Abstract
Similipal Biosphere Reserve (SBR) is a tropical moist deciduous forest dominated by the species Shorea robusta. To the best of our knowledge their rich biodiversity has not been explored in term of its microbial wealth. In the present investigation, soil samples were collected from ten selected sites inside SBR and studied for their physicochemical parameters and culturable soil fungal diversity. The soil samples were found to be acidic in nature with a pH ranging from of 5.1–6.0. Highest percentage of organic carbon and moisture content were observed in the samples collected from the sites, Chahala-1 and Chahala-2. The plate count revealed that fungal population ranged from 3.6 × 104–2.1 × 105 and 5.1 × 104–4.7 × 105 cfu/gm of soil in summer and winter seasons respectively. The soil fungus, Aspergillus niger was found to be the most dominant species and Species Important Values Index (SIVI) was 43.4 and 28.6 in summer and winter seasons respectively. Among the sites studied, highest fungal diversity indices were observed during summer in the sites, Natto-2 and Natto-1. The Shannon-Wiener and Simpson indices in these two sites were found to be 3.12 and 3.022 and 0.9425 and 0.9373 respectively. However, the highest Fisher’s alpha was observed during winter in the sites Joranda, Natto-2, Chahala-1 and Natto-1 and the values were 3.780, 3.683, 3.575 and 3.418 respectively. Our investigation revealed that, fungal population was dependent on moisture and organic carbon (%) of the soil but its diversity was found to be regulated by sporulating species like Aspergillus and Penicillium.
diversity indices; fungal population; SIVI
Introduction
In the forest ecosystems, the growth and diversity of microorganisms are regulated by different physical and chemical properties like pH, organic carbon, P and K contents of the soil (Wardle et al., 2004Wardle DA, Bardgett RD, Klironomos JN et al. (2004) Ecological linkages between aboveground and belowground biota. Science 304:1629e1633.; Ogram et al., 2006Ogram A, Bridgham S, Corstanje R et al. (2006) Linkages between microbial community composition and biogeochemical processes across scales. In: Verhoeven JTA, Beltman B, Bobbink R et al.(eds) Wetlands and Natural Resource Management. Springer, Berlin, pp. 239–268.). Rhizosphere, which is a complex environment of forest ecosystems, is an intense site of microbial activity that harbours a great diversity of microorganisms affecting plant growth and health (Khan et al., 2007Khan MS, Zaidi A, Wani PA (2007) Role of phosphate-solubilizing microorganisms in sustainable agriculture - A review Agronom for Sustain Develop 27:29–43.). Among various microorganisms, rhizospheric fungi play an important role for decomposition of different macromolecules like celluloses, hemicelluloses and peptides in forest ecosystem (Berg and McClaugherty, 2003Berg B, McClaugherty C (2003) Plant Litter: Decomposition, Humus Formation, Carbon Sequestration. Spinger, Berlin.; Hanson et al., 2008Hanson CA, Allison SD, Bradford MA et al. (2008) Fungal taxa target different carbon sources in forest soil. Ecosystems 11:1157–1167.; McGuire et al., 2010McGuire KL, Bent E, Borneman J et al. (2010) Functional diversity in resource use by fungi. Ecol 9:2324–2332.). The population size and distribution of these organisms is often influenced by the abundance and nature of the organic content of the soil, climatic conditions, surface vegetation and soil texture (Marschner et al., 2003Marschner P, Kandeler E, Marschner B (2003) Structure and function of the soil microbial community in a long- term fertilizer experiment. Soil Biol Bioch 35:453–461.).
Recent studies suggested that there may be around 1.5 to 5.1 million extant fungal species and about 1200 new species are being described in each year (Hibbett et al., 2011Hibbett DS, Ohman A, Glotzer D et al. (2011) Progress in molecular and morphological taxon discovery in Fungi and options for formal classification of environmental sequences. Fungal Biol Rev 25:38–47.). Further, O’Brien et al. (2005)O’Brien HE, Parrent JL, Jackson JA et al. (2005) Fungal community analysis by largescale sequencing of environmental samples. Appl Environ Microbiol 71:5544–5550. were of the opinion that less that 2–6% of the fungal species are currently described. This indicated that there are many more fungal species to be explored, named and identified. Therefore, studying fungi in tropical forests, unexplored habitats and lost or hidden species could contribute sustainable to global fungal diversity (Hawksworth and Rossman, 1997Hawksworth DL, Rossman AY (1997) Where are all the undescribed fungi? Phytopath 87:888–891.). The estimation of fungal species diversity at any given site of any ecosystem may be a difficult task for mycologist because of the fact that fungal communities are highly diverse, cryptic and ephemeral in nature. Besides, probability of encountering and recording all species present during any sampling effort is low (Tran et al., 2006Tran HTM, Stephenson SL, Hyde KD et al. (2006) Distribution and occurrence of myxomycetes in tropical forests of northern Thailand. Fungal Divers 22:227–242.; Aime and Brearley, 2012Aime MC, Brearley FQ (2012) Tropical fungal diversity: closing the gap between species estimates and species discovery. Biodivers Conserv 21:2177–2180.).
Similipal hills located in the midst of Mayurbhanj district of Odisha state, India and located in between 21°28′ to 22°08′N latitude and 86°04′ to 86°37′ E longitude and covering 5,578 km2 of forest land. The Similipal Biosphere Reserve has been divided into three zones, i.e. Core zone (845 km2), Buffer zone (2174 km2) and transitional zone (2559 km2). The climates of Similipal is tropical with warm and humid, summer temperature around 40 °C during peak of the season. The rainy season starts from mid of June to October with a rainfall of about 125 mm in the monsoon. Winter creeps in gradually from mid October and becomes severe in December lowering temperature up to 5 °C in many parts of the hill. This uneven geophysical condition influences the diversity of floral and faunal distribution. Except few sporadic reports (Bahuti et al., 2006Bahuti R, Rath CC, Mohapatra U (2006) Phsico- chemical and mycological studies of selected soil samples from Similipal Biosphere Reserve. Plant Sci 28:1–7.; Behera et al., 2009Behera M, Dandapat J, Rath CC (2009) Isolation, characterization and screening of bacteria isolates from Similipal Biosphere Reserve forest soil for their metal tolerance capacity and extracellular enzymatic activities. Biorem Biodiv Bioavail 3:72–78.) scientific studies of SBR in terms of microbial wealth is not available in literature. This prompted us to carry out the present investigation to study the fungal diversity of this unique ecosystem in different seasons with respect to their physicochemical factors, in order to explore the importance of fungal population in maintenance of soil quality and to exploit their biotechnological potentials in near future.
Materials and Methods
Collection of soil samples
Hundred soil samples were collected from ten different sites (Chahala-1&2, Bareipani, Natto1-&2, Gurgudia-1&2, Pithabata-1&2 and Joranda) particularly from rhizosphere during a time span of 2 years (2010–2012) representing both summer and winter seasons (Figure 1). All ten sites differ from each other on the basis of physical and chemical nature of soil. The Chahal-1&2 are within core region and covered by dense forest. Natto-1 &2 are characterized by dense vegetation with slope position of 20–30°. Bareipani is located at high elevation of 776 m above sea level. The vegetation of Joranda is characterized by numerous streams and water fall with dense forest cover, Pithabota- 1 &2 are located in between buffer and core areas. The Gurgudia-1&2 are located in the buffer area of the Biosphere reserve. Random sampling was carried out in each plot following the method of Baruah and Barthakur (1998)Baruah TC, Barthakur HP (1998) A Text Book Soil Analysis. Vikas publ, New Delhi.. Each soil sample represents composite of well mixed five sampling units collected from each plot of 20 × 20 m. Soil pH, temperature, humidity, P, K and percentages of organic carbon was determined by following standard soil analysis methods (Alexander, 1972Alexander M (1972) Introduction to Soil Microbiology, 2nd edition. Wiley and Sons, New York; Baruah and Barthakur, 1998Baruah TC, Barthakur HP (1998) A Text Book Soil Analysis. Vikas publ, New Delhi.).
Soil sample collection sites in Similipal Biophere Reserve: (1) Natto- 1, (2) Natto-2, (3) Chahala-1, (4) Chahala-2, (5) Gurgudia-1, (6) Gurgudia-2, (7) Joranda, (8) Bareipani, (9) Pithabata-1, and (10) Pithabata-2.
Isolation and enumeration of fungi
Fungi were isolated and enumerated following standard microbiological culture technique on Potato Dextrose Agar (PDA) by total plate count method (Cruickshank et al., 1973Cruickshank K, Duguid JP, Marmion BR et al. (1973) Medical Microbiology, A Guide to the Laboratory Diagnosis & Control of Infection, 12th edition. Edinburgh: ELBS and Churchill Livingstone, Great Britain.). The fungal isolates were identified based on their colony morphology, growth pattern on medium and reproductive characters using the standard identification manuals (Gilman, 1971Gilman JC (1971) A Manual of Soil Fungi. Iowa State College Press, Ames, Lowa.; Barnett and Hunter, 1996Barnett HL, Hunter BB (1996) Illustrated Genera of Imperfect Fungi. APS Press, St. Paul.).
Quantification of the species (Species Important Value Index, SIVI)
The relative abundance, relative frequency and relative density of species were determined using the following formulae (Dash, 2001Dash MC (2001) Fundamental of Ecology. Tata McGrow-Hill Publ., New Delhi.).
Relative Abundance (RA) = Abundance of a species per sampling sites divided by total number of abundance value based on number.
Relative Frequency (RF) = Number of sampling sites containing a species divided by sum of frequency of all species.
Relative density (RD) = Number of individuals of a species in all sampling sites divided by number of individuals of all species in all sampling sites.
Species Importance Values Index (SIVI) was calculated by the sum of three parameters (RA+RF+RD).
Diversity analyses
Fisher’s α, Shannon’s diversity index (H′), Shimpson’s diversity index (λ), Margalef’s richness index (RI), Menhinick’s richness index (R2), and Evenness indices (E1, E2, E3, E4 and E5) diversity indices were calculated using software of Paleontological Statistics (PAST ver. 2.0; Ryan et al., 1995Ryan PD, Harper DAT, Whalley JS (1995) PALSTAT, Statistics for Palaeontologists. Chapman and Hall, Dordt.).
Community ordination techniques
The variations of population size among the sampling area in two seasons were summarized and the relations between sites were determined by Principal Components Analysis (PCA). The logarithmic values of population of each site were arranged in relation to multi condition axes (axis 1, 2 and 3) on PCA. These data were used to calculate the Eigen value based on which co-ordinate axes were represented so as to produce information on the similarity of the habitat types. To test for linearity of population structure among the sites, the data was further evaluated to Detrended correspondence analysis. Thus, genera were calculated to be highest SIVI was considered for determination of inter species relation distribution in two different seasons by measuring Euclidean distance, using PAST.
Results
Physico chemical parameters of the soil
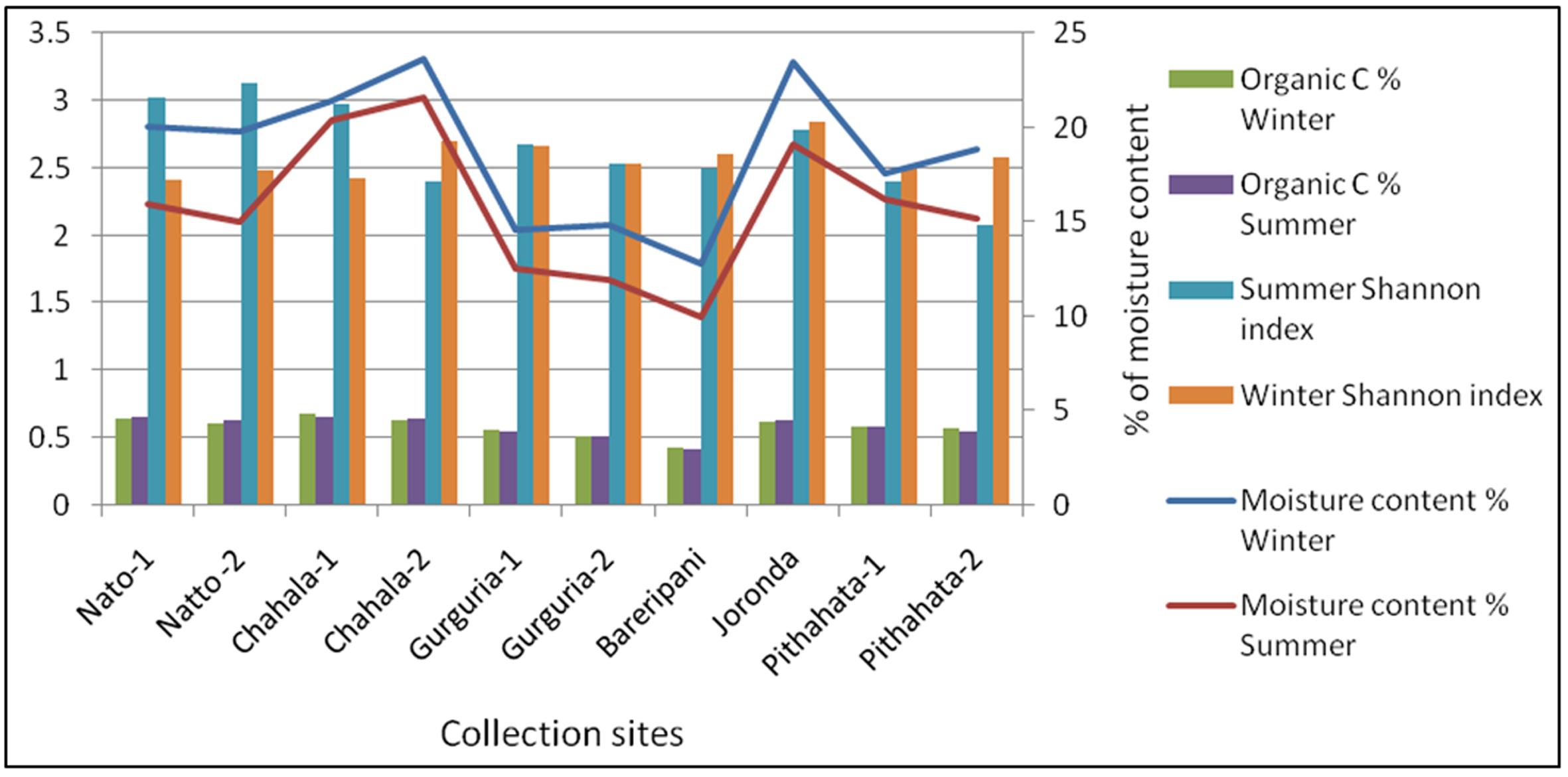
A total of 260 fungal isolates were obtained from 100 soil samples collected from 10 different sites of Similipal Biosphere Reserve in two seasons. The physico-chemical parameters of the soil samples and its associated plant species were studied (Table 1). All the soil samples were found to be acidic in nature, and highest acidity, moisture and organic carbon percentage were observed in the soil sample collected from Chahala-1. Whereas, low moisture content and organic carbon percentage were recorded from Bareripani site. The salinity of the soil samples were found to be even (0.5) except increasing trend was observed in the soil samples collected from Gurgudia. The dominant plant species occurring in the collected sites was Shorea robusta.
Fungal population
Occurrence of fungal propagules in the soil samples were enumerated by standard plate count methods (pour and spread plate methods) and expressed in cfu gm−1 of soil. Soil fungal population was observed to vary among different sites. The fungal population ranged from 3.6 × 104 to 2.1 × 105 in summer and 5.1 × 104 to 4.7 × 105during winter. In comparison, spread plate method showed higher number of fungal propagules than pour plate technique. Among the sites, highest fungal population was observed in the soil sample collected from Chahala-2 in both the seasons whereas, lowest fungal population was observed in the sample collected from Bareipani (Figure 2).
Enumeration of culturable fungi in different sites of Similipal Biosphere Reserve, through spread and pour plate methods at two different seasons.
Species important values index (SIVI)
Species important values index is used to determine the overall importance of identified species in the community structure (Table 2). It gives an idea of the sociological structure of a species in its totality in the community. The result indicated that Aspergillus niger and Aspergillus awamorishowed highest SIVI values. Aspergillus species were found to be the most dominant species among the community in all sampling sites. The dominant species of Aspergillus were identified as A. alliceus, A. flavus, A. fornscecous, A. humicola, A. luchuensis, A. niger, A. awamori, A. tamarine, A. terrus, A. wentii, A. ustus and A. panamensis.
Species diversity and richness
The species diversity and richness of soil fungi were studied by different diversity indices in different sites and seasons (Tables 3, 4). Shannon diversity index (H) was calculated as it represents the number of individuals as well as number of taxa in each collected site. Maximum value of H was observed at Natto-2 (3.12) and Joranda (2.84) in summer and winter seasons respectively (Figure 3). Simpson diversity index (1-D) was also studied as it measures the dominant species. Highest 1-D was observed at Natto-2 (0.942) during summer while during winter was observed in Chahala-2 (0.903). The Dominance (D) indicates dominancy of a particular species. The value was highest at Pithabata-2 (0.2127) during summer. But in Natto-2, the D value was higher in winter (0.1557) and lower in summer (0.05746). However, lowest D value was observed at Chahala-2 (0.09694) in winter. Besides, Fisher alpha diversity indicated highest value in the soil sample of Joranda in both seasons.
Diversity indices of fungi at different sites of Similipal Biosphere Reserve, during summer season.
Diversity indices of fungi at different sites of Similipal Biosphere Reserve, during winter season.
Sometimes one sample may contain a larger number of taxa, while the other has a larger diversity index. Therefore, a number of diversity indices may be compared to make sure that the diversity ordering is robust. A formal way of doing this is to define a family of diversity indices, dependent upon a single continuous parameter. So the diversity profile was carried out among the samples in different sites. The diversity profile of the sites, Natto-1 and Natto-2, Chahala-1, Chahala-2, Bareipani and Gurgudia did not intercept with each other, indicating the distinctness of their diversity indices (Figure 4). Further, species richness was studied by Menhinick- Margalef diversity indices (Smax). The result indicated that maximum species richness was reported in the sampling site of Joranda but less in Bareipani. Besides, Berger Parker dominance index was calculated which expresses the proportion of the total share that is due to the dominant species. In comparison to the species components in sampling sites with respect to their population size, Berger-Parker dominance index expressed total higher number of species sharing the communities in the sites Pithabata-2, followed by Bareipani, Pithabata-1, Joranda, Chahala-1, Chahala-2, Gurguria-2, Guruguria-1, Natto-1 and Natto-2 during summer season. While, it was found to be highest in Bareipani followed by Pithabata-1, Natto-2 and Chahala-1 during winter.
Depicting the diversity profile of fungi in different sites of Similipal Biosphere Reserve.
Species evenness
Species evenness is also an important component of diversity index as it calculates the distribution pattern of species among the sites. Simpson index (1-Shannon index) measures evenness of the community from zero to one. The calculated values indicated approximately towards zero, which means, less evenness species distribution. At Natto-1 and Natto-2 during summer season, the distribution of species was found to be even in nature whereas, in Pithabat-2 species distribution was less even. But during winter, species evenness was more in Pithabata-2 followed by Bareipani and Chahala-2. Little variation of equitability values were observed among the sites.
Component ordination
PCA is used for the reduction of information on a large number of variables into a small set while losing only a small amount of information. It also helps in breaking down or partitioning a resemblance matrix into a set of perpendicular axes or components.
The presences of variables (population of sampling units) in different axis were provided maximum information about the ecological similarities among sites. Each PCA axis or components correspond to an Eigen value is the variance accounted for, by the axes. The Eigen values are extracted in the descending order of magnitude. Such that the corresponding PCA components represented greater to lesser amount variation in the matrix (Muthukrishna et al., 2012Muthukrishnan S, Sanjayan KP, Jahir HK (2012) Species composition, seasonal changes and community ordination of alkalotolerant micro fungal diversity in a natural scrub jungle ecosystem of Tamil Nadu, India. Mycosphere 3:92–109.). The values of PCA as co- relation among the sites were represented (Figure 5). The PCA ordination plotting of Component 1 vs. Component 2 indicates that the data of the fungal population sites showed highest +ve values for Chahala-1 and Chahala-2 around 0.9 to be static and more significantly modulated the fungal population in this ecosystem. On the other hand Pithabat-2 and Bareipani were positioned towards the −ve sides of the axis and values were less to be −0.40289 and −0.64711 respectively. Whereas, variation in the fungal population was observed in both while studying in spread and pour plates. Plotting Component-1vs. Component-3 also showed relatively same effect as that of spread plate and pour plate methods for encountering the fungal population. But, the summer population and winter population fall on alternate sides when Component-1 vs. Component-3 and Component-2 vs. Component-3 were plotted. Moreover, the populations of fungi in Chahala-2 and Joranda-1 sites were depicted on same sides of plotting represented less variation between the sites.
A,B,C graphs showing the principal component ordination. Position of ten sites based on fungal species abundance. A: Principal Component 1 vs 2. B: Component 1 vs 3. C: Component 2 Vs 3. A (Natto-1), B (Natto-2), C (Joranda) D (Chahala-1), E (Chalala-2), F (Gurgudia-1), G (Gurgudia-2), H (Bareipani), I(Pithabata-1) J (Pithabata-2), Sp.W. (spread plate winter), Sp.S. (spread plate summer) P.S. (pour plate summer) and P.W. (pour plate winter).
Detrended correspondence analysis
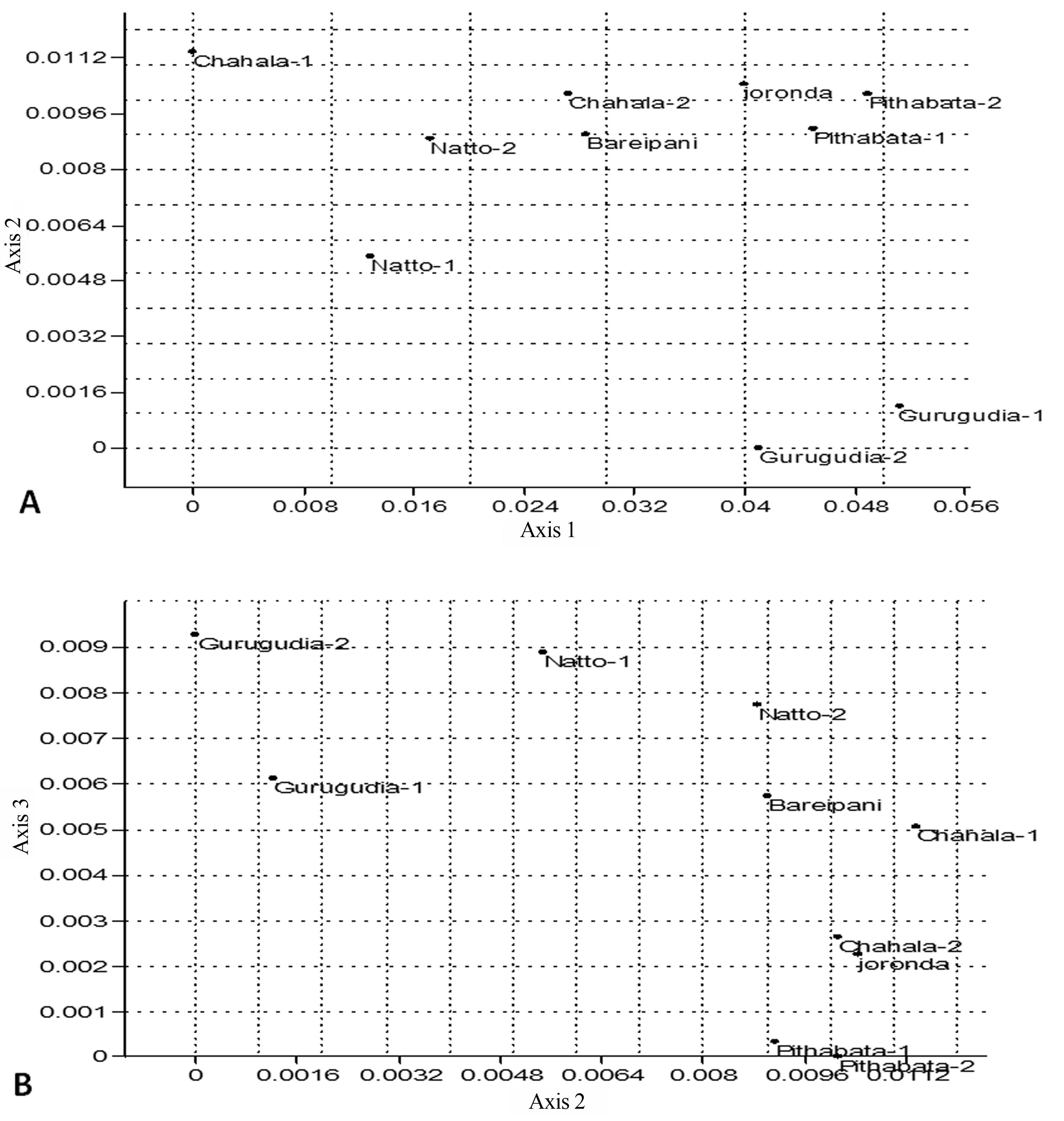
We have made an attempt to find out the linearity distribution of fungal population among the sampling sites. Detrended correspondence analysis plotting axis 1 and axis 2 and axis 2 and axis 3 indicated non linear arrangement of the samples. The values of the first three axis obtained by computing the detrended correspondence analysis is proved. In pictorial representation (Figure 6) of all the sites were plotted on sheet, to be observed scattered any where suggested that non linearity distribution of taxa among sampling sites.
Community ordination of Aspergillus species
The relationships between Aspergillus species in the community in different sites and seasons were analysed by community ordination to understand the natural system of grouping of species.
Overall 12 Aspergillus spp. reflected the community structure and population size. Dendrogram of the clustering of the species using Euclidean distance during summer is provided (Figure 7). The result indicated that Aspergillus nigerformed a separate group in the community. Further, A. awamorialso differed from other group indicating its distinctness. While other species clustered together within the community. Correspondingly, the dendrogram using Euclidean distance during winter also indicated similar results (Figure 8).
Dendrogarm clustering the 12 abundant fungal (Aspergillus) species of the forest in summer litter using Euclidean distance.
Dendrogarm clustering the 12 abundant fungal (Aspergillus) species of the forest in winter season litter using Euclidean distance.
Discussion
In terrestrial ecosystem, fungi are important components of microbial communities and play vital role in maintaining soil health and productivity. Studies suggest that soil fungal diversity and composition is governed by wide range of biotic and abiotic factors (Lauber et al., 2008Lauber CL, Strickland MS, Bradford MA et al. (2008) The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol Biochem 40:2407–2415.; Barcenas-Moreno et al., 2009Barcenas-Moreno G, Gomez-Brandon M, Rousk J et al.(2009) Adaptation of soil microbial communities to temperature: comparisonof fungi and bacteria in a laboratory experiment. Glob Chang Biol 15:2950–2957.; Hawkes et al., 2011Hawkes CV, Kivlin SN, Rocca JD et al. (2011) Fungal community responses to precipitation. Glob Chang Biol 17:1637–1645.). Das and Dkhar (2011)Das BB, Dkhar MS (2011) Rhizosphere microbial populations and physico chemical properties as affected by organic and inorganic farming practices. Amer-Eur J Agr Environ Sci 10:140–150. were of the opinion that microbial flora in moist deciduous forest is dependent on moisture content, pH, temperature, Nitrogen, Carbon sources. Several studies surveyed fungal diversity in different forest ecosystems, suggested a strong correlation between fungal and plant diversity, due to fungal host specificity (Peay et al., 2013Peay KG, Baraloto C, Fine PV (2013) Strong coupling of plant and fungal community structure across western Amazonian rainforests. ISME J 7:1852–1886.; Shi et al., 2013Shi LL, Mortimer PE, Slik JWF et al. (2013) Variation in forest soil fungal diversity along a latitudinal Gradient. Fungal Divers DOI 10.1007/s13225-013-0270-5.
https://doi.org/10.1007/s13225-013-0270-...
). Similipal Biosphere Reserve rich in plant diversity and dominated by Shorea robusta. The geography of this Biosphere Reserve is uneven. The soil physicochemical properties were found to be varied from site to site. Therefore, in this investigation, fungi occurring in soil samples of Similipal Biosphere Reserve were studied in respect to their diversity and physicochemical parameters of the soil. In the present investigation, we studied soil fungi of 100 sampling units collected from Similipal Biosphere Reserve, which are categorized into 10 major sites (Table 1). In the other words, the disturbances on fungal communities and population have been poorly studied in tropical regions, because these communities have been considered, likely wrongly, as both resistant and resilient to disturbance (Allison and Martiny, 2008Allison SD, Martiny JBH (2008) Resistance, resilience, and redundancy in microbial communities. Proc Natl Acad Sci 105:11512–11519.; Da Silva et al., 2012da Silva DAC, Pereira CMR, de Souza RG et al.(2012) Diversity of arbuscular mycorrhizal fungi in resting and dunes areas in Brazilian northeast. Biodiv Conserv 21:2361–2373.). Fungal population and diversity also varied with respect to the rhizopheric plants species(Hattori et al., 2012Hattori T, Yamashita S, Lee SS (2012) Diversity and conservation of wood-inhabiting polypores and other aphyllophoraceous fungi in Malaysia. Biodiv Conserv 21:2375–2396.). Here, the sampling sites were accomplished to the rhizopheric region of Shorea robusta asdominant flora. Gilbert (2005)Gilbert GS (2005) The dimensions of plant disease in tropical forests. In: Burslem DRFP, Pinard MA, Hartley S (eds) Biotic Interactions in the Tropics. Cambridge University Press, Cambridge, pp 141–164. suggested that the high plant diversity of tropical systems results in a decrease in fungal specificity due to a lack of selective pressure for specialization, often resulting in low species diversity. The Similipal soil was fertilized continuously by deposition of biomass of the plants. Therefore, dominance of fungal species in the soil samples could be recognized by organic substances deposited by dominant plant species. Another factor that, geographic distance monitoring the fungal diversity was reported by Green et al.(2004)Green JL, Holmes AJ, Westoby M et al. (2004) Spatial scaling of microbial eukaryote diversity. Nature 432:747–750.. According to him, fungal community became less similar with increasing geographic distance. Therefore, in our investigation larger variation of population was recorded among the above 10 sites could be attributable to the geographic position of the sites. It was also found that the similar fungal flora were recorded where soil samples were collected from closely located sites i.e. Chahala-1 &2, Natto-1 &2, Pithabata 1-&2, Gurgudia-1 &2. The varied population among the sites was also found due to the change in physicochemical parameters of the soil. Highest population was recorded in Chahala-1, followed by Chahala-2, Joranda and Natto-1 in winter season. Among the sites, the fungal population size was dependent on moisture content and organic carbon % of the soil (Table 1, Figure 2). Lowest fungal population was recorded in the site Bareipani i.e., 5.16 × 104 and 3.62 × 104 in winter and summer, seasons respectively.
In PCA analysis study it was summarized and calculated that winter and summer populations to be far change in population size in same place. However, in summer, fungal population fell down to half of the winter population among the sites as recorded could be attributable to the drying of soil. These findings are in corroboration with other workers (Gupta and Bhriguvanshi, 1997Gupta M, Bhriguvanshi SR (1997) Dissolution of phosphates as influenced by soil moisture regimes and P solubilizing microorganisms. J Ind Soc Soil Sci 45:613–614.; Narsian and Patel, 2009Narsian VT, Patel HH (2009) Relationship of physicochemical properties of rhizosphere soils with native population of mineral phosphate solubilizing fungi. Indian J Microbiol 49:60–67.). Yan et al.(2000)Yan A, McBratney B, Copeland L (2000) Functional substrate biodiversity of cultivated and uncultivated a horizons of vertisols in NW New South Wales. Geoderma 96:321–343. has drawn a relationship between organic carbon and functional diversity of fungal population, as observed in this investigation. Sharma et al. (1997)Sharma S, Piccolo A, Insam H (1997) Different carbon source utilization profiles from four tropical soils of Ethiopia. In: Insam H, Rangger A (eds) Microbial Communities. Springer, New York, pp 132–139., stated a linear relationship between (Shannon’s diversity index) H and microbial biomass. In this context, it was observed that Shannon index to be more in summer season than winter season because of the rise of dominance of single species. It was clearly understood that population size of ecosystem is directly dependent to organic carbon contents and moisture contents of the soil (Figures 2, 4). Both, Dominance and Simpson diversity index were found to vary from site to site, as a result of difference in physicochemical parameters and associated rhizopheric plants. Mycoflora differs in its composition from one ecological niche to the other have also been reported (Monoharachary, 2008Manoharchary C, Mohan KC, Kunwar IK et al. (2008) Phosphate solubilizing fungi associated withCasuarina equisetifolia. J Mycol Pl Pathol 38:507–513.). The dominant genera appeared abundantly in Indian soils regulate the relative frequency and relative density of other soil fungal genera (Bhagat and Pan, 2010Bhagat S, Pan S (2010) Cultural and phenotypic characterization of Trichoderma sp. From Andaman and Nicobar Islands. J Mycol Pl Pathol 40:145–157.) and had important role in leaf litter decomposition (Panda et al., 2009Panda T, Rout SD, Mishra N (2009) Decomposition of Casuarina leaf litter in coastal sand dunes of rissa. Int J Gen Mol Biol 1:137–143.). This may be due to the faster growth rate of these fungi in addition to their better intrinsic prolific sporulating capacity to utilize the substrate. Therefore, sporulating fungi were better established and studied by culturable methods. Panda et al. (2010)Panda T, Pani PK, Mishra N et al. (2010) A Comparative Account of the Diversity and Distribution of Fungi in Tropical Forest Soils and Sand Dunes of Orissa, India. J Biodiversity 1:27–41. also reported that fungal succession in plantation sites greatly differed from sites without plantation due to the presence of organic materials and that promote the domination of single species. Therefore, eveness (Evenness e^H/S) of fungal species also varied from site to site as reported by our study due to uneven geography, physico-chemical parameters of soil.
On the other hand, the exact evaluation of fungal population and diversity is very difficult (Smith et al., 2011Smith ME, Henkel TW, Aime MC et al. (2011) Ectomycorrhizal fungal diversity and community structure on three co-occurring leguminous canopy tree species in a Neotropical rainforest. New Phytologist 192:699–712.; Piepenbring et al., 2012Piepenbring M, Hofmann TA, Unterseher M et al.(2012) Species richness of plants and fungi in western Panama: towards a fungal inventory in the tropics. Biodivers Conserv 21:2181–2193.). As, fungi are cryptic and hyper diverse organisms that assemble in complex and dynamic communities. Perrone et al. (2011)Perrone G, Stea G, Epifani F et al. (2011) Aspergillus niger contains the cryptic phylogenetic species A. awamori. Fungal Biol 115:1138–1150. reported that species that produce spore-bearing structures can be easier to discover, although fruiting periods can be short and fructifications are ephemeral. In this investigation, we have measured the fungal population rather than fungal diversity. The species which are more dominant colonized better on the plate (during standard plate count method) than other species. On the other hand these species were better sporulating, therefore, soil fungal flora and population were maintained by these group of organisms. Total 17 genera were taken into account during this study, amongst all, Aspergillus was observed to be most dominant genera followed by Penicillium. The SIVI value suggested the floristic status of the species (Table 2). The Aspergillus niger showed SIVI 43.4 and 28.6 both in summer and winter seasons respectively, indicating the population size of the communities among the sampling sites being regulated by these two species, It is also called insurance hypothesis (Yachi and Loreau, 1999Yachi S, Loreau M (1999) Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proceeding of National Academy of Science 96:1463–1468.). In winter the Aspergillus niger was isolated in more numbers than summer season but the SIVI value showed the alternate results due to the well establishment of other species in winter rather than summer season. The dendrogarm of the 12 abundant Aspergillus species isolated from the Similipal Biosphere Reserve soil both in winter and summer season litter using Euclidean distance (Figures 7, 8) was drawn based on the SIVI values. The figure showed that Aspergillus niger form a separate group in community and minimize the role of other microbes in microbial ecosystem followed by cryptic species (Perrone et al., 2011Perrone G, Stea G, Epifani F et al. (2011) Aspergillus niger contains the cryptic phylogenetic species A. awamori. Fungal Biol 115:1138–1150.) like Aspergillus awamori. Shimpson’s diversity index of the site Chahala-2(0.850) and Bareipani (0.854) was reported to be same but in Chahala-1 the fungal population recorded to be more by two folds, than that of population of Bareipani (Figure 2). It clearly indicated that Aspergillus niger have not only better sustainability but has capacity to regulate the microbial structure in moist deciduous forest soil, could be due to the competitively superior under such conditions when there are favourable niches everywhere (Hall et al., 2000Hall SJ, Gray SA, Hammett ZL (2000) Biodiversity-productivity relations: an experimental evaluation of mechanisms. Oecologia 122:545–555.), called resource heterogeneity hypothesis. Secondly, the specific association of fungal species in different sites is because of their niche specialization. Senthilkumar et al. (1993)Senthilkumar K, Udaiyan K, Manian S (1993) Succession pattern of mycolfora associated with litter degradation in a Cymbopogoncaesius-dominated tropical grassland. Tropic Grasslands 27:121–127. reported that most of the fungal flora occurring in the later stages of decomposition was efficient degraders of cellulose and lignin. In the present study more fungal species were recorded from the sites with more moisture content and leaf litter deposition, indicating that these species could be efficient cellulose and lignin degraders.
A little change of diversity index and dominance index were observed in communities with uniform distribution of taxa among the sampling sites. But large variation of population of taxa among the sites were observed by studying the positioning of sites on Detrended correspondence analysis plot of axis-1 vs axis-2 and axis-2 and 3 (Figure 6).
Conclusion
It is concluded from our findings and other investigations, that the determination of complete structure of fungal flora is a difficult chapter. But, dominant and sporulating genera are the determinate of fungal diversity in the soil. The well evolved (sporulating) species are the dominant genera that contribute the maximum population to maintain the floral structure in the soil ecosystem that is attributable due to well dissemination of their spores in the soil. However, the moisture content and organic C percentage regulate the viability of their spores as well as the vegetative hyphae, as evidenced in this investigation, maintaining the fungal diversity in the soil ecosystem to a large extent.
Acknowledgments
This study was financially supported by Department of Science and Technology, Govt. Odisha (no. ST-(Bio)-54/2009). We thank the help of Field Director (Dr. A.K. Nayak) Similipal Triger Reserve, Baripada, permitting to carry out the research investigation inside SBR.
References
- Aime MC, Brearley FQ (2012) Tropical fungal diversity: closing the gap between species estimates and species discovery. Biodivers Conserv 21:2177–2180.
- Alexander M (1972) Introduction to Soil Microbiology, 2nd edition. Wiley and Sons, New York
- Allison SD, Martiny JBH (2008) Resistance, resilience, and redundancy in microbial communities. Proc Natl Acad Sci 105:11512–11519.
- Bahuti R, Rath CC, Mohapatra U (2006) Phsico- chemical and mycological studies of selected soil samples from Similipal Biosphere Reserve. Plant Sci 28:1–7.
- Barcenas-Moreno G, Gomez-Brandon M, Rousk J et al.(2009) Adaptation of soil microbial communities to temperature: comparisonof fungi and bacteria in a laboratory experiment. Glob Chang Biol 15:2950–2957.
- Barnett HL, Hunter BB (1996) Illustrated Genera of Imperfect Fungi. APS Press, St. Paul.
- Baruah TC, Barthakur HP (1998) A Text Book Soil Analysis. Vikas publ, New Delhi.
- Behera M, Dandapat J, Rath CC (2009) Isolation, characterization and screening of bacteria isolates from Similipal Biosphere Reserve forest soil for their metal tolerance capacity and extracellular enzymatic activities. Biorem Biodiv Bioavail 3:72–78.
- Berg B, McClaugherty C (2003) Plant Litter: Decomposition, Humus Formation, Carbon Sequestration. Spinger, Berlin.
- Bhagat S, Pan S (2010) Cultural and phenotypic characterization of Trichoderma sp. From Andaman and Nicobar Islands. J Mycol Pl Pathol 40:145–157.
- Cruickshank K, Duguid JP, Marmion BR et al. (1973) Medical Microbiology, A Guide to the Laboratory Diagnosis & Control of Infection, 12th edition. Edinburgh: ELBS and Churchill Livingstone, Great Britain.
- da Silva DAC, Pereira CMR, de Souza RG et al.(2012) Diversity of arbuscular mycorrhizal fungi in resting and dunes areas in Brazilian northeast. Biodiv Conserv 21:2361–2373.
- Das BB, Dkhar MS (2011) Rhizosphere microbial populations and physico chemical properties as affected by organic and inorganic farming practices. Amer-Eur J Agr Environ Sci 10:140–150.
- Dash MC (2001) Fundamental of Ecology. Tata McGrow-Hill Publ., New Delhi.
- Gilbert GS (2005) The dimensions of plant disease in tropical forests. In: Burslem DRFP, Pinard MA, Hartley S (eds) Biotic Interactions in the Tropics. Cambridge University Press, Cambridge, pp 141–164.
- Gilman JC (1971) A Manual of Soil Fungi. Iowa State College Press, Ames, Lowa.
- Green JL, Holmes AJ, Westoby M et al. (2004) Spatial scaling of microbial eukaryote diversity. Nature 432:747–750.
- Gupta M, Bhriguvanshi SR (1997) Dissolution of phosphates as influenced by soil moisture regimes and P solubilizing microorganisms. J Ind Soc Soil Sci 45:613–614.
- Hall SJ, Gray SA, Hammett ZL (2000) Biodiversity-productivity relations: an experimental evaluation of mechanisms. Oecologia 122:545–555.
- Hanson CA, Allison SD, Bradford MA et al. (2008) Fungal taxa target different carbon sources in forest soil. Ecosystems 11:1157–1167.
- Hattori T, Yamashita S, Lee SS (2012) Diversity and conservation of wood-inhabiting polypores and other aphyllophoraceous fungi in Malaysia. Biodiv Conserv 21:2375–2396.
- Hawkes CV, Kivlin SN, Rocca JD et al. (2011) Fungal community responses to precipitation. Glob Chang Biol 17:1637–1645.
- Hawksworth DL, Rossman AY (1997) Where are all the undescribed fungi? Phytopath 87:888–891.
- Hibbett DS, Ohman A, Glotzer D et al. (2011) Progress in molecular and morphological taxon discovery in Fungi and options for formal classification of environmental sequences. Fungal Biol Rev 25:38–47.
- Khan MS, Zaidi A, Wani PA (2007) Role of phosphate-solubilizing microorganisms in sustainable agriculture - A review Agronom for Sustain Develop 27:29–43.
- Lauber CL, Strickland MS, Bradford MA et al. (2008) The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol Biochem 40:2407–2415.
- Manoharchary C, Mohan KC, Kunwar IK et al. (2008) Phosphate solubilizing fungi associated withCasuarina equisetifolia. J Mycol Pl Pathol 38:507–513.
- Marschner P, Kandeler E, Marschner B (2003) Structure and function of the soil microbial community in a long- term fertilizer experiment. Soil Biol Bioch 35:453–461.
- McGuire KL, Bent E, Borneman J et al. (2010) Functional diversity in resource use by fungi. Ecol 9:2324–2332.
- Muthukrishnan S, Sanjayan KP, Jahir HK (2012) Species composition, seasonal changes and community ordination of alkalotolerant micro fungal diversity in a natural scrub jungle ecosystem of Tamil Nadu, India. Mycosphere 3:92–109.
- Narsian VT, Patel HH (2009) Relationship of physicochemical properties of rhizosphere soils with native population of mineral phosphate solubilizing fungi. Indian J Microbiol 49:60–67.
- O’Brien HE, Parrent JL, Jackson JA et al. (2005) Fungal community analysis by largescale sequencing of environmental samples. Appl Environ Microbiol 71:5544–5550.
- Ogram A, Bridgham S, Corstanje R et al. (2006) Linkages between microbial community composition and biogeochemical processes across scales. In: Verhoeven JTA, Beltman B, Bobbink R et al.(eds) Wetlands and Natural Resource Management. Springer, Berlin, pp. 239–268.
- Panda T, Pani PK, Mishra N et al. (2010) A Comparative Account of the Diversity and Distribution of Fungi in Tropical Forest Soils and Sand Dunes of Orissa, India. J Biodiversity 1:27–41.
- Panda T, Rout SD, Mishra N (2009) Decomposition of Casuarina leaf litter in coastal sand dunes of rissa. Int J Gen Mol Biol 1:137–143.
- Peay KG, Baraloto C, Fine PV (2013) Strong coupling of plant and fungal community structure across western Amazonian rainforests. ISME J 7:1852–1886.
- Perrone G, Stea G, Epifani F et al. (2011) Aspergillus niger contains the cryptic phylogenetic species A. awamori Fungal Biol 115:1138–1150.
- Piepenbring M, Hofmann TA, Unterseher M et al.(2012) Species richness of plants and fungi in western Panama: towards a fungal inventory in the tropics. Biodivers Conserv 21:2181–2193.
- Ryan PD, Harper DAT, Whalley JS (1995) PALSTAT, Statistics for Palaeontologists. Chapman and Hall, Dordt.
- Senthilkumar K, Udaiyan K, Manian S (1993) Succession pattern of mycolfora associated with litter degradation in a Cymbopogoncaesius-dominated tropical grassland. Tropic Grasslands 27:121–127.
- Sharma S, Piccolo A, Insam H (1997) Different carbon source utilization profiles from four tropical soils of Ethiopia. In: Insam H, Rangger A (eds) Microbial Communities. Springer, New York, pp 132–139.
- Shi LL, Mortimer PE, Slik JWF et al. (2013) Variation in forest soil fungal diversity along a latitudinal Gradient. Fungal Divers DOI 10.1007/s13225-013-0270-5.
» https://doi.org/10.1007/s13225-013-0270-5 - Smith ME, Henkel TW, Aime MC et al. (2011) Ectomycorrhizal fungal diversity and community structure on three co-occurring leguminous canopy tree species in a Neotropical rainforest. New Phytologist 192:699–712.
- Tran HTM, Stephenson SL, Hyde KD et al. (2006) Distribution and occurrence of myxomycetes in tropical forests of northern Thailand. Fungal Divers 22:227–242.
- Wardle DA, Bardgett RD, Klironomos JN et al. (2004) Ecological linkages between aboveground and belowground biota. Science 304:1629e1633.
- Yachi S, Loreau M (1999) Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proceeding of National Academy of Science 96:1463–1468.
- Yan A, McBratney B, Copeland L (2000) Functional substrate biodiversity of cultivated and uncultivated a horizons of vertisols in NW New South Wales. Geoderma 96:321–343.
Publication Dates
-
Publication in this collection
Jan-Mar 2015
History
-
Received
30 Dec 2013 -
Accepted
06 June 2014