 42(4):398-403.
- Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH et al. (1995) Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 33(9):2233-2239.
- World Health Organization (2009) World alliance for patient safety: WHO guidelines on hand hygiene in health care. First global patient safety challenge clean care is safer care. Geneva, Switzerland.
Publication Dates
-
Publication in this collection
04 Nov 2014 -
Date of issue
Sept 2014
History
-
Received
11 Feb 2013 -
Accepted
13 Dec 2013