ABSTRACT
The goal of this investigation was to isolate competent polynuclear aromatic hydrocarbons degraders that can utilize polynuclear aromatic hydrocarbons of former industrial sites at McDoel Switchyard in Bloomington, Indiana. Using conventional enrichment method based on soil slurry, we isolated, screened and purified two bacterial species strains PB1 and PB2. Applying the ribotyping technique using the 16S rRNA gene analysis, the strains were assigned to the genus Pseudomonas (Pseudomonas plecoglossicida strain PB1 and Pseudomonas sp. PB2). Both isolates showed promising metabolic capacity on pyrene sprayed MS agar plates during the preliminary investigations. Using time course studies in the liquid cultures at calculated concentrations 123, 64, 97 and 94 ppm for naphthalene, chrysene, fluroanthene and pyrene, P. plecoglossicida strain PB1 and Pseudomonas sp. PB2 showed partial utilization of the polynuclear aromatic hydrocarbons. Naphthalene was degraded between 26% and 40%, chrysene 14% and 16%, fluroanthene 5% and 7%; pyrene 8% and 13% by P. plecoglossicida strain PB1 and Pseudomonas sp. PB2 respectively. Based on their growth profile, we developed a model R2 = 1 to predict the degradation rate of slow polynuclear aromatic hydrocarbon-degraders where all the necessary parameters are constant. From this investigation, we confirm that the former industrial site soil microbial communities may be explored for the biorestoration of the industrial site.
Keywords:
16S rRNA gene analysis; Former industrial sites; Soil microbial communities; Competent PAH degraders
Introduction
Global industrialization is without consequences, particularly with the deposition of hydrophobic contaminants in sediments, surface soil and dump sites. Polynuclear aromatic hydrocarbons (PAHs), a hydrophobic contaminant have been listed as one of priority/toxic environmental contaminants. They are chemical compounds comprising mainly of carbon (C) and hydrogen (H); arranged in form of two or more aromatic rings with various structural configurations.11 Jacques RJS, Okeke BC, Bento FM, Peralba MCR, Camaro FAV. Improved enrichment and isolation of polycyclic aromatic hydrocarbons (PAH)-degrading microorganisms in soil using Anthracene as a Model PAH. Curr Microbiol. 2009;58:628-634. PAHs can be classified as alternant (e.g., benzo[a]pyrene, benz]a]anthracene, chrysene, dibenz[a,h]anthracene) or non-alternant (e.g., fluoranthene, benzo[k]fluoranthene, benzol[j]fluoranthene, indeno[1,2,3-c,d]pyrene). The alternant PAHs comprises of aromatic rings with six carbon atoms while the non-alternant ones contain aromatic rings with less or more than six carbons. This peculiarity is based on the electron density associated with the molecule. Alternant PAHs have an equally distributed electron density, whereas non-alternant PAHs behave almost as if they were two different molecules because of an uneven distribution of electron density from one portion of the molecule to another. PAHs being a derivative of benzene, and thermodynamically stable, have two or more fused aromatic rings arranged in linear, angular, or clustered structures.22 Cheung P-Y, Kinkle BK. Mycobacterium diversity and pyrene mineralization in petroleum-contaminated soils. Appl Environ Microbiol. 2001;67:2222-2229.,33 Peng RH, Xiong AS, Xue Y, et al. Microbial biodegradation of polyaromatic hydrocarbons. FEMS Microbiol Rev. 2008;32:927-955. PAHs are ubiquitous environmental pollutant, entering the environment from natural and anthropogenic sources such as natural fires, volcanic eruptions, aluminum smelting, coke production, and creosote preservation. The anthropogenic sources typically are as a result of incomplete combustion of the organic substances that make up such substances.44 Maliszewska-Kordybach B. Sources, concentrations, fate and effects of polycyclic aromatic hydrocarbons (PAHs) in the environment. Part A: PAHs in air. Pol J Environ Stud. 1999;8:131-136.,55 Nwinyi OC, Picardal FW, Thuy A, Amund OO. Aerobic degradation of naphthalene, fluoranthene, pyrene and chrysene using indigenous strains of bacteria isolated from an old polluted industrial site. Can J Pure Appl Sci. 2013;7:2303-2314.
The human body could be exposed to PAHs through inhalation, dermal contact and ingestion. Within the body, PAHs being highly lipid-soluble are quickly absorbed by the fatty tissues such as the kidney, liver and gastro-intestinal tract of mammals. In humans, the highest metabolizing capacity present is the liver, then the lungs, intestinal mucosa, skin and kidneys. Metabolism may also take place in nasal tissues, mammary glands, spleen, brain follicles, erythrocytes, platelets, leukocytes, placenta and uterus. In the liver, PAHs are adapted by cytochrome P450 into epoxides a major intermediates that are reactive and enzymatically metabolized to dihydrodiols and phenols. The dihydrodiols and phenols react against DNA and proteins causing mutagenic damage to cells.66 Samanta SK, Singh OV, Jain RK. Polycyclic aromatic hydrocarbons: environmental pollution and bioremediation. Trends Biotechnol. 2002;20:243-248.
Naphthalene has been noted to be a hazardous air pollutant.77 USEPA Clean Air Act (42 USC, 7401-7626). Washington, DC: United States Environmental Protection Agency. Available at http://www.epa.gov/air/caa/2004; Accessed 2014.
http://www.epa.gov/air/caa/2004...
When experimental organisms are exposed to naphthalene, it causes decrease in their hemoglobin concentration and inhibits their oxygen consumption. Naphthalene is used as raw chemical for industrial synthases of phthatic anhydride.88 Preuss R, Angerer JR, Drexler H. Naphthalene - an environmental and occupational toxicant. Int Arch Occup Environ Health. 2003;76:556-576. Naphthalene at high concentration may cause hemolytic anemia and some other conditions, whereas the tumorigenic potential is presently considered low.99 Umweltbundesamt. Naphthalin/naphthole and human biomonitoring. Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz. 2007;50:1357-1364.,1010 Bogen KT, Beson JM, Yost GS, Morris JB, Dahl AR, Clewell HJ. Naphthalene metabolism in relation to target tissue anatomy, physiology, cytotoxicity and tumorigenic mechanism of action. Regul Toxicol Pharmacol. 2008;51:27-36. Naphthalene has been often used as a model PAH due to high speed of utilization by microorganisms compared to other PAH and the relatively simple structure of the intermediates in the catabolic pathways. Thus information on bacterial degradation of naphthalene has been used to understand and predict pathways of most PAHs.
Pyrene is a hydrophobic compounds and its persistence within ecosystems is due to low water solubility, dense clouds of p-electrons on both sides of the ring structures, making them resistant to nucleophilic attack.1111 Johnsen AR, Wick LY, Harms L. Principles of microbial PAH-degradation in soil. Environ Poll. 2005;133:71-84.
Soil can act as a sink for carbon. It could receive considerable amount of PAHs that may likely remain persistent in the environment due to low solubility and sequestration in soil and sediments; to lack of versatile metabolic capacity of microorganisms to degrade these compound.1111 Johnsen AR, Wick LY, Harms L. Principles of microbial PAH-degradation in soil. Environ Poll. 2005;133:71-84. According to Regonne and co-workers, for effective cleanup of PAH- contaminated soils, cheaper and more ecologically friendly options are proposed over chemical and physical processes.1212 Regonne RK, Martin F, Mbawala A, Ngassoum MR, Jouanneau Y. Identification of soil bacteria able to degrade phenanthrene bound to a hydrophobic sorbent in situ. Environ Poll. 2013;180:145-151. These options will involve greater understanding of the processes involved and factors that limit the degradation of high molecular and low molecular weight PAHs. Bioremediation is a proficient and safe method to clean up PAH from contaminated sites. It has been applied to both terrestrial and aquatic ecosystems; and may possibly provide a position in biorestoration of contaminated soils. Microorganisms transform the PAHs to CO2 and water through metabolism or co-metabolism. They PAHs serve as carbon and energy sources, thus reducing the associated toxicity and co-metabolic substrates of PAH.1111 Johnsen AR, Wick LY, Harms L. Principles of microbial PAH-degradation in soil. Environ Poll. 2005;133:71-84. Since the 1950s efforts have been made to select microorganisms with ability to degrade PAHs from pure cultures.1313 Cerniglia CE. Fungal metabolism of polycyclic aromatic hydrocarbons: past, present and future applications in bioremediation. J Ind Microbiol Biotechnol. 1997;19:324-333. Since then, many bacteria strains have been isolated for their ability to transform, degrade and utilize PAHs as a source of energy and carbon.55 Nwinyi OC, Picardal FW, Thuy A, Amund OO. Aerobic degradation of naphthalene, fluoranthene, pyrene and chrysene using indigenous strains of bacteria isolated from an old polluted industrial site. Can J Pure Appl Sci. 2013;7:2303-2314.,1414 Wikada J, Nojiri H, Kasuga K, Yoshida T, Habe H, Omori T. Molecular detection and diversity of polycyclic aromatic hydrocarbon-degrading bacteria isolated from geographically diverse sites. Appl Microbiol Biotechnol. 2002;58:202-209.
15 Mroczek A, Piotrowska-Seget S, Labuzek A. Bacterial degradation and bioremediation of polycyclic aromatic hydrocarbons. Polish J Environ Stud. 2003;12:15-25.
16 Andreoni V, Cavalca L, Rao MA, et al. Bacterial communities and enzyme activities of PAHs polluted soils. Chemosphere. 2004;57:401-412.-1717 Hickey AM, Gordon L, Dobson AD, Kelly CT, Doyle EM. Effect of surfactants on fluoranthene degradation by Pseudomonas alcaligenes PA-10. Appl Microbiol Biotechnol. 2007;74:851-856. Hitherto, it has been recognized that few bacteria have been isolated that are capable of utilizing PAHs with four or more aromatic rings as sole sources of carbon and energy.1818 Kanaly RA, Harayama S. Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons by bacteria. J Bacteriol. 2000;182:2059-2067.,1919 Davidova IA, Gieg LM, Duncan KE, Suflita JM. Anaerobic phenanthrene mineralization by a carboxylating sulfate-reducing bacterial enrichment. ISME J. 2007;1:436-442. Consequently, it is of paramount importance to isolate and investigate versatile degraders of PAHs. In this study, we report for the first time the isolation and characterization of bacterial strains from a former industrial site in Bloomington in Indiana using conventional enrichment of soil slurry. The bacteria Pseudomonas plecoglossicida strain PB1 and Pseudomonas sp. PB2 were screened for their growth and degradation fluxes in naphthalene, fluoranthene, pyrene and chrysene. We proposed a mathematical model to assist in the simulation of the degradation rates of our bacterial species on the selected PAHs (naphthalene, fluoranthene, pyrene and chrysene).
Materials and methods
Chemicals
The naphthalene, fluoranthene, pyrene and chrysene of analytical grades were purchased from Sigma Aldrich Corp. (St. Louis, MO, USA). Sodium benzoate (99+% purity), 2,2,4,4,6,8,8-heptamethylnonane (HMN), and all other organic solvents were obtained from Fisher Scientific Co. (Springfield, NJ, USA). Hexane, a high purity solvent for GC-chromatograph was obtained from EMD Chemicals Inc. Merck. The PAH analytical standards were procured from Accustandard Inc. (New Haven, CT 06513). All other chemicals and reagents used were of reagent grade or better.
Stock solutions and media
For the enrichment and degradation experiments, chloride free minimal salts (MS) medium as described by2020 Kim S, Picardal FW. A novel bacterium that utilizes monochlorobiphenyls and 4-chlorobenzoate as growth substrates. FEMS Microbiol Lett. 2000;185:225-229.
21 Nwinyi OC, Nwodo CS, Amund OO. Biodegradation potential of two Rhodococcus strains capable of utilizing aniline as carbon source in tropical ecosystem. Res J Microbiol. 2008;3:99-104.
22 Nwinyi OC. Degradation of askarel (PCB blend) by indigenous aerobic bacteria isolates from dumpsites in Ore, Ondo State, Nigeria. Aust J Basic Appl Sci. 2010;4:3938-3948.-2323 Nwinyi OC. Enrichment and Identification of Askarel oil (PCB blend) degrading bacteria enriched from landfill sites in Edo State, Nigeria. Agric Biol J North Am. 2011;2:89-100.,55 Nwinyi OC, Picardal FW, Thuy A, Amund OO. Aerobic degradation of naphthalene, fluoranthene, pyrene and chrysene using indigenous strains of bacteria isolated from an old polluted industrial site. Can J Pure Appl Sci. 2013;7:2303-2314. were used. The medium consisted of (g) 0.5(NH4)2SO4, 0.1MgSO4·7H2O, 0.076Ca(NO3)2·4H2O and 1.0 mL each of trace metal and vitamin solutions per liter of 40 mM phosphate buffer (pH 7.25). Naphthalene stock solution were prepared in HMN, a non-degradable carrier to provide an initial concentration of ca. 123 ppm. The concentration represents the total mass in both the aqueous and HMN phases, divided by the aqueous volume. The appropriate stock solution was added using a gas-tight syringe in 250-µL aliquots to provide test compound concentration of ca. 100 ppm in the final medium. Alongside, chrysene, fluoranthene and pyrene stock solution were prepared differently by dissolving the weighted test compounds in acetone respectively. Fluoranthene, chrysene and pyrene were added from the different stock solution of the test compound into the balch tubes using a Hamilton gas-tight syringe in 250-µL aliquots, to provide test compound concentration of ca. 97 ppm for fluoranthene, ca. 64 ppm for chrysene, and pyrene ca. 94 ppm in the final medium. Solid MS medium was made by the addition of 1.8% Bacto-agar (Difco Laboratories, Detroit, MI, USA).
The naphthalene solution was added with Hamilton gas tight syringe 250 µL aliquots into the balch tubes to provide test compound concentration of 100 ppm in the final medium. The MS medium was supplemented with the test compound, achieving an experiment dependent concentration. The cultures were incubated at room temperature on a shaker table to aid slow mass transfer of the test compound into the aqueous phase. Initial investigations were carried out using MS medium supplemented with HMN as the sole carbon and energy sources to determine that HMN did not serve as growth substrate.
Soil sample collection and enrichment of PAH degrading bacteria
The soil samples were collected from former industrial sites at the McDoel switchyard in Bloomington, Indiana. For decades, the site had been contaminated with PAHs, other organic and inorganic pollutants. Soil samples were taken from 6 to 11 cm layer of the contaminated soil at three locations, with indications of low to high level of PAH-contamination based on preliminary environmental audit. The soil samples were placed in separate sterile jars and transported back to the lab at ambient temperatures. The samples were dark in appearance. PAH-degrading bacteria were initially isolated by the conventional enrichment methods. For this, 5.0 g of the different soil samples were weighed into 160 mL serum bottles mixed with 30 mL of sterile MS medium. PAH-contaminated soils may have limited bioavailability due to sorption and strong hydrophobicity of PAH. Thus pyrene was added in the enrichment bottles to serve as supplemental carbon and energy source. All the 160 mL serum bottle bioreactor was set up in triplicates. The serum bottles were crimp-sealed with teflon-coated, butyl rubber stoppers to prevent losses due to volatilization and/or sorption. These were incubated horizontally on an orbital shaker table (Labline Instruments Inc., Melrose Park, IL, USA) at ambient temperature. Air sparging was done weekly to re-aerate the headspace and biweekly periodic transfers were made using about 15% inoculum into new MS medium supplemented with the test PAHs. The procedure was repeated for seven successive times.
Determination of carbon, hydrogen and nitrogen (CHN) ratio of the soil samples
The carbon, hydrogen and oxygen ratio of the obtained soil samples from the spatial distribution at McDoel switchyard were determined using the PerkinElmer 2400 series CHN analyzer. The sieved soil samples were thoroughly mixed and oven-dried at 85 °C for 24 h. Approximately 6 mg of the oven-dried soil were weighed into tin capsules. The capsules were then placed into the PerkinElmer 2400 series CHN analyzer for determination of carbon, hydrogen and nitrogen ratio.
Isolation, purification and phylogenetic analysis
Pure cultures from pyrene-enriched media were isolated by directly plating aliquots (0.2 mL) of highly-enriched cultures onto MS agar. Because we wished to prevent loss of catabolic plasmids from capable isolates, we used MS agar medium supplemented with pyrene rather than nutrient agar to maintain selective pressure. The pyrene was added to the medium using the spray plate technique as described by Kiyohara et al.2424 Kiyohara H, Nagao K, Yana K. Rapid screen for bacteria degrading water insoluble, solid hydrocarbon on agar plates. Appl Environ Microbiol. 1982;43:454-457. Immediately after spread-plating the 0.2 mL aliquot of enrichment culture, an ethereal solution of pyrene was uniformly sprayed onto the surface of the agar. The plates were sealed with parafilm film and incubated for 1 week at 30 °C in dark. Pyrene-degrading microorganisms were identified by cleared zones around an individual colony. The selected colony was purified by repetitive streaking on MS agar sprayed with pyrene. The most efficient bacterial isolates were selected by comparison of growth rate, generation time, and generation number.
The pure culture were routinely cultured and sustained on solid MS agar plates containing 2.5 mM pyrene. For 16S rRNA gene analysis, genomic DNA was isolated from overnight cultures of isolates growing on 2.5 mM benzoate using an UltraClean Microbial DNA Isolation kit (Mo Bio Laboratories, Solana Beach, CA, USA). Three eubacterial PCR primers; forward primer 8fm (AGAGTTTGATCMTGGTCAG) and reverse primers 926r (CCGTCAATTCCTTTRAGTTT) and 1387r (GGGCGGWGTGTACAAGGC) were used to amplify the 16S rRNA gene. The reaction mixtures were incubated at 95 °C for 2.5 min and then cycled 33 times through the following temperature profile: 95 °C for 30 s, 48 °C for 30 s, and 72 °C for 1.5 min, followed by a single 10 min incubation at 72 °C. About 2 µL of each amplification mixture was analyzed by agarose gel electrophoresis 10.0 µg mL-1 (w/v) ethidium bromide to establish that amplicons were of the expected length. The PCR amplicons were subsequently cleaned using QIAquick Nucleotide Removal Kit from Qiagen Inc. (Turnberry lane, CA 91355). For the 16S rRNA sequencing, the PCR products were sequenced following an ABI Big Dye Terminator Cycle Sequencing reaction using an Applied Biosystems 3730 automated sequencing system (Applied Biosystems, Inc., Foster City, CA, USA). The following settings were applied: denaturation for 3 min at 94 °C, 25 cycles at 96 °C for 10 s, 50 °C for 5 s and 60 °C for 4 min. The resultant sequences were edited and aligned using Bio Edit software version 4.8.7 to check for reading errors and when possible, clarify ambiguities.2525 Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98NT. Nucleic Acids Symp Ser. 1999;41:95-98. Sequences were subsequently compared with deposited sequences in GenBank database using the BLAST algorithm available at URL http://www.ncbi.nlm.nih.gov/BLAST/.2626 Altschul SF, Madden TL, Schaffer AA, et al. Gapped BLAST and PSI-Blast: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389-3402.
Growth on the different carbon and energy sources
Investigations of the potentials of the pure cultures to grow on naphthalene, fluoranthene, pyrene and chrysene were carried out. The tests were carried out in MS medium supplemented with each PAH test compound as sole carbon source. These investigations were conducted in crimp-sealed tubes (balch tubes) usually utilized for anaerobic studies. For issues of quality control/assurance, tubes utilized for this study were baked in muffle furnace at 500 °C to remove organic contaminants. Growth and degradation studies were performed in balch tubes containing 10 mL of MS medium, the tested PAH, inoculum, and approximately 15 mL air headspace to maintain aerobic conditions. The different tubes were supplemented with different PAHs respectively. Naphthalene was added from an HMN stock solution at a concentration of ca. 123 ppm as described above and inoculated with 105 cells/mL of phosphate buffer (pH 7.25) washed cells pre-grown in 2.5 mM benzoate. All the stock solutions were aseptically prepared before use. Fluoranthene, chrysene and pyrene were added from the stock solution into the balch tubes using a gas-tight syringe in 250-µL aliquots, to provide test compound concentration ca. 97 ppm for fluoranthene, ca. 64 ppm for chrysene, and pyrene ca. 94 ppm in the final medium. Balch tubes were crimp-sealed with teflon-coated, butyl rubber stoppers to prevent losses due to volatilization or sorption. The tubes were incubated horizontally on a shaker table at (120 rev/min) at ambient temperature. Epifluorescence microscopic examination was utilized to monitor growth by counting the cells numbers using replicate tubes. The cells were stained with acridine orange stain after fixation with 50 µL of glutraldehyde. The acridine orange stain bind to the DNA of the cells and is usually used to determine the total bacteria cells present. Visual examinations in agreement with periodic GC analyses to measure the test compound disappearance was also done. In this study, growth was positive when there is an increase in turbidity greater than the killed or abiotic control that was used. For statistical estimate, at least 10 microscopic fields were randomly chosen and a minimum of 1000 cells were counted. Data are presented as the mean cell numbers ± the SEM.
Transformation of PAH compounds - naphthalene, fluoranthene, pyrene and chrysene experiments
The degradation studies of the PAHs - naphthalene, fluoranthene, pyrene and chrysene were likewise conducted in the balch tubes. The tubes were inoculated with the different bacterial cultures, crimp sealed and incubated horizontally on the shaker table at ambient temperature. After 14 days, the degradation reactions were stopped for naphthalene while experiments with fluoranthene, pyrene and chrysene were stopped after 21 days. 5 mL of hexane was added, vortexing for 1-2 min and subsequently, mixed continuously on a tube rotator for 12 h to stop the degradation study. Beckman GS-6 series centrifuge at 2190 rpm for 20 min was used to separate the hexane fraction and the aqueous phase. The hexane extracts were collected for further analysis. The extracts were stored in target vials with a headspace of 1 mL and crimp sealed using an 11 mm Teflon rubber stopper from National scientific and preserved at 4 °C prior to analysis.
Analytical methods
Gas chromatography coupled flame ionization detector and statistical analysis
The hexane extracts were analyzed on an HP 5890 Series II gas chromatography GC (Hewlett Packard Co., Palo Alto, CA, USA) fitted with an HP 3396 series II integrator and equipped with a flame ionization detector (FID). Hexane extracts (5 µL injection volume) were injected using a 10-µL Hamilton gas-tight syringe through a 30 m HP-5 megabore fused-silica capillary column (J & W Scientific, Folsom, CA, USA; 0.32 mm id, 0.25 µm film thickness). The GC utilized helium (He) as the carrier gas with a linear velocity He flow rate = ∼3.33 mL/min; H2 = ∼30 mL/min; air = ∼400 mL/min. The injector and detector temperatures ranged between 180 and 300 °C with two different rates. The program cycle for naphthalene was at an initial temperature of 50 °C; this was held for 5 min then ramped at 30 °C/min to 180 °C for 2 min, then ramped to 300 °C at 40 °C/min for 4 min. Analytical standards of PAHs were prepared in hexane. Typical coefficients of correlation for standard curves were 0.98-0.99. Statistical tests was performed using the Prism 4.0 computer software program (Graph Pad Software, San Diego, CA, USA) and statistical package for social scientist (SPSS) 15.0.
Results
Isolation and phylogenetic characterization of the PAH degrading strains
Ten morphological different microbial colonies were selected from the MS agar plates following initial enrichment on pyrene. Upon screening individual isolates for growth on MS salicyclic acid, MS benzoate and we selected two isolates for further study. The colony morphology of some isolates observed under the fluorescent microscope showed uniform bacillary rods, producing non-fluorescent diffusible blue pigment. 16S rRNA phylogenetic analyses placed our strains PB-1 and PB-2 within the genus Pseudomonas (Table 1). The closest relative of strain PB-1 had 99% similarity as P. plecoglossicida strain 2-3 (EU594553),2727 Shi Y. Studies on Communities of Endophytic Microorganisms and Dynamic Analysis from Sugar Beet on the North Slope of Xinjiang Tianshan Mountain; 2008. www.ncbi.nlm.gov; Accessed 2014.
www.ncbi.nlm.gov...
a root organism isolated from root of sugar beet. Strain PB-2 had 98% homology as Pseudomonas sp. strain MTQ15 (HQ143608),2828 Jin F, Ding Y, Ding W, Reddy MS, Fernando WG, Du B. Genetic diversity and phylogeny of antagonistic bacteria against Phytophthora nicotianae isolated from Tobacco Rhizosphere. Int J Mol Sci. 2011;12:3055-3071. isolated from the soil around the rhizosphere surrounding tobacco plant in China. We have classified our isolates as P. plecoglossicida strain PB1, and Pseudomonas sp. strain PB2 (GenBank database accession numbers JN624752 and JN624753 issued).
Determination of CHN ratio
The obtained quantity of carbon, nitrogen and hydrogen from the soil samples from McDoel switchyard from different locations within the site is as shown in Table 2. It was apparent that each of the soil sample examined had similar trend where nitrogen being the lowest values and carbon the highest values.
Degradation of naphthalene
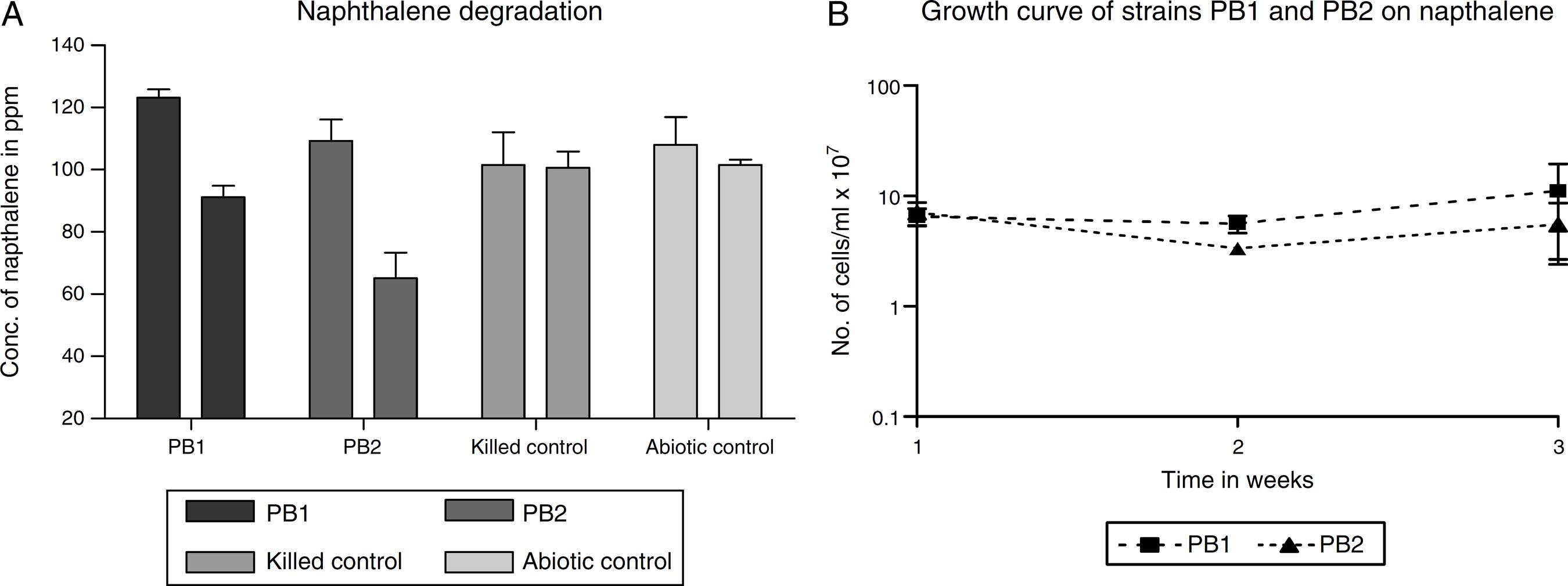
In this study, we observed that the initial and final values (data not shown) obtained for the abiotic and biotic controls had no significant difference with the values obtained at the initial stage for the test samples. The isolated strains PB-1 and PB-2 were evaluated on naphthalene to determine its degradative potential on the test compound. The evaluation was done with strains PB-1 and PB-2 washed cells grown on MS-benzoate. We employed no other carbon source other than the provided naphthalene. Following 14 days of incubation, strains PB-1 and PB-2 ability to utilize naphthalene was appraised by comparing the GC peak areas of the initial day time (0) and the final time (t). We established the growth of our strains by the intense increase in the turbidity of the test sample and considerable reduction in the concentration of naphthalene. (Fig. 1A) shows the values of the net reduction (percent reduction in total naphthalene content) in naphthalene concentration. These were 26% and 40% respectively for strain PB-1 and PB-2. The initial concentration of naphthalene at time zero was ca. 123 ppm while the final concentration ranged between 65 and 91 ppm. The mean biodegradation rate of naphthalene by strain PB-1 was 0.095 ± 0.004 mg L-1 h-1. The mean PB-2 biodegradation rate for naphthalene was 0.131 ± 0.005 mg L-1 h-1.
(A) Degradation of naphthalene by MS-benzoate grown cells of PB-1, PB-2, incubated for 14 days. Data represent the mean and standard deviation of triplicate determination of initial and final concentration respectively. The error bars (Standard Deviation) were due to differential response of cells in triplicate tubes. (B) Naphthlane-dependent growth and cell numbers distribution of strains PB-1, PB-2, in naphthalene incubated for 14 days. Data represent the mean of replicates tubes for initial time (0) cell density represented as (1) and final time (14 days) represented as (3) respectively. The x-axis value range was chosen as such to allow for even spread of the growth curve. The large error bars (Standard Deviation) were due to differential response of cells in triplicate tubes.
Growth of PB-1 and PB-2 on naphthalene
In this study, we defined growth as increase in the cell number of at least one-order-of-magnitude and concomitant disappearance of the parent compound when compared to the biotic and abiotic control. We determined that growth observed was not from HMN by supplementing it as the only carbon source. It was observed that no appreciable growth occurred for strains PB-1 and PB-2 in HMN. Consequently in all cases, cell numbers increased by a significant orders-of-magnitude than the balch tubes of the abiotic and biotic control. This evidently demonstrated growth on naphthalene. (Fig. 1B) shows the results of growth profiles of strains PB-1,2. Cell numbers were counted after 7 days. Strains PB-1 and PB-2, had decline in the cell number when compared at time (0) after one week. Over the course of this investigation, strain PB-2, exhibited an increase in the cell numbers that occurred until the end of 14 days incubation period. Possibly the observed decline in cell numbers may be due to stress experienced by the cells that were active at log phase having been pre-grown in fresh MS-benzoate.
Degradation of chrysene
The strains PB-1 and PB-2 consumed chrysene as a source of its energy and carbon sources. Evidently, the strains exhibited increases in their cell numbers. However, each of the strains had a different growth pattern as shown by the growth profile graph (Fig. 2B). The strain PB-2 was able to degrade more of the chrysene than strain PB-1. The (mean and standard deviation values) of chrysene used in this investigation was ca. 64 ppm. At the end of the 21 days incubation period, the strains degraded about 14-16% of the chrysene. The final concentration (mean and standard deviation) assessed was 54 ppm. Strain PB-1 consumed the chrysene at 14% at volume biodegradation rate of 0.017 ± 0.011 mg L-1 h-1. Strain PB-2 utilized 16% of chrysene at the volume biodegradation rate of 0.021 ± 0.009 mg L-1 h-1. It was evident from the growth profile study in Fig. 2B that after the second week, there was a decline in the cell numbers of PB-1 and PB-2, this may perhaps be due to dead end products or intolerance to the produced intermediate products.
(A) Degradation of chrysene by MS-benzoate grown cells of PB-1 and PB-2, incubated for 21 days. Data represent the mean and standard deviation of triplicate determination of initial and final concentration respectively. The error bars (Standard Deviation) were due to differential response of cells in triplicate tubes. (B) Chrysene dependent growth and cell numbers distribution of strains PB-1 and PB-2, in chrysene incubated for 21 days. Data represent the mean of replicates tubes for initial time (0) cell density represented as (1) and final time (21 days) represented as (4) respectively. The x-axis value range was chosen as such to allow for even spread of the growth curve. The error bars (Standard Deviation) were due to differential response of cells in triplicate tubes.
Degradation of fluoranthene
The abilities of strains PB-1 and PB-2 to degrade fluoranthene was assessed using washed benzoate-grown cells. The percentage net reductions for fluoranthene are 5% and 7% for strains PB-1 and PB-2 respectively. Representing the concentration in ppm the initial concentration ca. 97 ppm and the final ca. 91 ppm thus this signifies minimal utilization of fluoranthene (Fig. 3A). The mean biodegradation rate used by strain PB-1 was 0.009 ± 0.0001 mg L-1 h-1. Strain PB-2 consumed fluoranthene at the rate of 0.013 ± 0.005 mg L-1 h-1. From the growth profile in Fig. 3B, the strains did not show any lag phase possibly due to endogenous utilization of the substrates. This lasted for about a week period. Thereafter, there was sharp decline in the cell numbers that continued to the end of the investigation. This may suggest that the organisms could not tolerate high concentration of fluoranthene. In addition, the rate of decline for strain PB-1 and PB-2 were similar.
(A) Degradation of fluoranthene by MS-benzoate grown cells of PB-1 and PB-2, incubated for 21 days. Data represent the mean and standard deviation of triplicate determination of initial and final concentration respectively. The error bars (Standard Deviation) were due to differential response of cells in triplicate tubes. (B) Fluoranthene dependent growth and cell numbers distribution of strains PB-1 and PB-2, in chrysene incubated for 21 days. Data represent the mean of replicates tubes for initial time (0) cell density represented as (1) and final time (21 days) represented as (4) respectively. The x-axis value range was chosen as such to allow for even spread of the growth curve. The error bars (Standard Deviation) were due to differential response of cells in triplicate tubes.
Degradation of pyrene
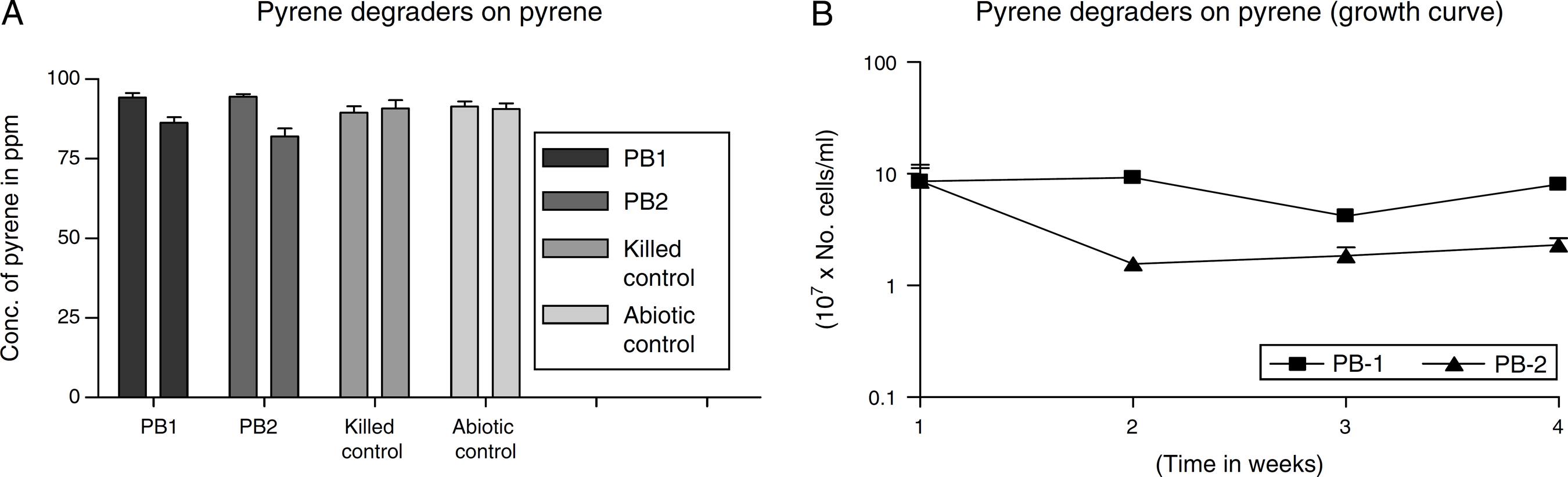
The degradation potential of the MS-benzoate washed cells of strains PB-1 and PB-2 were evaluated on pyrene, having exhibited optimum potential in degrading pyrene on MS-agar sprayed pyrene. Nonetheless, the organisms exhibited low consumption of pyrene in balch tubes supplemented with initial concentration of pyrene ca. 94 ppm and the final concentration ca. 83 ppm After 21 days of incubation, strain PB-1 were able to consume about 8% of pyrene at the biodegradation rate of 0.016 ± 0.001 mg L-1 h-1 while strain PB-2 utilized 13% of pyrene at the biodegradation rate of 0.024 ± 0.005 mg L-1 h-1. In the growth profile in Fig. 4B), it showed that the organisms had a lag period, that resulted in the decline of cell numbers following the assay carried out the first week. After the third week, there was an increase in the cell numbers. Nevertheless, the multiplication in cell numbers was not a sharp logarithmic growth. It showed a gradual increase. There are possibilities, that the organisms might be synthesizing different metabolic pathway having been grown in MS-benzoate before this evaluation. The slow rate of the biodegradation suggests that these strains could utilize pyrene minimally in a liquid medium when compared with growth on the sprayed solid MS-agar. In addition, it is evident that these organisms may require long adaptation period for effective utilization of pyrene.
(A) Degradation of pyrene by MS-benzoate grown cells of PB-1 and PB-2, incubated for 21 days. Data represent the mean and standard deviation of triplicate determination of initial and final concentration respectively. The error bars (Standard Deviation) were due to differential response of cells in triplicate tubes. (B) Pyrene dependent growth and cell numbers distribution of strains PB-1 and PB-2, in chrysene incubated for 21 days. Data represent the mean of replicates tubes for initial time (0) cell density represented as (1) and final time (21 days) represented as (4) respectively. The x-axis value range was chosen as such to allow for even spread of the growth curve. The error bars (Standard Deviation) were due to differential response of cells in triplicate tubes.
Discussion
Many PAHs have been noted to be toxic and as such interest in understanding the physicochemical processes and microbial degradation activities that influences these PAHs in soil are important. Soils subjected to a constant contamination can yield a natural selection of autochthonous pollutant - biodegrading microbial population.2929 Kastner M, Breuer-Jammali M, Mahro B. Enumeration and characterization of the soil microflora from hydrocarbon-contaminated soil sites able to mineralize polycyclic aromatic hydrocarbons. Appl Microbiol Biotechnol. 1994;41:267-273. Microorganisms obtained from such polluted soil are efficient in using hydrocarbons as carbon and energy sources. Thus the art of application of microorganisms for the bioremediation of PAH-contaminated environment is an attractive technology for the reclamation and sustainable development of polluted sites. In this study, we have isolated and characterized P. plecoglossicida strain PB1, and Pseudomonas sp. strain PB2 with capacity to grow and partially degrade the selected PAHs - naphthalene, fluoranthene, pyrene and chrysene.
Often times, sites contaminated with hydrocarbon could be difficult reclaiming, partly due to the imbalance in the carbon:nitrogen:phosphorus (C:N:P) ratio, caused by high carbon and low nitrogen levels might slow down or even prevent biodegradation. The percentage of C:H:N ratio obtained from the soil analyses (samples 1, 2, 3) from the McDoel switchyard is as shown in Table 2. An imbalance in the C:N:P ratio has been found to reduce the capacity of microbes to form viable biomass in using PAHs as carbon/energy source. Van Hamme and co-workers3030 Van Hamme JD, Singh A, Ward OP. Recent advances in petroleum microbiology. Microbiol Mol Biol Rev. 2003;67:503-549. revealed that nitrogen and phosphorus contents greatly affect microbial degradation of hydrocarbon. Besides organic compounds as carbon source (C), productive PAH-metabolizing microorganisms require inorganic macronutrients such as nitrogen and phosphorus for biomass production. Thus the macronutrients requirements for C, N and P for bacterial growth are not constant but vary with type, carbon source utilized and the environmental habitat. In addition, Verde and co-workers,3131 Verde K, Heldal M, Norland S, Bratbak G. Elemental composition (C, N, P) and cell volume of exponentially growing and nutrient-limited bacterioplankton. Appl Environ Microbiol. 2002;68:2965-2971. reported that the amount of N and P required for metabolism of a certain amount of PAHs depends on the bacterial specific growth rate, the elemental composition of the bacterial cells formed and the maximum carbon conversion efficiency. By itself, the PAH conversion rate and growth of PAH-degrading species in a soil environment very much relies on the available concentrations of N and P. Thus from the obtained results of C:H:N ratio, these variations in these essential nutrients might have accounted for the high levels of PAH recorded during the environmental audit.
The evolution of degradative abilities among bacterial strains may be as a result of the plasmids. Plasmids allow bacterial populations to access the horizontal gene pool for adaptive traits that may be useful for their survival under local selective pressures imposed by organic pollutants.
On the assessment of our strains PB-1 and PB-2 for their degradation and growth studies on naphthalene, we believed that the HMN a highly branched alkane did not induce aromatic dioxygenase, thus functioned as intended, i.e., reduction in volatility and facilitated mass transfer of naphthalene into the medium. For naphthalene, the amount utilized by strains PB-1 and PB-2 were 26% and 40% respectively from initial concentration ca. 123 ppm. These values seemed low when compared to other bacterial strains known to degrade naphthalene. This may suggest that previous exposure of our strains to pyrene during enrichment may influence the type of plasmid/catabolic enzymes expressed by the bacterial species. From the growth profile in Fig. 1B, there was a decline in the cell number which suggests that the organisms may had to adapt to the new substrate and synthesize new pathway for utilization of naphthalene. It may also be that naphthalene caused a physiological stress on strain PB-1 and PB-2. From studies of Foght and Westlake,3232 Foght JM, Westlake DW. Transposon and spontaneous deletion mutants of plasmid-borne genes encoding polycyclic aromatic degradation by a strain of Pseudomonas fluorescens. Biodegradation. 1996;7:353-366. a plasmid may encode a complete degradative pathway or partial degradative step. Some other plasmids may code for enzymes that have specificity for several substrates. In addition, Foght and Westlake in their investigation reported of genes encoding the upper and lower pathways of naphthalene within the NAH plasmids of several pseudomonads. The noted of broad specificities that allowed the host to grow on several two and three-ring PAHs, as sole carbon and energy sources. In view of this, we assessed the degradative capacity of strain PB-1 and PB-2 on chrysene. From the obtained values, it was evident that they could utilize chrysene partially at 13% and 16% from the original concentration of about 79 ppm. In fluoranthene, the bacterial strains (PB-1 and PB-2) utilized 4% and 7% from the initial concentration of 98 ppm respectively. On pyrene, 8% and 13% were utilized from the initial concentration of 94 ppm. It is obvious from the growth profile, that chrysene and pyrene were partially degraded by these bacterial strains. However, a decline in cell numbers was evident in the fluoranthene study. It can be deduced from our obtained results, that the strains utilized naphthalene, pyrene and chrysene at slow rate. The slow rate of utilization of pyrene may be that the organisms were not able synthesize enough biosurfactants that will aid mass transfer of the substrate.
Pyrene a HMW PAH of ubiquitous distribution, environmental persistence, and potentially with deleterious effect on human health has prompted the development of clean up of pyrene contaminated soils. Conversely, most bacterial species show limited ability to degrade these hydrophobic compounds.3333 Lundstedt S [M.Sc. dissertation] Analysis of PAHs and Their Transformation Products in Contaminated Soil and Remedial Processes. Sweden: Department of Chemistry-Environmental Chemistry, Umeå Unversity; 2003, 45 pp.,3434 Wilson SC, Jones KC. Bioremediation of soil contaminated with polynuclear aromatic hydrocarbon (PAHs) - a review. Environ Poll. 1993;81:229-249. From this study, the strains PB-1 and PB-2 may possess broad spectrum catabolic plasmids having shown potentials of utilizing the HMW PAHs - pyrene and chrysene. In addition, the limited degradation of chrysene and pyrene may be explained based on the stereochemistry of their structures. Chrysene and pyrene have four of six membered rings. The only difference is in their arrangement, where pyrene is fused together to form a crystalline ball structure while chrysene is almost linear combination of all rings. In this study, strains PB-1 and PB-2 were able to exhibit similar behavior to chrysene and pyrene but not to fluoranthene with three six membered ring and one five membered ring. It goes to show that the stereochemistry influences the recalcitrance of most aromatic hydrocarbons. Boldrin and other workers3535 Boldrin B, Tiehm A, Fritzsche C. Degradation of phenanthrene, fluorine, fluoranthene and pyrene by a Mycobacterium sp. Appl Environ Microbiol. 1993;59:1927-1930. isolated Mycobacterium strain BB1 from a former coal gasification site. The organism had an exponential growth on solid pyrene (0.04 h-1) as sole carbon and energy source. Kastner and co-workers2929 Kastner M, Breuer-Jammali M, Mahro B. Enumeration and characterization of the soil microflora from hydrocarbon-contaminated soil sites able to mineralize polycyclic aromatic hydrocarbons. Appl Microbiol Biotechnol. 1994;41:267-273. isolated Gordona sp BP9 capable of using pyrene as a sole carbon and energy source from PAH contaminated site using the plate screening technique.
For the predictive models based on the growth curve plots Figs. 5-8(A and B), it showed that a nonlinear relationship of the polynomial type describes adequately the relationship between both the PB-1 and PB-2 with time. Thus, based on the results of the statistical nonlinear regression modeling presented by the predictive growth plots, the values of the measure of explanatory power of the model (R2) is indicative that the models predicts accurately changes in the growth pattern as the time in weeks changes. Hence, variability in the growth patterns of the PBs is associated with the length of time of action.
In conclusion, P. plecoglossicida strain PB1 and Pseudomonas sp. PB2 could partially degrade pyrene, chrysene, naphthalene and fluoranthene at low concentrations. The predictive model (R2) revalidates the growth profile of our bacterial strains PB1 and PB2. However, this model may be applied to determine degradation rates of slow-growing PAH degraders. As a whole, the results suggested that P. plecoglossicida strain PB1 and Pseudomonas sp. PB2 would be an excellent candidates with a potential for future application in the bioremediation of PAH-contaminated sites.
-
Associate Editor: Cynthia Canêdo da Silva
Acknowledgements
The authors would like to acknowledge the financial support of Institute of International Education, administrators of the Fulbright Scholarship which supported O. Nwinyi and the School of Public and Environmental Affairs Indiana University, Bloomington, IN, USA, for additional support. The authors appreciate the inputs of Associate Professor Flynn W. Picardal and An Thuy of the School of Public and Environmental Affairs Indiana University, Bloomington, IN, USA.
REFERENCES
-
1Jacques RJS, Okeke BC, Bento FM, Peralba MCR, Camaro FAV. Improved enrichment and isolation of polycyclic aromatic hydrocarbons (PAH)-degrading microorganisms in soil using Anthracene as a Model PAH. Curr Microbiol 2009;58:628-634.
-
2Cheung P-Y, Kinkle BK. Mycobacterium diversity and pyrene mineralization in petroleum-contaminated soils. Appl Environ Microbiol 2001;67:2222-2229.
-
3Peng RH, Xiong AS, Xue Y, et al. Microbial biodegradation of polyaromatic hydrocarbons. FEMS Microbiol Rev 2008;32:927-955.
-
4Maliszewska-Kordybach B. Sources, concentrations, fate and effects of polycyclic aromatic hydrocarbons (PAHs) in the environment. Part A: PAHs in air. Pol J Environ Stud 1999;8:131-136.
-
5Nwinyi OC, Picardal FW, Thuy A, Amund OO. Aerobic degradation of naphthalene, fluoranthene, pyrene and chrysene using indigenous strains of bacteria isolated from an old polluted industrial site. Can J Pure Appl Sci 2013;7:2303-2314.
-
6Samanta SK, Singh OV, Jain RK. Polycyclic aromatic hydrocarbons: environmental pollution and bioremediation. Trends Biotechnol 2002;20:243-248.
-
7USEPA Clean Air Act (42 USC, 7401-7626). Washington, DC: United States Environmental Protection Agency. Available at http://www.epa.gov/air/caa/2004; Accessed 2014.
» http://www.epa.gov/air/caa/2004 -
8Preuss R, Angerer JR, Drexler H. Naphthalene - an environmental and occupational toxicant. Int Arch Occup Environ Health 2003;76:556-576.
-
9Umweltbundesamt. Naphthalin/naphthole and human biomonitoring. Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz 2007;50:1357-1364.
-
10Bogen KT, Beson JM, Yost GS, Morris JB, Dahl AR, Clewell HJ. Naphthalene metabolism in relation to target tissue anatomy, physiology, cytotoxicity and tumorigenic mechanism of action. Regul Toxicol Pharmacol 2008;51:27-36.
-
11Johnsen AR, Wick LY, Harms L. Principles of microbial PAH-degradation in soil. Environ Poll 2005;133:71-84.
-
12Regonne RK, Martin F, Mbawala A, Ngassoum MR, Jouanneau Y. Identification of soil bacteria able to degrade phenanthrene bound to a hydrophobic sorbent in situ. Environ Poll 2013;180:145-151.
-
13Cerniglia CE. Fungal metabolism of polycyclic aromatic hydrocarbons: past, present and future applications in bioremediation. J Ind Microbiol Biotechnol 1997;19:324-333.
-
14Wikada J, Nojiri H, Kasuga K, Yoshida T, Habe H, Omori T. Molecular detection and diversity of polycyclic aromatic hydrocarbon-degrading bacteria isolated from geographically diverse sites. Appl Microbiol Biotechnol 2002;58:202-209.
-
15Mroczek A, Piotrowska-Seget S, Labuzek A. Bacterial degradation and bioremediation of polycyclic aromatic hydrocarbons. Polish J Environ Stud 2003;12:15-25.
-
16Andreoni V, Cavalca L, Rao MA, et al. Bacterial communities and enzyme activities of PAHs polluted soils. Chemosphere 2004;57:401-412.
-
17Hickey AM, Gordon L, Dobson AD, Kelly CT, Doyle EM. Effect of surfactants on fluoranthene degradation by Pseudomonas alcaligenes PA-10. Appl Microbiol Biotechnol 2007;74:851-856.
-
18Kanaly RA, Harayama S. Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons by bacteria. J Bacteriol 2000;182:2059-2067.
-
19Davidova IA, Gieg LM, Duncan KE, Suflita JM. Anaerobic phenanthrene mineralization by a carboxylating sulfate-reducing bacterial enrichment. ISME J 2007;1:436-442.
-
20Kim S, Picardal FW. A novel bacterium that utilizes monochlorobiphenyls and 4-chlorobenzoate as growth substrates. FEMS Microbiol Lett 2000;185:225-229.
-
21Nwinyi OC, Nwodo CS, Amund OO. Biodegradation potential of two Rhodococcus strains capable of utilizing aniline as carbon source in tropical ecosystem. Res J Microbiol 2008;3:99-104.
-
22Nwinyi OC. Degradation of askarel (PCB blend) by indigenous aerobic bacteria isolates from dumpsites in Ore, Ondo State, Nigeria. Aust J Basic Appl Sci 2010;4:3938-3948.
-
23Nwinyi OC. Enrichment and Identification of Askarel oil (PCB blend) degrading bacteria enriched from landfill sites in Edo State, Nigeria. Agric Biol J North Am 2011;2:89-100.
-
24Kiyohara H, Nagao K, Yana K. Rapid screen for bacteria degrading water insoluble, solid hydrocarbon on agar plates. Appl Environ Microbiol 1982;43:454-457.
-
25Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98NT. Nucleic Acids Symp Ser 1999;41:95-98.
-
26Altschul SF, Madden TL, Schaffer AA, et al. Gapped BLAST and PSI-Blast: a new generation of protein database search programs. Nucleic Acids Res 1997;25:3389-3402.
-
27Shi Y. Studies on Communities of Endophytic Microorganisms and Dynamic Analysis from Sugar Beet on the North Slope of Xinjiang Tianshan Mountain; 2008. www.ncbi.nlm.gov; Accessed 2014.
» www.ncbi.nlm.gov -
28Jin F, Ding Y, Ding W, Reddy MS, Fernando WG, Du B. Genetic diversity and phylogeny of antagonistic bacteria against Phytophthora nicotianae isolated from Tobacco Rhizosphere. Int J Mol Sci. 2011;12:3055-3071.
-
29Kastner M, Breuer-Jammali M, Mahro B. Enumeration and characterization of the soil microflora from hydrocarbon-contaminated soil sites able to mineralize polycyclic aromatic hydrocarbons. Appl Microbiol Biotechnol 1994;41:267-273.
-
30Van Hamme JD, Singh A, Ward OP. Recent advances in petroleum microbiology. Microbiol Mol Biol Rev 2003;67:503-549.
-
31Verde K, Heldal M, Norland S, Bratbak G. Elemental composition (C, N, P) and cell volume of exponentially growing and nutrient-limited bacterioplankton. Appl Environ Microbiol 2002;68:2965-2971.
-
32Foght JM, Westlake DW. Transposon and spontaneous deletion mutants of plasmid-borne genes encoding polycyclic aromatic degradation by a strain of Pseudomonas fluorescens Biodegradation 1996;7:353-366.
-
33Lundstedt S [M.Sc. dissertation] Analysis of PAHs and Their Transformation Products in Contaminated Soil and Remedial Processes. Sweden: Department of Chemistry-Environmental Chemistry, Umeå Unversity; 2003, 45 pp.
-
34Wilson SC, Jones KC. Bioremediation of soil contaminated with polynuclear aromatic hydrocarbon (PAHs) - a review. Environ Poll 1993;81:229-249.
-
35Boldrin B, Tiehm A, Fritzsche C. Degradation of phenanthrene, fluorine, fluoranthene and pyrene by a Mycobacterium sp. Appl Environ Microbiol 1993;59:1927-1930.
Publication Dates
-
Publication in this collection
Jul-Sep 2016
History
-
Received
23 Jan 2015 -
Accepted
22 Dec 2015