Abstract
The entomopathogenic fungi Beauveria bassiana, Metarhizium anisopliae, Lecanicillium lecanii and Isaria fumosorosea were tested for their efficacy in managing the exotic spiraling whitefly Aleurodicus dispersus (Hemiptera, Aleyrodidae) on cassava (Manihot esculenta) during 2 seasons (2011-2012 and 2012-2013). The fungi I. fumosorosea and L. lecanii exhibited promising levels of control (> 70% mortality of the A. dispersus population). The percent mortality increased over time in both seasons. Application of I. fumosorosea was highly pathogenic to A. dispersus in both seasons compared to the other entomopathogenic fungi. Analysis of the percent mortality in both seasons revealed differences in efficacy between 3 and 15 days after treatment. The season also influenced the effects of the fungi on the A. dispersus population. Thus, entomopathogenic fungi have the potential to manage A. dispersus infestation of cassava.
Key words:
Aleurodicus dispersus; Manihot esculenta; biocontrol; entomopathogenic fungi; mortality
Introduction
Cassava (M. esculenta Cranrz.) is the most important starchy root crop grown in the tropics (Sánchez et al., 2009Sánchez T, Salcedo E, Ceballos H et al. (2009) Screening of starch quality traits in cassava (Manihot esculenta Crantz). Starch/Stärke 61:12-19.) and the main crop cultivated in the Southern Peninsular regions of India. Among the various insect pests of cassava, the exotic spiraling whitefly A. dispersus Russell (Hemiptera, Aleyrodidae) can cause losses that reach 53% (Geetha, 2000Geetha B (2000) Biology and Management of Spiralling Whitefly, Aleurodicus dispersus Russell (Homoptera: Aleyrodidae). Ph.D. Dissertation, Tamil Nadu Agricultural University, 105 pp.). A. dispersus is a polyphagous pest with an extensive host range covering 481 plant species belonging to 295 genera from 90 families of vegetables, fruits and ornamental trees (Srinivasa, 2000Srinivasa MV (2000) Host plants of the spiraling whitefly Aleurodicus dispersus Russell (Hemiptera: Aleyrodidae). Pest Manage Horticul Ecosys 6:79-105.; Boopathi and Karuppuchamy, 2013Boopathi T, Karuppuchamy P (2013) Evaluation of ecofriendly agents against spiralling whitefly, Aleurodicus dispersus Russell on brinjal. Madras Agricul J 100:559-661.; Boopathi et al., 2012Boopathi T, Karuppuchamy P, Kalyanasundaram M et al. (2012) Relative efficacy of botanicals, an organic salt and fish oil rosin soap in controlling the spiralling whitefly, Aleurodicus dispersus Russell on cassava. Hexapoda 19:59-66.; Boopathi et al., 2014cBoopathi T, Mohankumar S, Karuppuchamy P et al. (2014c) Genetic evidence for diversity of spiralling whitefly, Aleurodicus dispersus (Hemiptera: Aleyrodidae) populations in India. Fla Entomol 97:1115-1122.). Infestation causes premature leaf drop. Moreover, the production of copious amounts of honeydew serves as a substrate for sooty mold growth (Akinlosotu et al., 1993Akinlosotu TA, Jackai LEN, Nitonifor NN et al. (1993) Spiralling whitefly, Aleurodicus dispersus in Nigeria. FAO Plant Protec Bull 41:127-129.; Boopathi et al., 2013Boopathi T, Karuppuchamy P, Kalyanasundaram M et al. (2013) Pathogenicity, ovicidal action, and median lethal concentrations (LC50) of entomopathogenic fungi against exotic spiralling whitefly, Aleurodicus dispersus Russell. J Path 1-7. Available at: http://dx.doi.org/10.1155/2013/393787 Accessed 1 November 2013.
http://dx.doi.org/10.1155/2013/393787...
), which in turn reduces the photosynthetic activity and plant vigor (Kumashiro et al., 1983Kumashiro BR, Lai PY, Funasaki GY et al. (1983) Efficacy of Nephaspis amnicola and Encarsia haitiensis in controlling Aleurodicus dispersus in Hawaii. Proc Hawaii Entomol Soc 24:261-269.; John et al., 2007John B, Darryl H, Greg P (2007) Spiralling whitefly Aleurodicus dispersus exotic threat to Western Australia. Hort Guard TM Initiative AGWEST. Available at: www.agric.wa.gov.au/objtwr/imported_assets/…/pw/…/fs01800.pdf. Accessed May 2007.
www.agric.wa.gov.au/objtwr/imported_asse...
; Boopathi et al., 2014aBoopathi T, Karuppuchamy P, Kalyanasundaram M et al. (2014a) Effect of botanicals, fish oil rosin soap and organic salt on eggs of spiralling whitefly, Aleurodicus dispersus. Indian J Plant Protec 42:86-88.; Boopathi et al., 2014bBoopathi T, Karuppuchamy P, Kalyanasundaram M et al. (2014b) Toxicity of newer insecticides against spiralling whitefly, Aleurodicus dispersus under laboratory conditions. Indian J Plant Protec 42:178-180.). The insect's natural enemies, especially the parasitoids Encarsia guadeloupae Viggiani and Encarsia sp. nr. meritoria Gahan, (Geetha, 2000Geetha B (2000) Biology and Management of Spiralling Whitefly, Aleurodicus dispersus Russell (Homoptera: Aleyrodidae). Ph.D. Dissertation, Tamil Nadu Agricultural University, 105 pp.) and predators, Mallada astur (Banks) and Cybocephalus spp., (Mani and Krishnamoorthy, 1999Mani M, Krishnamoorthy A (1999) Development and predatory potential of the green lacewing, Mallada astur (Banks) (Neuroptera: Chrysopidae) on the spiralling whitefly Aleurodicus dispersus Russell (Homoptera: Aleyrodidae). J Biol Cont 13:45-49.) have proven to be highly useful in suppressing the spiraling whitefly.
The entomopathogenic fungi have shown good epizootic potential against whiteflies, i.e., Bemisia spp. and Trialeurodes spp., in both field environments and under greenhouse conditions (Fang et al., 1983Fang QX, Gong YX, Zhou YY et al. (1983) Paecilomyces fumosoroseus var. beijingensis. Acta Mycol Sinica 2:168-172.; Osborne and Landa, 1992Osborne LS, Landa Z (1992) Biological control of whiteflies with entomopathogenic fungi. The Fla Entomol 75:456-471.; Carruthers et al., 1993Carruthers RI, Wraight SP, Jones WA (1993) An overview of biological control of the sweetpotato whitefly, Bemisia tabaci. In: D.J. Herber and D.A. Richter (eds) Proceedings Beltwide Cotton Conferences, vol. 2. National Cotton Council of America, Memphis, pp 680-685.; Lacey et al., 1996Lacey LA, Fransen JJ, Carruthers RI (1996) Global distribution of naturally occurring fungi of Bemisia, their biologies and use as biological control agents. In: D. Gerling and R.T. Mayer (eds.). Bemisia 1995: Taxonomy, biology, damage, control and management. Intercept Limited, Andover, pp 401-433.). I. fumosorosea (Wize) caused the highest mortality to nymphs of the silver leaf whitefly (Bemisia argentifolii Bellows and Perring) under laboratory conditions (Wraight et al., 1998Wraight SP, Carruthers RI, Bradley CA et al. (1998) Pathogenicity of the entomopathogenic fungi Paecilomyces spp. and Beauveria bassiana against the silverleaf whitefly, Bemisia argentifolii. J Invert Pathol 71:217-226., 2000Wraight SP, Carruthers RI, Jaronski ST et al. (2000) Evaluation of the entomopathogenic fungi Beauveria bassiana and Paecilomyces fumosoroseus for microbial control of the silverleaf whitefly, Bemisia argentifolii. Biol Cont 17:203-217.) and 100% mortality to A. dispersus nymphs 15 days after treatment (DAT) under laboratory conditions (Boopathi et al., 2013Boopathi T, Karuppuchamy P, Kalyanasundaram M et al. (2013) Pathogenicity, ovicidal action, and median lethal concentrations (LC50) of entomopathogenic fungi against exotic spiralling whitefly, Aleurodicus dispersus Russell. J Path 1-7. Available at: http://dx.doi.org/10.1155/2013/393787 Accessed 1 November 2013.
http://dx.doi.org/10.1155/2013/393787...
). L. lecanii (Zimmerm.) Zare and Gams produced 80-90% mortality in A. dispersus 15 days after application under in vivo conditions (Aiswariaya et al., 2007Aiswariaya KK, Manjunatha M, Naik M (2007) Evaluation of fungi Fusarium semitectum Berk and Ravenel and Verticillium lecanii (Zimm.) Viegas against spiraling whitefly Aleurodicus dispersus Russell on guava. Karnataka J Agricul Sci 20:283-287.) and 97.8% mortality to A. dispersus nymphs 15 DAT under laboratory conditions (Boopathi et al., 2013Boopathi T, Karuppuchamy P, Kalyanasundaram M et al. (2013) Pathogenicity, ovicidal action, and median lethal concentrations (LC50) of entomopathogenic fungi against exotic spiralling whitefly, Aleurodicus dispersus Russell. J Path 1-7. Available at: http://dx.doi.org/10.1155/2013/393787 Accessed 1 November 2013.
http://dx.doi.org/10.1155/2013/393787...
). The entomopathogenic fungus B. bassiana (Balsamo) Vuillemin produced higher mortality to the first instars and adults of the silver leaf whitefly (Nagasi et al., 1998Nagasi A, Paster BL, Brownsbridge M (1998) Screening and bioassay of entomopathogenic fungi for the control of silver leaf whitefly, Bemisia argentifolia. Insect Sci Appl 18:37-44.) and 52-98% mortality to Bemisia at concentrations of 1-4 x 106 conidia mL-1 (Eyal et al., 1992Eyal J, Mabud MDA, Fischdein KL et al. (1992) Assessment of Beauveria bassiana Nov. EO-1 strain, which produce red pigment for microbial control. Appl Biochem Biotechnol 44:65-80.).
In India, substantial scientific research into crop pest management using entomopathogenic fungi against agricultural and horticultural insect pests began in the early 1990s. Many of the economically important vegetable insect pest species from Hemiptera, Lepidoptera, Coleoptera and Isoptera have been shown to be susceptible to various entomopathogenic fungal isolates (Geetha, 2000Geetha B (2000) Biology and Management of Spiralling Whitefly, Aleurodicus dispersus Russell (Homoptera: Aleyrodidae). Ph.D. Dissertation, Tamil Nadu Agricultural University, 105 pp.; Aiswariaya et al., 2007Aiswariaya KK, Manjunatha M, Naik M (2007) Evaluation of fungi Fusarium semitectum Berk and Ravenel and Verticillium lecanii (Zimm.) Viegas against spiraling whitefly Aleurodicus dispersus Russell on guava. Karnataka J Agricul Sci 20:283-287.; Boopathi et al., 2013Boopathi T, Karuppuchamy P, Kalyanasundaram M et al. (2013) Pathogenicity, ovicidal action, and median lethal concentrations (LC50) of entomopathogenic fungi against exotic spiralling whitefly, Aleurodicus dispersus Russell. J Path 1-7. Available at: http://dx.doi.org/10.1155/2013/393787 Accessed 1 November 2013.
http://dx.doi.org/10.1155/2013/393787...
; Boopathi et al., 2015Boopathi T, Karuppuchamy P, Kalyanasundaram M et al. (2015) Microbial control of the exotic spiralling whitefly, Aleurodicus dispersus (Hemiptera: Aleyrodidae) on eggplant using entomopathogenic fungi. Afr J Microbiol Res 9:39-46.), including A. dispersus. The present study investigated the usefulness of the entomopathogenic fungi B. bassiana, M. anisopliae (Metschnikoff) Sorokin, L. lecanii and I. fumosorosea as effective biocontrol agents against the most destructive pest of cassava, A. dispersus.
Materials and Methods
Fungal isolates
Strains of the entomopathogenic fungi and their sources are listed in Table 1. Isolates were maintained on potato dextrose agar (PDA) in test tubes and stored at 4 °C. Continuous cultures were maintained on slants; subcultures were grown for 14 days at 25 °C and then stored at 4 °C.
Production and enumeration of spores
Spore suspensions of each fungal isolate were prepared in 0.5% aqueous Tween® 80 and homogenized with a vortex mixer for two minutes (Lacey, 1997Lacey L (1997) Manual of Techniques in Insect Pathology. Academic Press, San Diego, 409 pp.). Then, the spores were counted using a hemocytometer. The conidial suspension was further diluted with 0.5% aqueous Tween® 80 solution in test tubes to obtain concentrations of 2 × 109 conidia.mL-1.
Spore production
Air-dried conidia were produced as follows. Maize (150 g) suspended in 60 mL of sterile water was autoclaved in polythene bags (10 x 25 cm) at 121 °C and a pressure of 1.05 kg cm-3 for 15 min. and cooled to room temperature for 24 h. One milliliter of the 2 x 109 conidia mL-1 suspension was introduced to each polythene bag and incubated for 2 weeks at 26 ± 3 °C. The two-week-old cultures were harvested, and the air-dried conidia/spores were filtered through a sieve with a particle size of 125 μm.
Spore harvesting and drying
The cultures were allowed to air dry overnight in a room with a temperature of 25 ± 5 °C and a relative humidity of 50 ± 5%. Air-dried conidia remained viable and active for up to 8 months without any loss in efficacy for the management of insect pests.
Formulation and application equipment
A wettable powder formulation was prepared by mixing air-dried conidia with commercial diluent clay (Kaolin, Ashapura Group of Industries, Chennai, Tamil Nadu, India) at a ratio of 1:4 (20% w/w a.i., active ingredient) in a sterile room. All treatments were sprayed using a single-nozzle atomizing (air-assist) sprayer (pneumatic knapsack sprayer). The spray nozzle was carried near ground level for each spray and directed at a right angle to the row. Each row was treated twice, once on each side of the row. The spray volume was 500-700 Lha-1. Spraying was performed in the late evening to reduce the possible oppressive effect of solar radiation on conidial/spore germination.
Field evaluation
Field experiments were conducted in cassava for two seasons at Pollachi, Coimbatore, Tamil Nadu, India, in 2011-2012 (Season 1) and 2012-2013 (Season 2). Rooted sets of cassava (cv. CO-2) were planted in 10 x 10 m plots at a spacing of 90 x 90 cm. Treatments were applied to 5 replicates arranged in a randomized complete block design (RCBD). Weeding, application of manures and fertilizers, and other cultural operations were performed as per crop production guidelines (TNAU, 2012TNAU (2012) Crop Production Guide 2005. Chennai and Tamil Nadu Agricultural University, Tamil Nadu.). Furrow irrigation (approximately 700-800 Lplot-1) was applied every 2-3 weeks in the absence of rain. The respective wettable powder formulated entomopathogenic fungi were suspended in 1.0% Teepol® excluding the control. Two applications of fungi were applied 15 days apart due to heavy infestation (> 200 A. dispersus per leaf) of A. dispersus (more than the economic threshold level, ETL) at the rate of 2 x 109 conidiamL-1. The first application was applied during the vegetative/reproductive stage of the cassava. The second application was applied to new leaves and shoots that had high populations of newly emerged nymphs and adults of A. dispersus. Both applications were applied to the same plants. Pre-treatment observations of the A. dispersus population (no. of nymphs and adults of A. dispersus per leaf) were taken 24 h. before each application of fungi, and post-treatment observations were taken at 3, 7, 10 and 15 days after each treatment (DAT). Observations of the A. dispersus populations (no. of nymphs and adults of A. dispersus per leaf) were recorded on leaves from the top, middle and bottom of 5 tagged plants per plot after the first and second applications.
Data analysis
Statistical analysis of the data was performed using the methods of Gomez and Gomez (1984)Gomez KA, Gomez AA (1984) Statistical Procedures for Agricultural Research. John Wiley and Sons, New York. with SAS Software Version 9.3 (SAS Institute Inc., 2011SAS Institute Inc. (2011) SAS® 9.3 System Options: Reference, Second Edition. SAS Institute Inc., SAS Campus Drive, Cary, NC.). The data were analyzed using four-way ANOVA. All ANOVA analyses were performed on original values, and the means were separated using the least significant difference (LSD) test at p < 0.05 or p < 0.01. The percent mortality of A. dispersus populations was determined and corrected using the control method of Henderson and Tilton (1955)Henderson CF, Tilton EW (1955) Tests with acaricides against brown wheat mite. J Econ Entomol 48:157-161. as follows:
where Ta = number of insects in the treatment after spraying, Tb = number of insects in the treatment before spraying, Cb = number of insects in the untreated control before spraying and Ca = number of insects in the untreated control after spraying.
Results and Discussion
All entomopathogenic fungal treatments caused medium to high mortality of A. dispersus. Individual A. dispersus killed by these entomopathogenic fungi dried rapidly on the cassava leaves, and the cadavers remained attached to the cassava leaves. A. dispersus individuals infected by the white muscardine fungus B. bassiana were red to red-brown in color. Mycelia and sporulation of entomopathogenic fungi on the cadaver occurred during extended periods of rainfall, higher relative humidity over many nights, or late in the experimental trials. M. anisopliae produced green conidia/spores from tightly packed and parallel-oriented conidiogenous cells. The amounts of I. fumosorosea hyphal growth and sporulation were obviously greater 3-5 days after spraying than the other entomopathogenic fungi; however, the postmortem development of the entomopathogenic fungi was not monitored and quantified (Figure 1).
All entomopathogenic fungi caused substantial reductions in A. dispersus populations on cassava following both applications in both seasons. The percent mortality of all entomopathogenic fungi increased over time in both applications and seasons, whereas the highest A. dispersus population (> 180 per leaf) was observed in the control populations in both seasons 1 and 2. There were differences between the effects of fungi (F = 8284.393; p < 0.0000), applications (F = 706.327; p < 0.0000), observation dates (F = 1489.155; p < 0.0000), seasons (F = 40.523; p < 0.0000) and the interactions on the mortality of A. dispersus: fungi x applications (F = 47.459; p < 0.0000), fungi x observation dates (F = 93.773; p < 0.0000), fungi x seasons (F = 8.416; p < 0.0000), applications x observation dates (F = 19.655; p < 0.0000), applications x seasons (F = 15.256; p < 0.0000), observation dates x seasons (F = 11.249; p < 0.0000), fungi x applications x observation dates (F = 1.998; p < 0.0240), fungi x observation dates x seasons (F = 2.287; p < 0.0085), applications x observation dates x seasons (F = 12.252; p < 0.0000), and fungi x applications x observation dates x seasons (F = 3.056; p < 0.0004) (Table 2). However, there was not a significant difference between the fungi x applications x seasons (F = 2.329; p < 0.0560) interaction on the mortality of A. dispersus.
The percent mortality resulting from both applications in season 1 demonstrated differences in efficacy between pathogens (Table 3). Higher mortality occurred with I. fumosorosea in both application 1 (59.04%, 100.35 A. dispersus per leaf) and application 2 (68.34%, 22.55 A. dispersus per leaf) in season 1 than that of the other fungi, whereas in the control treatment, the highest A. dispersus populations, 228.30 per leaf and 237.30 per leaf, were observed in season 1 following application 1 and application 2, respectively. Similarly, in season 2 I. fumosorosea produced higher mortality following both application 1 (60.70%, 80.66 A. dispersus per leaf) and application 2 (70.63%, 15.55 A. dispersus per leaf) than that of the other fungi. However, the highest A. dispersus population in the control treatment was registered for both application 1 (187.34 per leaf) and application 2 (208.14 per leaf) in season 2. Therefore, the season influenced the effect of the fungi on the reduction of A. dispersus. Furthermore, a higher rate of mortality was observed in cassava in season 2 than in season 1.
Percent corrected mortality of A. dispersus following both applications on cassava in season 1 and season 2.
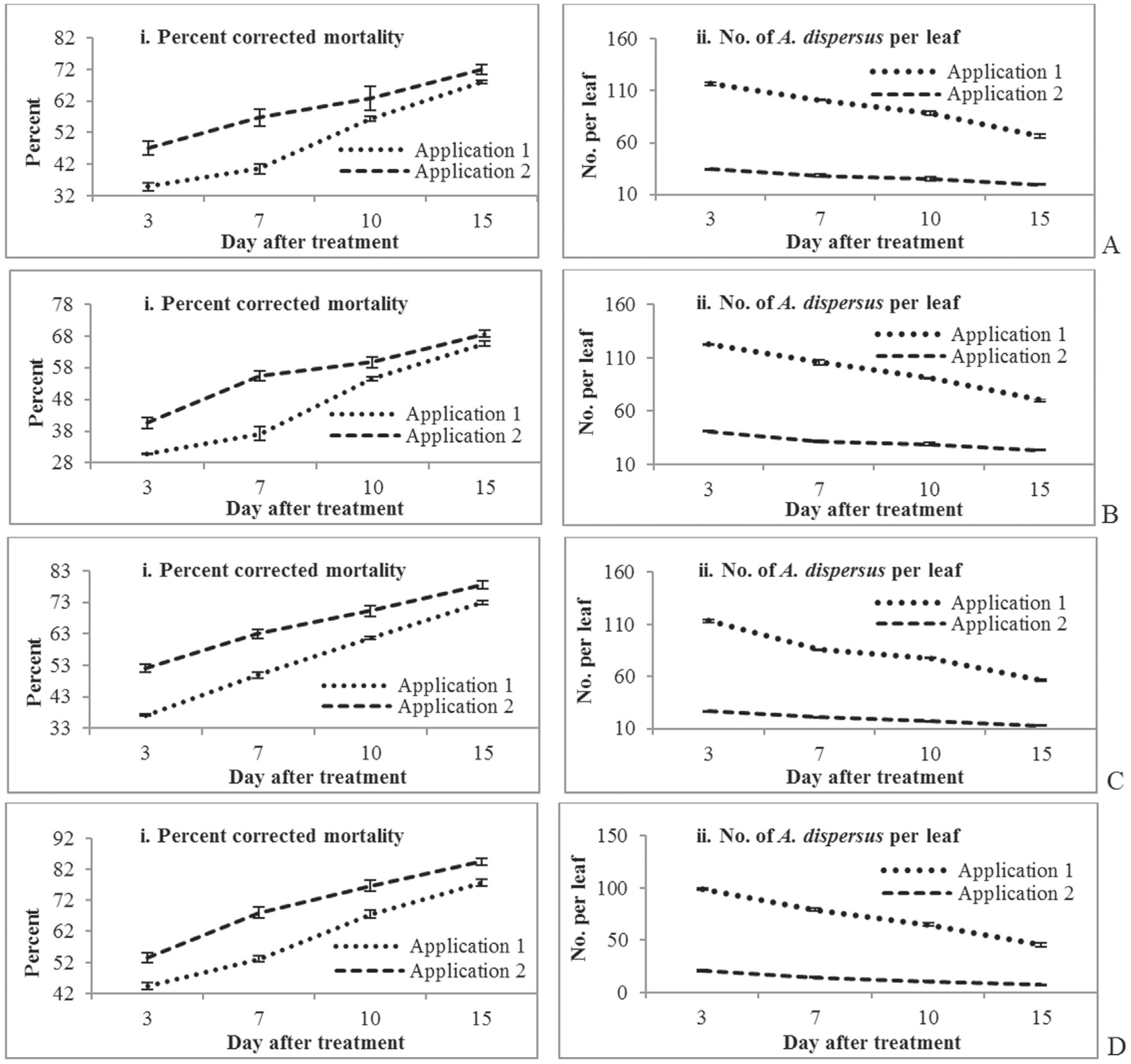
The differences in the mortality of A. dispersus induced by B. bassiana on cassava indicated that there were differences in efficacy between days 3-15 post-treatment (Figure 2a, 2b). B. bassiana produced the highest mortality after application 2 in both seasons. The percent mortality of A. dispersus induced by B. bassiana increased over time when comparing both applications and seasons. The highest mortality was observed at 15 DAT for both application 1 (66.45%, 81.00 A. dispersus per leaf) and application 2 (70.13%, 24.80 A. dispersus per leaf) in season 1 (Figure 2a, 2b). Similar trends were also observed in season 2, with 68.13% mortality following application 1 (66.36 A. dispersus per leaf) and 71.95% mortality following application 2 (19.60 A. dispersus per leaf) (Figure 2b). The lowest mortality was observed 3 DAT for both application 1 (37.90%, 141.80 A. dispersus per leaf) and application 2 (44.09%, 44.88 A. dispersus per leaf) in season 1. Similarly, in season 2 the lowest mortality was observed following application 1 (34.84%, 116.72 A. dispersus per leaf) and application 2 (47.07%, 34.36 A. dispersus per leaf) at 3 DAT. Weather parameters such as relative humidity, temperature and rainfall were 75-85.0%, 22.0-32.3 °C and 14.0 mm in season 1 and 72-81%, 20.8-31.1 °C and 9.0 mm in season 2, respectively. Previously, Eyal et al. (1992)Eyal J, Mabud MDA, Fischdein KL et al. (1992) Assessment of Beauveria bassiana Nov. EO-1 strain, which produce red pigment for microbial control. Appl Biochem Biotechnol 44:65-80. reported that 52-98% mortality of Bemisia tabaci (Gennadius) was induced by B. bassiana. Wright and Chandler (1992)Wright JE, Chandler LD (1992) Development of a biorational mycoinsecticide: Beauveria bassiana conidial formulation and its application against boll weevil population (Coleoptera: Curculionidae). J Econ Entomol 85:1131-1135. also reported that B. bassiana caused comparatively lesser mortality than Anthonomus grandis Boheman (boll weevil) in the field. Nagasi et al. (1998)Nagasi A, Paster BL, Brownsbridge M (1998) Screening and bioassay of entomopathogenic fungi for the control of silver leaf whitefly, Bemisia argentifolia. Insect Sci Appl 18:37-44. reported that B. bassiana produced higher mortality of the first instars and adults of the silver leaf whitefly. Wraight et al. (1998Wraight SP, Carruthers RI, Bradley CA et al. (1998) Pathogenicity of the entomopathogenic fungi Paecilomyces spp. and Beauveria bassiana against the silverleaf whitefly, Bemisia argentifolii. J Invert Pathol 71:217-226., 2000Wraight SP, Carruthers RI, Jaronski ST et al. (2000) Evaluation of the entomopathogenic fungi Beauveria bassiana and Paecilomyces fumosoroseus for microbial control of the silverleaf whitefly, Bemisia argentifolii. Biol Cont 17:203-217.) observed that B. bassiana caused the highest mortality to nymphs of B. argentifolii under laboratory conditions. Furthermore, Wraight and Knaf (1994)Wraight JE, Knaf TA (1994) Evaluation of Naturalis-L for control of cotton insects. In: Proceedings Brighton Crop Protection Conference. Pests and diseases, vol. 1. BCPC Publications, Brighton. used a higher dose of 5 x 1013 conidia (2.5 conidia.mL-1) and achieved 90% control of B. tabaci nymphs 7 DAT, and Boopathi et al. (2013)Boopathi T, Karuppuchamy P, Kalyanasundaram M et al. (2013) Pathogenicity, ovicidal action, and median lethal concentrations (LC50) of entomopathogenic fungi against exotic spiralling whitefly, Aleurodicus dispersus Russell. J Path 1-7. Available at: http://dx.doi.org/10.1155/2013/393787 Accessed 1 November 2013.
http://dx.doi.org/10.1155/2013/393787...
reported that B. bassiana had a comparatively higher efficacy against A. dispersus under laboratory conditions than against M. anisopliae.
Efficacy of entomopathogenic fungi on the mortality of A. dispersus on cassava in season 1 between 3 and 15 days after treatment (A) B. bassiana (B) M. anisopliae (C) L. lecanii (D) I. fumosorosea.
Metarhizium anisopliae produced the highest mortality following application 2 in both seasons (Figure 2c, 2d). The percent mortality increased over time for both applications and seasons. The highest mortality was observed at 15 DAT following both application 1 (59.36%, 95.92 A. dispersus per leaf) and application 2 (65.51%, 33.72 A. dispersus per leaf) in season 1 (Figure 2c). Similar results were also observed in season 2, with 65.70% mortality following application 1 (70.48 A. dispersus per leaf) and 68.71% mortality following application 2 (23.32 A. dispersus per leaf) (Figure 2d). The next highest mortality rate was observed at 10 DAT, whereas the lowest mortality rate was produced at 3 DAT of both application 1 and application 2 in both seasons.
The fungus L. lecanii caused a higher mortality to A. dispersus following application 2 than following application 1 in both seasons (Figure 3a, 3b). The percent mortality of A. dispersus induced by L. lecanii increased over time for both applications and seasons. L. lecanii produced the highest mortality at 15 DAT following both application 1 (70.13%, 71.68 A. dispersus per leaf) and application 2 (73.75%, 19.24 A. dispersus per leaf) in season 1 (Figure 3a). Similarly, in season 2 L. lecanii caused the highest mortality following application 1 (72.94%, 56.48 A. dispersus per leaf) and application 2 (78.59%, 12.76 A. dispersus per leaf) at 15 DAT (Figure 3b). Similar results were reported by Aiswariaya et al. (2007)Aiswariaya KK, Manjunatha M, Naik M (2007) Evaluation of fungi Fusarium semitectum Berk and Ravenel and Verticillium lecanii (Zimm.) Viegas against spiraling whitefly Aleurodicus dispersus Russell on guava. Karnataka J Agricul Sci 20:283-287., with 3.6 x 109 spores mL-1 of L. lecanii inducing ~90% mortality of nymphs and ~80% mortality of adults of A. dispersus 15 days after application. However, Boopathi et al. (2013)Boopathi T, Karuppuchamy P, Kalyanasundaram M et al. (2013) Pathogenicity, ovicidal action, and median lethal concentrations (LC50) of entomopathogenic fungi against exotic spiralling whitefly, Aleurodicus dispersus Russell. J Path 1-7. Available at: http://dx.doi.org/10.1155/2013/393787 Accessed 1 November 2013.
http://dx.doi.org/10.1155/2013/393787...
reported that L. lecanii had higher mortality against A. dispersus under laboratory conditions.
Efficacy of entomopathogenic fungi on the mortality of A. dispersus on cassava in season 2 between 3 and 15 days after treatment (A) B. bassiana (B) M. anisopliae (C) L. lecanii (D) I. fumosorosea.
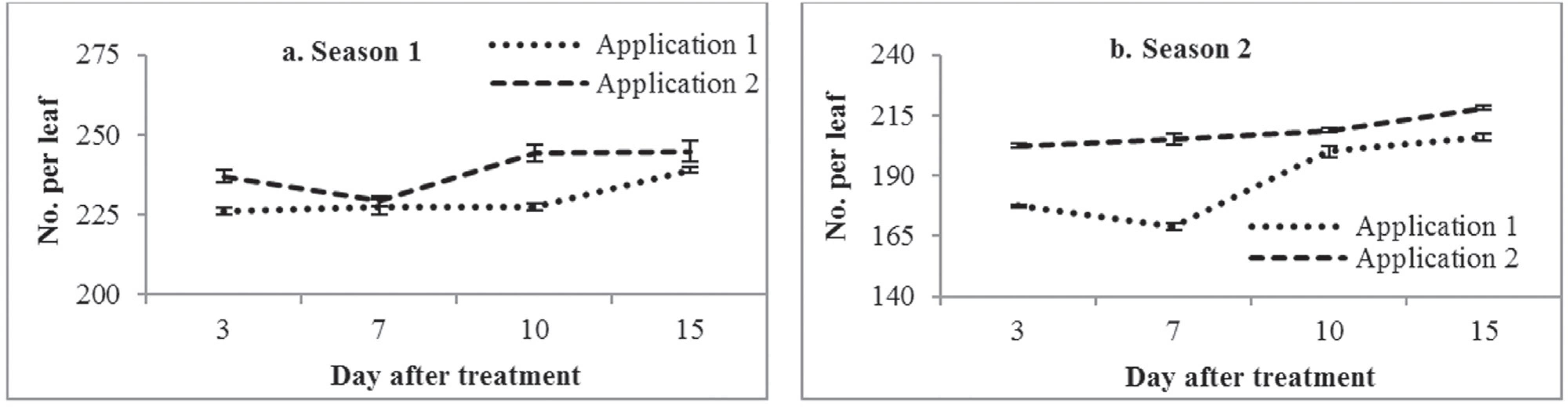
Isaria fumosorosea produced higher mortality following application 2 than following application 1 in both seasons (Figure 2d). Similar to the other fungi, the percent mortality induced by I. fumosorosea increased over time for both applications and seasons. The highest mortality was due to both application 1 (73.70%, 62.68 A. dispersus per leaf) and application 2 (79.96%, 12.80 A. dispersus per leaf) at 15 DAT in season 1 (Figure 3c). Similar trends were also observed in season 2, with 77.73% mortality following application 1 (45.88 A. dispersus per leaf) and 84.50% mortality following application 2 (7.48 A. dispersus per leaf) (Figure 3d). The next highest mortality was observed at 10 DAT, whereas the lowest mortality was produced at 3 DAT following both application 1 and application 2 in both seasons. However, the control of the A. dispersus population was the higher in both season 1 (> 200 A. dispersus per leaf) (Figure 4) and season 2 (> 150 A. dispersus per leaf) than for any other enthomopathogenic fungi (Figure 4). Similar results were reported by Boopathi et al. (2013)Boopathi T, Karuppuchamy P, Kalyanasundaram M et al. (2013) Pathogenicity, ovicidal action, and median lethal concentrations (LC50) of entomopathogenic fungi against exotic spiralling whitefly, Aleurodicus dispersus Russell. J Path 1-7. Available at: http://dx.doi.org/10.1155/2013/393787 Accessed 1 November 2013.
http://dx.doi.org/10.1155/2013/393787...
, who reported that 2 x 109 conidia mL-1 of I. fumosorosea produced 100% mortality in A. dispersus 15 DAT under laboratory conditions. This result is in agreement with the present findings. Similarly, Wraight et al. (1998Wraight SP, Carruthers RI, Bradley CA et al. (1998) Pathogenicity of the entomopathogenic fungi Paecilomyces spp. and Beauveria bassiana against the silverleaf whitefly, Bemisia argentifolii. J Invert Pathol 71:217-226., 2000Wraight SP, Carruthers RI, Jaronski ST et al. (2000) Evaluation of the entomopathogenic fungi Beauveria bassiana and Paecilomyces fumosoroseus for microbial control of the silverleaf whitefly, Bemisia argentifolii. Biol Cont 17:203-217.) observed that I. fumosorosea caused higher mortality to nymphs of B. argentifolii under laboratory conditions, while Ayhan and Kubilay (2005)Ayhan G, Kubilay M (2005) Pathogenicity of Paecilomyces spp. to the glasshouse whitefly, Trialeurodes vaporariorum, with some observations on the fungal infection process. Turk J Agric For 29:331-339. and Avery et al. (2008)Avery PB, Faull J, Simmonds MSJ (2008) Effects of Paecilomyces fumosoroseus and Encarsia formosa on the control of the greenhouse whitefly: preliminary assessment of a compatability study. Bio Control 53:303-316. reported that I. fumosorosea produced the highest mortality of the greenhouse whitefly Trialeurodes vaporariorum (Westwood).
A. dispersus population (no. per leaf) in control treatment on cassava in both season 1 and season 2 between 3 and 15 days after treatment.
Temperature and relative humidity are important microclimatic factors that improve the pathogenicity of enthomopathogenic fungi under field conditions. Rainfall (9.0-14.0 mm), relative humidity (72-85.0%), and temperature (20.8-32.3 °C) in both season 1 and season 2 favored enthomopathogenic fungal infection and growth. This finding is in agreement with previous studies with I. fumosorosea (Boopathi et al., 2013Boopathi T, Karuppuchamy P, Kalyanasundaram M et al. (2013) Pathogenicity, ovicidal action, and median lethal concentrations (LC50) of entomopathogenic fungi against exotic spiralling whitefly, Aleurodicus dispersus Russell. J Path 1-7. Available at: http://dx.doi.org/10.1155/2013/393787 Accessed 1 November 2013.
http://dx.doi.org/10.1155/2013/393787...
; Boopathi et al., 2015Boopathi T, Karuppuchamy P, Kalyanasundaram M et al. (2015) Microbial control of the exotic spiralling whitefly, Aleurodicus dispersus (Hemiptera: Aleyrodidae) on eggplant using entomopathogenic fungi. Afr J Microbiol Res 9:39-46.). Our results suggest that I. fumosorosea was more effective in suppressing the exotic A. dispersus in the field than in suppressing B. bassiana, M. anisopliae or L. lecanii; this finding is consistent with the lower LC50 value reported for I. fumosorosea in a previously conducted pathogenicity test (Boopathi et al., 2013Boopathi T, Karuppuchamy P, Kalyanasundaram M et al. (2013) Pathogenicity, ovicidal action, and median lethal concentrations (LC50) of entomopathogenic fungi against exotic spiralling whitefly, Aleurodicus dispersus Russell. J Path 1-7. Available at: http://dx.doi.org/10.1155/2013/393787 Accessed 1 November 2013.
http://dx.doi.org/10.1155/2013/393787...
). M. anisopliae did not produce an added advantage because it could not suppress the rapidly growing A. dispersus population in the field. Thus, effective control of A. dispersus was not achieved compared to that of I. fumosorosea. This result could be because M. anisopliae was the least effective against A. dispersus and contained a lower density of conidia. Similarly, Bateman et al. (1993)Bateman RP, Carey M, Moore D et al. (1993) The enhance infectivity of Metarhizium flavoviridae in oil formulation to desert locust at low humidities. Ann Appl Biol 122:145-152. reported that M. anisopliae was ineffective against the desert locust at low humidity. Two repeated sprays fifteen days apart were required before I. fumosorosea could totally suppress the A. dispersus population, indicating that I. fumosorosea virulence was maintained throughout the duration of the field experiment.
Among the four enthomopathogenic fungi evaluated, I. fumosorosea and L. lecanii showed promising levels of virulence to A. dispersus in both applications and seasons compared to that of M. anisopliae and B. bassiana. Similarly, Boopathi et al. (2015)Boopathi T, Karuppuchamy P, Kalyanasundaram M et al. (2015) Microbial control of the exotic spiralling whitefly, Aleurodicus dispersus (Hemiptera: Aleyrodidae) on eggplant using entomopathogenic fungi. Afr J Microbiol Res 9:39-46. reported that I. fumosorosea and L. lecanii caused the highest mortality to A. dispersus on eggplants. Thus, these fungi (I. fumosorosea and L. lecanii) can be potentially used as an alternate pest control method to combat the pest insect A. dispersus on cassava. Their wide application as mycoinsecticides could be utilized after exploring their pathogenicity in field trials. However, additional testing with yield assessments and economic analyses must be conducted before ultimate conclusions are drawn.
Acknowledgments
The authors are grateful to the Professor and Head, Department of Agricultural Entomology and the Director, Centre for Plant Protection Studies, Tamil Nadu Agricultural University, Coimbatore, Tamil Nadu, India, for providing the facilities and support. The assistance given by Mr. Muthumariappan, Mr. Mani and Mr. Arulnayagam in recording the observations is acknowledged.
References
- Aiswariaya KK, Manjunatha M, Naik M (2007) Evaluation of fungi Fusarium semitectum Berk and Ravenel and Verticillium lecanii (Zimm.) Viegas against spiraling whitefly Aleurodicus dispersus Russell on guava. Karnataka J Agricul Sci 20:283-287.
- Akinlosotu TA, Jackai LEN, Nitonifor NN et al. (1993) Spiralling whitefly, Aleurodicus dispersus in Nigeria. FAO Plant Protec Bull 41:127-129.
- Avery PB, Faull J, Simmonds MSJ (2008) Effects of Paecilomyces fumosoroseus and Encarsia formosa on the control of the greenhouse whitefly: preliminary assessment of a compatability study. Bio Control 53:303-316.
- Ayhan G, Kubilay M (2005) Pathogenicity of Paecilomyces spp. to the glasshouse whitefly, Trialeurodes vaporariorum, with some observations on the fungal infection process. Turk J Agric For 29:331-339.
- Bateman RP, Carey M, Moore D et al. (1993) The enhance infectivity of Metarhizium flavoviridae in oil formulation to desert locust at low humidities. Ann Appl Biol 122:145-152.
- Boopathi T, Karuppuchamy P (2013) Evaluation of ecofriendly agents against spiralling whitefly, Aleurodicus dispersus Russell on brinjal. Madras Agricul J 100:559-661.
- Boopathi T, Karuppuchamy P, Kalyanasundaram M et al. (2012) Relative efficacy of botanicals, an organic salt and fish oil rosin soap in controlling the spiralling whitefly, Aleurodicus dispersus Russell on cassava. Hexapoda 19:59-66.
- Boopathi T, Karuppuchamy P, Kalyanasundaram M et al. (2013) Pathogenicity, ovicidal action, and median lethal concentrations (LC50) of entomopathogenic fungi against exotic spiralling whitefly, Aleurodicus dispersus Russell. J Path 1-7. Available at: http://dx.doi.org/10.1155/2013/393787 Accessed 1 November 2013.
» http://dx.doi.org/10.1155/2013/393787 - Boopathi T, Karuppuchamy P, Kalyanasundaram M et al. (2014a) Effect of botanicals, fish oil rosin soap and organic salt on eggs of spiralling whitefly, Aleurodicus dispersus Indian J Plant Protec 42:86-88.
- Boopathi T, Karuppuchamy P, Kalyanasundaram M et al. (2014b) Toxicity of newer insecticides against spiralling whitefly, Aleurodicus dispersus under laboratory conditions. Indian J Plant Protec 42:178-180.
- Boopathi T, Mohankumar S, Karuppuchamy P et al. (2014c) Genetic evidence for diversity of spiralling whitefly, Aleurodicus dispersus (Hemiptera: Aleyrodidae) populations in India. Fla Entomol 97:1115-1122.
- Boopathi T, Karuppuchamy P, Kalyanasundaram M et al. (2015) Microbial control of the exotic spiralling whitefly, Aleurodicus dispersus (Hemiptera: Aleyrodidae) on eggplant using entomopathogenic fungi. Afr J Microbiol Res 9:39-46.
- Carruthers RI, Wraight SP, Jones WA (1993) An overview of biological control of the sweetpotato whitefly, Bemisia tabaci In: D.J. Herber and D.A. Richter (eds) Proceedings Beltwide Cotton Conferences, vol. 2. National Cotton Council of America, Memphis, pp 680-685.
- Eyal J, Mabud MDA, Fischdein KL et al. (1992) Assessment of Beauveria bassiana Nov. EO-1 strain, which produce red pigment for microbial control. Appl Biochem Biotechnol 44:65-80.
- Fang QX, Gong YX, Zhou YY et al. (1983) Paecilomyces fumosoroseus var. beijingensis Acta Mycol Sinica 2:168-172.
- Geetha B (2000) Biology and Management of Spiralling Whitefly, Aleurodicus dispersus Russell (Homoptera: Aleyrodidae). Ph.D. Dissertation, Tamil Nadu Agricultural University, 105 pp.
- Gomez KA, Gomez AA (1984) Statistical Procedures for Agricultural Research. John Wiley and Sons, New York.
- Henderson CF, Tilton EW (1955) Tests with acaricides against brown wheat mite. J Econ Entomol 48:157-161.
- John B, Darryl H, Greg P (2007) Spiralling whitefly Aleurodicus dispersus exotic threat to Western Australia. Hort Guard TM Initiative AGWEST. Available at: www.agric.wa.gov.au/objtwr/imported_assets/…/pw/…/fs01800.pdf. Accessed May 2007.
» www.agric.wa.gov.au/objtwr/imported_assets/…/pw/…/fs01800.pdf - Kumashiro BR, Lai PY, Funasaki GY et al. (1983) Efficacy of Nephaspis amnicola and Encarsia haitiensis in controlling Aleurodicus dispersus in Hawaii. Proc Hawaii Entomol Soc 24:261-269.
- Lacey L (1997) Manual of Techniques in Insect Pathology. Academic Press, San Diego, 409 pp.
- Lacey LA, Fransen JJ, Carruthers RI (1996) Global distribution of naturally occurring fungi of Bemisia, their biologies and use as biological control agents. In: D. Gerling and R.T. Mayer (eds.). Bemisia 1995: Taxonomy, biology, damage, control and management. Intercept Limited, Andover, pp 401-433.
- Mani M, Krishnamoorthy A (1999) Development and predatory potential of the green lacewing, Mallada astur (Banks) (Neuroptera: Chrysopidae) on the spiralling whitefly Aleurodicus dispersus Russell (Homoptera: Aleyrodidae). J Biol Cont 13:45-49.
- Nagasi A, Paster BL, Brownsbridge M (1998) Screening and bioassay of entomopathogenic fungi for the control of silver leaf whitefly, Bemisia argentifolia Insect Sci Appl 18:37-44.
- Osborne LS, Landa Z (1992) Biological control of whiteflies with entomopathogenic fungi. The Fla Entomol 75:456-471.
- Sánchez T, Salcedo E, Ceballos H et al. (2009) Screening of starch quality traits in cassava (Manihot esculenta Crantz). Starch/Stärke 61:12-19.
- SAS Institute Inc. (2011) SAS® 9.3 System Options: Reference, Second Edition. SAS Institute Inc., SAS Campus Drive, Cary, NC.
- Srinivasa MV (2000) Host plants of the spiraling whitefly Aleurodicus dispersus Russell (Hemiptera: Aleyrodidae). Pest Manage Horticul Ecosys 6:79-105.
- TNAU (2012) Crop Production Guide 2005. Chennai and Tamil Nadu Agricultural University, Tamil Nadu.
- Wraight JE, Knaf TA (1994) Evaluation of Naturalis-L for control of cotton insects. In: Proceedings Brighton Crop Protection Conference. Pests and diseases, vol. 1. BCPC Publications, Brighton.
- Wraight SP, Carruthers RI, Bradley CA et al. (1998) Pathogenicity of the entomopathogenic fungi Paecilomyces spp. and Beauveria bassiana against the silverleaf whitefly, Bemisia argentifolii J Invert Pathol 71:217-226.
- Wraight SP, Carruthers RI, Jaronski ST et al. (2000) Evaluation of the entomopathogenic fungi Beauveria bassiana and Paecilomyces fumosoroseus for microbial control of the silverleaf whitefly, Bemisia argentifolii Biol Cont 17:203-217.
- Wright JE, Chandler LD (1992) Development of a biorational mycoinsecticide: Beauveria bassiana conidial formulation and its application against boll weevil population (Coleoptera: Curculionidae). J Econ Entomol 85:1131-1135.
Publication Dates
-
Publication in this collection
09 Oct 2015 -
Date of issue
Oct-Dec 2015
History
-
Received
19 Dec 2014 -
Accepted
11 Mar 2015