ABSTRACT
Streptomyces lunalinharesii strain 235 produces an antimicrobial substance that is active against sulfate reducing bacteria, the major bacterial group responsible for biofilm formation and biocorrosion in petroleum reservoirs. The use of this antimicrobial substance for sulfate reducing bacteria control is therefore a promising alternative to chemical biocides. In this study the antimicrobial substance did not interfere with the biofilm stability, but the sulfate reducing bacteria biofilm formation was six-fold smaller in carbon steel coupons treated with the antimicrobial substance when compared to the untreated control. A reduction in the most probable number counts of planktonic cells of sulfate reducing bacteria was observed after treatments with the sub-minimal inhibitory concentration, minimal inhibitory concentration, and supra-minimal inhibitory concentration of the antimicrobial substance. Additionally, when the treated coupons were analyzed by scanning electron microscopy, the biofilm formation was found to be substantially reduced when the supra-minimal inhibitory concentration of the antimicrobial substance was used. The coupons used for the biofilm formation had a small weight loss after antimicrobial substance treatment, but corrosion damage was not observed by scanning electron microscopy. The absence of the dsrA gene fragment in the scraped cell suspension after treatment with the supra-minimal inhibitory concentration of the antimicrobial substance suggests that Desulfovibrio alaskensis was not able to adhere to the coupons. This is the first report on an antimicrobial substance produced by Streptomyces active against sulfate reducing bacteria biofilm formation. The application of antimicrobial substance as a potential biocide for sulfate reducing bacteria growth control could be of great interest to the petroleum industry.
Keywords:
Streptomyces; Antimicrobial substance; Biofilm; Biocorrosion; Sulfate reducing bacteria
Introduction
Biofilms are microbial communities of surface-attached cells embedded in a self-produced extracellular polymeric matrix.11 Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinical relevant microorganisms. Clin Microbiol Rev. 2002;15:167-193. In the petroleum industry, the formation of biofilms on carbon steel causes many problems, such as pipe clogging, biofouling of the distribution systems and generating microbiologically influenced corrosion (or biocorrosion).22 Flemming HC. Biofouling in water systems - cases, causes and countermeasures. Appl Microbiol Biotechnol. 2002;59:629-640.
3 Coetser SE, Cloete TE. Biofouling and biocorrosion in industrial water systems. Crit Rev Microbiol. 2005;31:213-232.-44 Videla HA, Herrera LK. Microbiologically influenced corrosion: looking to the future. Int Microbiol. 2005;8:169-180. Biocorrosion is an electrochemical process in which microorganisms initiate, facilitate, or accelerate a corrosion reaction on a metal surface.55 Rajasekar A, Anandkumar B, Maruthamuthu S, Ting Y-P, Rahman PKSM. Characterization of corrosive bacterial consortia isolated from petroleum-product-transporting pipelines. Appl Microbiol Biotechnol. 2010;85:1175-1188. It is a well-documented phenomenon and produces a deterioration of petroleum product pipelines and storage tanks.66 Benka-Coker MO, Metseagharun W, Ekundayo JA. Abundance of sulphate-reducing bacteria in Niger Delta oilfield waters. Bioresour Technol. 1995;54:151-154. Previous studies have emphasized the economic importance of undesirable microbial growth for the petroleum industry.77 Costerton JW, Boivin J. Biofilms and biocorrosion. In: Flemming H-C, Geesey GG, eds. Biofouling and Biocorrosion in Industrial Water Systems. 1991:195–204. Heidelberg, Berlin.
8 Beech IB. Corrosion of technical materials in the presence of biofilms - current understanding and state-of-the-art methods of study. Int Biodeter Biodegrad. 2004;53:177-183.-99 Voordouw G. Production-related petroleum microbiology: progress and prospects. Curr Opin Biotechnol. 2011;22:401-405. It has been estimated that 40% of all internal pipeline corrosion in the petroleum industry can be attributed to biocorrosion.1010 Graves JW, Sullivan EH. International corrosion in gas gathering systems and transmission lines. Mater Prot. 1966;5:33-37. The main bacteria involved in these harmful processes in petroleum industries are the sulfate reducing bacteria (SRB).1111 Postgate JR. The Sulphate-reducing Bacteria. 2nd ed. Cambridge, England: Cambridge University Press; 1984.,1212 Korenblum E, Goulart FRV, Rodrigues IA, et al. Antimicrobial action and anti-corrosion effect against sulfate reducing bacteria by lemongrass (Cymbopogon citratus) essential oil and its major component, the citral. AMB Express. 2013;3:44. These bacteria may lead to the biodegradation of the metal surfaces of tanks and pipes through biofilm formation.1313 Zuo R. Biofilms: strategies for metal corrosion inhibition employing microorganisms. Appl Microbiol Biotechnol. 2007;76:1245-1253.
In petroleum industries, biocides, such as chloride, glutaraldehyde and quaternary ammonium salts, are used for controlling and inhibiting SRB growth.1414 Videla HA. Prevention and control of biocorrosion. Int Biodeter Biodegrad. 2002;49:259-270. However, the appearance of biocide-resistant bacteria and the difficulty of biocides in penetrating the biofilms present serious problems. In addition, the residual concentration, toxicity and persistence of biocides in industrial effluents are of high environmental concern.1212 Korenblum E, Goulart FRV, Rodrigues IA, et al. Antimicrobial action and anti-corrosion effect against sulfate reducing bacteria by lemongrass (Cymbopogon citratus) essential oil and its major component, the citral. AMB Express. 2013;3:44. For these reasons, alternative sources for SRB control are of great interest to the petroleum industry.1515 Nemati T, Jenneman GE, Voordouw G. Impact of nitrate-mediated microbial control of souring in oil reservoirs on the extend of corrosion. Biotechnol Prog. 2001;17:852-859.,1616 Stewart P. Mechanisms of antibiotics resistance in bacterial biofilms. Int J Med Microbiol. 2002;292:107-113. One alternative method to control SRB growth with a reduced environmental impact is the use of antimicrobial substances produced by microorganisms.
Actinobacteria are biotechnologically valuable bacteria.1717 Balagurunathan R, Radhakrishnan M. Biotechnological, genetic engineering and nanotechnological potential of actinomycetes. In: Maheshwari DK, Dubey RC, Saravanamurthu R, eds. Industrial Exploitation of Microorganisms. 1st ed. 2010:302–436. New Delhi, India.,1818 Satheeja SV, Jebakumar SRD. Phylogenetic analysis and antimicrobial activities of Streptomyces isolates from mangrove sediment. J Basic Microbiol. 2011;51:71-79. The bioactive secondary metabolites produced by Actinobacteria are of clinical use and include antibiotics, antitumor agents, immunosuppressive agents and enzymes.1919 Kekuda TRP, Shobha KS, Onkarappa R. Studies on antioxidant and antihelmintic activity of two Streptomyces species isolated from Western Ghat soils of Agumbe, Karnataka. J Pharm Res. 2010;3:26-29.,2020 Ravikumar S, Inbaneson SJ, Uthiraselvam M, Priya SR, Ramu A, Banerjee MB. Diversity of endophytic actinomycetes from Karangkadu mangrove ecosystem and its antibacterial potential against bacterial pathogens. J Pharm Res. 2011;4:294-296. Among the Actinobacteria, approximately 7600 compounds are produced by Streptomyces species.2121 Berdy J. Bioactive microbial metabolites. J Antibiot (Tokyo). 2005;58:1-26. An antimicrobial substance (AMS) produced by strain 235 of S. lunalinharesii, which was originally isolated from a tropical Brazilian soil, was previously shown to inhibit the growth of Desulfovibrio alaskensis NCIMB 13491.2222 Rosa JP, Korenblum E, Franco-Cirigliano MN, et al. Streptomyces lunalinharesii strain 235 shows the potential to inhibit bacteria involved in biocorrosion process. BioMed Res Int. 2013;2013:1-10. This antimicrobial substance showed to be stable at a variety of temperatures and in a wide pH range.2222 Rosa JP, Korenblum E, Franco-Cirigliano MN, et al. Streptomyces lunalinharesii strain 235 shows the potential to inhibit bacteria involved in biocorrosion process. BioMed Res Int. 2013;2013:1-10. In this study, for the first time, the effects of the AMS produced by S. lunalinharesii strain 235 on the formation and stability of the D. alaskensis NCIMB 13491 biofilm on carbon steel surfaces and the consequences of AMS treatment on biocorrosion were evaluated.
Materials and methods
Bacterial strains and growth conditions
The Actinobacteria Streptomyces lunalinharesii strain 235 used in this study was originally isolated from soil of the Atlantic Forest (Rio de Janeiro, Brazil)2323 Coelho RRR, Lopes A, Semêdo LTAS, Cruz FS. Culture filtrates of actinomycetes isolated from tropical soils inhibit Trypanosoma cruzi replication in vitro. Rev Microbiol. 1995;26:307-313. and was identified by our group.2222 Rosa JP, Korenblum E, Franco-Cirigliano MN, et al. Streptomyces lunalinharesii strain 235 shows the potential to inhibit bacteria involved in biocorrosion process. BioMed Res Int. 2013;2013:1-10. It was previously selected as a promising agent for the production of AMS against different microbial strains,2323 Coelho RRR, Lopes A, Semêdo LTAS, Cruz FS. Culture filtrates of actinomycetes isolated from tropical soils inhibit Trypanosoma cruzi replication in vitro. Rev Microbiol. 1995;26:307-313.
24 Semêdo LTAS, Linhares AA, Gomes RC, et al. Isolation and characterization of actinomycetes from Brazilian tropical soils. Microbiol Res. 2001;155:291-299.-2525 Reis SA, Costa LV, Cavalcanti EDC, et al. Protein synthesis inhibitory activity in culture filtrates from new strains of Streptomyces isolated from Brazilian tropical soils. Lett Appl Microbiol. 2003;37:138-143. including aerobic and anaerobic bacteria involved in biocorrosion processes.2222 Rosa JP, Korenblum E, Franco-Cirigliano MN, et al. Streptomyces lunalinharesii strain 235 shows the potential to inhibit bacteria involved in biocorrosion process. BioMed Res Int. 2013;2013:1-10. This strain was grown in yeast extract-malt extract-agar (YMA)2626 Shirling EB, Gottlieb D. Methods for characterization of Streptomyces species. Int J Syst Bacteriol. 1966;16:313-340. under aerobic conditions at 28 °C for 7 days.
The sulfate reducing bacterium Desulfovibrio alaskensis NCIMB 13491 was isolated from a soured oil reservoir2727 Feio MJ, Zinkevich V, Beech IB, et al. Desulfovibrio alaskensis sp. nov., a sulfate-reducing bacterium from a soured oil reservoir. Int J Syst Evol Microbiol. 2004;54:1747-1752. and grown in Postgate C medium1111 Postgate JR. The Sulphate-reducing Bacteria. 2nd ed. Cambridge, England: Cambridge University Press; 1984. at 30 °C for 3 days, in anaerobic conditions using sealed serum bottles (10 mL). The bottles were purged with a N2 flux to achieve anaerobiosis.
Production of the antimicrobial substance (AMS)
Concentrated supernatant containing the AMS produced by strain 235 was obtained as described by Rosa et al.2222 Rosa JP, Korenblum E, Franco-Cirigliano MN, et al. Streptomyces lunalinharesii strain 235 shows the potential to inhibit bacteria involved in biocorrosion process. BioMed Res Int. 2013;2013:1-10. Briefly, a spore suspension (108 spores/mL) was inoculated in 50 mL of liquid chemically defined medium containing glucose2828 von der Weid I, Alviano DS, Santos ALS, Soares RM, Alviano CS, Seldin L. Antimicrobial activity of Paenibacillus peoriae strain NRRL BD-62 against a broad spectrum of phytopathogenic bacteria and fungi. J Appl Microbiol. 2003;95:1143-1151. at pH 7.0. After 7 days of incubation at 28 °C in stationary conditions, 5 mL of the culture was transferred into 2000 mL Erlenmeyer flasks containing 500 mL of the same liquid medium. After 7 days of incubation under the same conditions, the resulting supernatant was filtered through filter paper (Whatman n° 1), and the filtrate lyophilized (Free Zone 4.5) and resuspended in sterile Milli Q water (1.6 mL). The concentrated supernatant containing the AMS was used in the following experiments.
Short-term tests - the effect of AMS on the biofilm formation and stability
The short-term action of AMS on the D. alaskensis biofilm was evaluated during biofilm formation and after its formation, to evaluate its stability. The tests were conducted as described by Clark et al.2929 Clark ME, Edelmann RE, Duley ML, Wall JD, Fields MW. Biofilm formation in Desulfovibrio vulgaris Hildenborough is dependent upon proteins filaments. Environ Microbiol. 2007;9:2844-2854. with some modifications. Petri dishes (5 cm) containing a single carbon steel coupon (10 mm × 10 mm × 2 mm) were filled with AMS (0.5 mL), a reducing solution (0.5 mL) containing sodium thioglycolate 0.0124%, ascorbic acid 0.01% and resazurin 0.4% (pH 7.5) and a cell suspension of D. alaskensis grown in Postgate C medium (107 cells/mL, 1.0 mL). When testing the effect of AMS on the biofilm formation, AMS was introduced at the same time as the cell suspension, as described above. However, when testing the effect of AMS on the biofilm stability, the AMS was inoculated only after biofilm formation. In this case, Petri dishes containing the carbon steel coupon were filled solely with the cell suspension of D. alaskensis grown in Postgate C medium (107 cells/mL, 2.0 mL) and incubated for 24 h. Afterwards, the cells were removed, and 1 mL of the reducing solution and 1 mL of AMS were added. Prior to beginning the experiments, the coupon surfaces were treated with a sandblasting technique, cleaned in 18% HCl, and neutralized by immersion in a sodium bicarbonate solution. Finally, the coupons were washed with distilled water, rinsed in acetone, and dried in an air stream.1515 Nemati T, Jenneman GE, Voordouw G. Impact of nitrate-mediated microbial control of souring in oil reservoirs on the extend of corrosion. Biotechnol Prog. 2001;17:852-859. As an experimental control, Petri dishes containing the reducing solution and the cell suspension of D. alaskensis were used. After 7 days of incubation at 32 °C, the liquid mixture was removed and the coupons were rinsed 3 times in the reducing solution. The coupons were then submerged in a crystal violet dye (0.1%) for 10 min, rinsed 3 times in Milli Q water and destained in an ethanol/acetone solution (80:20, v/v) for 20 min. The destained solution was measured spectrophotometrically at OD580. The original planktonic cell suspension was measured at OD600, and the ratio of OD580 to OD600 was calculated to determine relative biofilm formation and/or stability.
All inoculation and incubation procedures were conducted in an anaerobic chamber (Plas Labs, Lansing, MI, USA), and all experiments were performed in triplicate.
Long-term tests - evaluation of AMS treatment during biofilm formation and its effects on biocorrosion
Two long-term experiments were conducted. The first (Test 1) aimed to analyze the effect of AMS during D. alaskensis biofilm formation. The second (Test 2) evaluated the effects of AMS on the D. alaskensis-produced biocorrosion of a carbon steel coupon (10 mm × 10 mm × 2 mm). Prior to beginning the experiments, the coupon surfaces were sandblasted and cleaned as described by Nemati et al.1515 Nemati T, Jenneman GE, Voordouw G. Impact of nitrate-mediated microbial control of souring in oil reservoirs on the extend of corrosion. Biotechnol Prog. 2001;17:852-859.
The experiments were performed in sealed serum bottles (10 mL) containing a single carbon steel coupon, an SRB cell suspension (107 cells/mL, 0.2 mL) and different amounts of AMS (0.05, 0.03, 0.01 g protein/mL, 1 mL) diluted in Postgate C medium, in order to obtain successive AMS dilutions (of 1/2, 1/4, 1/8). The AMS dilutions corresponded to the supra-MIC (Minimal Inhibitory Concentration) (2× MIC), the MIC and the sub-MIC (0.5× MIC) and were determined by our group.2222 Rosa JP, Korenblum E, Franco-Cirigliano MN, et al. Streptomyces lunalinharesii strain 235 shows the potential to inhibit bacteria involved in biocorrosion process. BioMed Res Int. 2013;2013:1-10. Serum bottles containing only Postgate C medium and serum bottles containing Postgate C medium and a cell suspension of SRB were used as the experimental controls. The experiments were performed in triplicate under anoxic conditions, incubated with stirring (50 rev/min) and maintained at 32 °C. After 30 days of incubation, the coupons were removed aseptically from each experiment inside a sterile laminar flow hood and used for imaging, cell counts and molecular analyses.
All inoculation and incubation procedures were conducted in an anaerobic chamber, and the experiments were performed in triplicate.
Scanning electronic microscopy (SEM) of the biofilms
To visualize biofilm formation by SEM (Test 1), the coupons were rinsed with a synthetic seawater solution (NaF 0.003%, SrCl2.6H2O 0.002%, H3BO3 0.03%, KBr 0.1%, KCl 0.7%, CaCl2 1.113%, Na2SO4 4%, MgCl2·6H2O 10.78%, NaCl 23.5%, NaSiO3·9H2O 0.02%, Na2EDTA 0.01% and NaHCO3 0.2%, pH 7.5), fixed with 2.5% glutaraldehyde (w/v) in 0.1 M cacodylate buffer (pH 7.2) and kept for 24 h at 4 °C. Then, the coupons were rinsed three times with the same cacodylate buffer for 10 min, dehydrated in ethanol solutions of increasing concentration (30, 50, 70 and 90%) and washed three times in 100% ethanol for 10 min at each step. After this step, the coupons were chemically dried by immersion in a 50% HMDS solution (hexamethyldisilazane solvent - Sigma) for 3 min.3030 Araujo JC, Téran FC, Oliveira RA, et al. Comparison of hexamethyldisilazane and critical point drying treatments for SEM analysis of anaerobic biofilms and granular sludge. J Electron Microsc (Tokyo). 2003;52:429-433. Finally, the samples were mounted on stubs, and coupon surfaces were sputter coated (Balzers SCD-040) with a 15 nm gold layer. The samples were then examined with a FEI QUANTA 250 microscope (FEI Company, Netherlands).
Corrosion tests
The surface corrosion (Test 2) and coupon weight loss were analyzed. To remove any adherent bacteria, the coupons were scraped with a sterile wooden stick in sterile glass bottles with 9 mL of a reducing solution. For the weight loss test, the coupon surfaces were cleaned (washed in acid, neutralized with sodium bicarbonate, rinsed in water and acetone, and dried in an air stream) as described by Nemati et al.1515 Nemati T, Jenneman GE, Voordouw G. Impact of nitrate-mediated microbial control of souring in oil reservoirs on the extend of corrosion. Biotechnol Prog. 2001;17:852-859. The determination of the exact weight of the coupons was carried out just before starting and after finishing the experiments. The coupon weight (in grams) was determined by using an electronic balance (Bioprecisa, model FA2104N). To calculate the weight loss resulting from corrosion, the weight of the coupon at the end of the experiments and after cleaning was subtracted from the original weight of the coupon. The corrosion rate (CR) of carbon steel coupons for each experiment (including the controls) was calculated and is expressed in mm/year,3131 ASTM G-4/95. Standard guide for conducting corrosion coupon tests in field applications. In: Annual Book of American Society for Testing and Materials Standards 3.02.; 2001:49–57. Philadelphia, PA, USA. using 7.84 g/cm3 as the density of carbon steel. A two-sample t test was performed on the treated and control coupons.3232 Motta VT, Wagner MB. Bioestatística. Caxias do Sul, RS/São Paulo, SP: Educs/Robe Editorial; 2003. In addition, after the coupon surfaces were scraped and cleaned,1515 Nemati T, Jenneman GE, Voordouw G. Impact of nitrate-mediated microbial control of souring in oil reservoirs on the extend of corrosion. Biotechnol Prog. 2001;17:852-859. they were gold coated and examined by SEM (FEI QUANTA 250 microscope) to observe their corrosion patterns. The images were acquired at a resolution of 7192 × 3090 pixels.
Most probable number
The most probable number (MPN) technique was performed as previously described.3333 Coutinho CMLM, Magalhães FC, Araújo Jorge TC. Scanning electron microscope study of biofilm formation on different flow rates over metal surfaces using sulfate-reducing bacteria. Biofouling. 1993;7:19-27. To count the sulfate-reducing bacteria (SRB), an aliquot (1 mL) of the scraped cell suspension solution from the coupons (Test 2) described above was used as the initial inoculum. Decimal dilutions (up to 10-8) were prepared in a synthetic seawater solution. Aliquots (1 mL) from each dilution were then transferred to Postgate E medium1111 Postgate JR. The Sulphate-reducing Bacteria. 2nd ed. Cambridge, England: Cambridge University Press; 1984. in triplicate and incubated at 32 °C. The MPN was determined using the MPN Reference Table.3434 Taylor J. The estimation of members of bacteria by tenfold dilution serial. J Appl Microbiol. 1962;25:54-61. This experiment was conducted under anaerobic conditions.
Molecular analysis
The scraped cell suspension from the coupons (Test 2) and the liquid phase were used to detect the dissimilatory sulfite reductase (DSR) gene. The presence of the dsrA gene indicates the presence of the reducing sulfate bacterium D. alaskensis. The dissimilatory sulfite reductase enzyme catalyzes the six-electron reduction of (bi)sulfite to sulfide, which is the central energy-conserving step of sulfate respiration.3535 Odom JM, Peck HD. Hydrogenase, electron transfer proteins, and energy coupling in the sulfate-reducing bacteria Desulfovibrio. Annu Rev Microbiol. 1984;38:551-592.
Aliquots (3 mL) of the scraped cell suspension and of the liquid phase (2 mL) were filtered through a Millipore membrane (0.45 µm). The DNA extraction of filtrates was accomplished using a direct lyses method with a commercial Fast DNA Spin Kit for soil (Bio Systems, Q Bio Gene, USA), according to the manufacturer's instructions. The extracted DNA was visualized on 0.8% (w/v) agarose gels to assess the DNA integrity and stored at 4 °C prior to use in PCR reactions.
The DNA extracted from the filtrates was amplified using the primers DSR-1F (5'-AC[C/G]CACTGGAAGCACG-3') and DSR-R (5'-GTGGMRCCGTGCAKRTTGG-3'),3636 Wagner M, Roger AJ, Flax JL, Brusseau GA, Stahl DA. Phylogeny of dissimilatory sulfite reductases supports an early origin of sulfate respiration. J Bacteriol. 1998;180:2975-2982.,3737 Kondo J, Nedwell DB, Purdy KJ, Silva SQ. Detection and enumeration of sulphate-reducing bacteria in estuarine sediments by competitive PCR. Geomicrobiol J. 2004;21:145-157. which are specific for the dsrA gene. The 25 µl reaction mixture contained 1 µl (10 ng) of DNA, 1× Taq polymerase buffer (10 mmol Tris-HCl [pH 8.3], 10 mmol KCl), 0.2 mM of each dNTP, 100 nM of each primer, 2.5 mM MgCl2, 1.25 U of Taq DNA polymerase (Promega, Madison, USA) and sterile Milli-Q water. The amplification conditions were as follows: 1× (15 s, 94 °C), 30× (15 s, 94 °C; 20 s 54 °C; 54 s, 72 °C), and 1× (1 min, 72 °C). Positive (D. alaskensis strain NCIMB 13491) and negative controls (without DNA) were run in all amplifications. The PCR products were visualized by electrophoresis on 1.4% agarose gels stained with ethidium bromide (2 µg/mL).
Results
Short-term tests - the effect of AMS on biofilm formation and stability
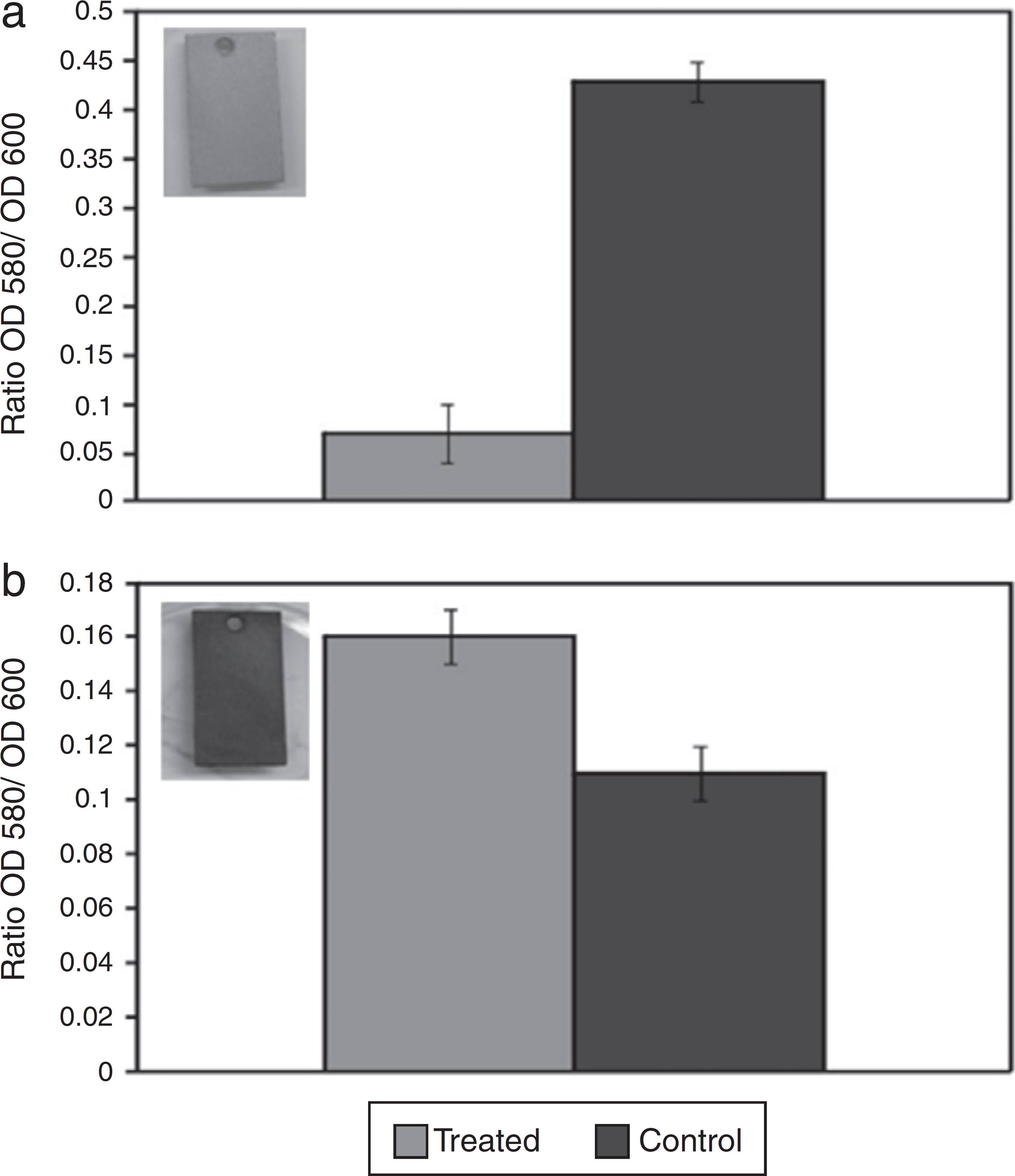
After confirming the inhibitory activity of AMS against D. alaskensis, the action of AMS on the D. alaskensis biofilm was assessed by a short-term test. Quantification of the crystal violet dye retained in the biofilm cells revealed that biofilm formation was six fold smaller in the coupon treated with AMS (Ratio OD580/OD600 = 0.07) when compared to the untreated control (Ratio OD580/OD600 = 0.43) (Fig. 1A). However, it was observed that AMS had no effect on the biofilm stability of D. alaskensis, as it was observed that the coupon that was treated with AMS (Ratio OD580/OD600 = 0.16) retained even more dye than the coupon that received no treatment (Ratio OD580/OD600 = 0.11) (Fig. 1B).
Quantification of the crystal violet dye retained in the biofilm formed by D. alaskensis cells on carbon steel coupons. The effect of the antimicrobial substance (AMS) on biofilm formation (A) and on biofilm stability (B) is shown. The values are expressed as a ratio of the optical densities of 580 and 600. The coupons treated with AMS are shown in the left of each graphic.
The coupons treated with AMS, obtained from both tests, were visually different. The coupon obtained from the first test remained clean without any blackish deposits (Fig. 1A, insert). However, the coupon obtained from the second test was covered in blackish deposits, suggesting iron sulfide formation and therefore the presence of D. alaskensis growth (Fig. 1B, insert).
Long-term tests - evaluation of the effects of AMS treatment during biofilm formation and on biocorrosion
Two experiments (Test 1 and Test 2) were carried out to evaluate the effects of AMS on D. alaskensis biofilm formation and on biocorrosion of the coupon surface. At the end of both experiments, the liquid phases of the experiments containing the 1/4 and 1/8 AMS dilutions and the control cells experiments were blackish, suggesting iron sulfide formation and therefore the presence of D. alaskensis. However, in the experiments containing the 1/2 dilution of AMS (0.05 g protein/mL) and the control without cells, the liquid phases maintained their yellowish color. The SEM comparison between the AMS-treated coupons, the blank coupons without cells (Fig. 2A) and the control cells with biofilm growth (Fig. 2B) shows that the biofilm was substantially reduced in the coupons treated with supra-MIC (1/2 of AMS) (Fig. 2C). However, the coupons treated with MIC (1/4 dilution of AMS) and sub-MIC (1/8 of AMS) (Fig. 2D and E, respectively) were not affected, and biofilms were subsequently formed.
Scanning electronic microscopy (SEM) micrographs showing the coupons treated with different concentrations of the antimicrobial substance (AMS). Blank coupons - without biofilm formation (A); control - coupons not treated with AMS (B); coupons treated with supra-Minimal Inhibitory Concentration (MIC) of AMS (C); and coupons treated with MIC (D) and sub-MIC of AMS (E). Scale bars represent 100 µm.
Corrosion tests
Smaller corrosion rates (CR) were observed on the coupons treated with supra-MIC (the 1/2 dilution of AMS), MIC (the 1/4 dilution of AMS) and sub-MIC (the 1/8 dilution of AMS) (0.27, 0.28 and 0.21 mm/year, respectively) when compared with the control cell experiment (0.38 mm/year). However, these differences were not statistically significant (p > 0.05). Additionally, the corrosion damage on the carbon steel surface was not observed by scanning electron micrographs after treatment with AMS and on the control cells (data not shown).
Most probable number
The impact of AMS on the SRB cells present in the biofilms formed on the coupons from Test 2 were determined using the most probable number (MPN) estimation of the SRB cells (Fig. 3). Biofilm samples from the control cell coupons indicated that the SRB counts were approximately 103 cells/mL. The numbers of SRB cells decreased after treatment with AMS. After treatment with MIC and sub-MIC concentrations of AMS (the 1/4 and 1/8 dilutions of AMS, respectively) the SRB counts were approximately 102 cells/mL. In addition, after treatment with supra-MIC of AMS (dilution 1/2) SRB counts were not detectable.
Most probable number (MPN) of sulfate reducing bacteria (SRB) after the treatment of the coupons with the Minimal Inhibitory Concentration (MIC - 1/4), supra-MIC (1/2) and sub-MIC (1/8) dilutions of AMS. The controls did not receive any treatment.
Molecular analysis
A PCR using primers specific for the dsrA gene, encoding the dissimilatory sulfite reductase enzyme, was used to evaluate the effects of AMS on the D. alaskensis cells from the scraped cell suspension and the liquid phase (Test 2). According to Fig. 4a, in the liquid phase, a fragment with the expected size (approximately 200 bp) was observed after treatment with the supra-MIC, MIC and sub-MIC concentrations of AMS. However, in the scraped cell suspension (Fig. 4b), a fragment with the expected size was not detected after treatment with the supra-MIC of AMS. These results suggest that D. alaskensis could either be absent, or present at quantities below the PCR detection limit, after treatment with the lowest dilution (1/2) of AMS.
Agarose gel electrophoresis showing the PCR fragments (approximately 200 bp) obtained after the amplification with specific primers for the dsrA gene (encoding the dissimilatory sulfite reductase) of the scraped cell suspension (A) and the liquid phase (B) treated with Minimal Inhibitory Concentration (MIC), supra-MIC and sub-MIC dilutions of AMS as templates.
Discussion
The present study examined the effects of AMS produced by S. lunalinharesii strain 235 on the formation and stability of SRB biofilms and on the biocorrosion of carbon steel coupons caused by these bacteria. Biocides are traditionally used to control biofilm formation; however, the environmental impact and cost of using these compounds should be considered. Streptomyces are the prime producers of bioactive compounds for the biotechnology industry. Several clinically significant antibiotics, as well as other widely used drugs targeting common diseases, have been derived from this unique genus affiliated with the order Actinomycetales.3838 Medema MH, Breitling R, Takano E. Synthetic biology in Streptomyces bacteria. Methods Enzymol. 2011;497:485-502. The AMS produced by S. lunalinharesii strain 235 has already been shown to inhibit the growth of SRB.2222 Rosa JP, Korenblum E, Franco-Cirigliano MN, et al. Streptomyces lunalinharesii strain 235 shows the potential to inhibit bacteria involved in biocorrosion process. BioMed Res Int. 2013;2013:1-10. However, further studies on this AMS had not yet been conducted. Several experiments in the present study have demonstrated that the AMS produced by S. lunalinharesii 235 is also able to affect the biofilms of SRB, a bacterial group involved in biofilm formation and pipe corrosion in the oil industry.
Since crystal violet staining was first described by Christensen et al.,3939 Christensen GD, Simpson WA, Younger JJ, et al. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985;22:996-1006. it has been modified to increase its accuracy and to allow biofilm biomass quantification.4040 Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic-Vlahovic M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods. 2000;40:175-179. Crystal violet is a basic dye which binds to negatively charged surface molecules and polysaccharides in the extracellular matrix.4141 Li X, Yan Z, Xu J. Quantitative variation of biofilms among strains in natural populations of Candida albicans. Microbiology. 2003;149:353-362. Crystal violet dye was used for the quantification of cells remaining in the SRB biofilm after AMS treatment in a seven-day experiment, and a reduction of about six fold was observed in the D. alaskensis cells. Consistent with this result, the coupons remained clean and free of blackish deposits, suggesting the absence of ferrous sulfide production from the biocorrosive action of D. alaskensis. However, the AMS was not able to interfere with the stability of the biofilms because when treating biofilms that had already formed with AMS, no significant differences were observed. Using the same method, Clark et al.2929 Clark ME, Edelmann RE, Duley ML, Wall JD, Fields MW. Biofilm formation in Desulfovibrio vulgaris Hildenborough is dependent upon proteins filaments. Environ Microbiol. 2007;9:2844-2854. observed that biofilm formation by Desulfovibrio vulgaris was inhibited approximately two fold after protease treatment. Korenblum et al.1212 Korenblum E, Goulart FRV, Rodrigues IA, et al. Antimicrobial action and anti-corrosion effect against sulfate reducing bacteria by lemongrass (Cymbopogon citratus) essential oil and its major component, the citral. AMB Express. 2013;3:44. also detected a reduction in the biofilm formation of D. alaskensis after treatment with a plant essential oil.
In another experiment, when D. alaskensis cells were incubated with different AMS dilutions for 30 days, the influence of AMS on the biofilm formation was again established. Scanning electron micrographs have shown biofilm formation on the coupons treated with MIC and sub-MIC concentrations of AMS; however, on the coupons treated with supra-MIC, the observed biofilm formation was substantially reduced, confirming that higher concentrations of AMS can inhibit biofilm formation to a significant degree. In addition, the most probable number counts of SRB were reduced after treatment with all three dilutions tested. In order to further verify this result, a more sensible molecular approach was used in the same experiment, and this approach also supported the efficiency of AMS in interfering with the biofilm formation of SRB on carbon steel coupons. After treatment with the lowest dilution (1/2) of AMS, the presence of a fragment with the expected size of the dsrA gene in the liquid phase and the absence of the same fragment in the scraped cell suspension suggested that D. alaskensis was unable to adhere to coupons treated with this dilution of AMS. Our study has revealed that in the experimental conditions used here, the AMS produced by S. lunalinharesii was able to slightly reduce the weight loss of the coupons and thus the amount of biocorrosion. Moreover, corrosion damage was not observed using scanning electronic microscopy. The golden rule in order to avoid biocorrosion in all industrial systems is to keep the system clean.1414 Videla HA. Prevention and control of biocorrosion. Int Biodeter Biodegrad. 2002;49:259-270. Thus, the use of AMS, produced by S. lunalinharesii strain 235, to control SRB biofilm formation would be incredibly useful for preventing metal surface corrosion. However, additional experiments will be necessary to allow for increased AMS production, so it can be detected without a supernatant concentration step.
-
Associate Editor: Raquel Silva Peixoto
Acknowledgements
This study was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and PETROBRAS. We thank Fernando Pereira de Almeida for the SEM images.
REFERENCES
-
1Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinical relevant microorganisms. Clin Microbiol Rev 2002;15:167-193.
-
2Flemming HC. Biofouling in water systems - cases, causes and countermeasures. Appl Microbiol Biotechnol 2002;59:629-640.
-
3Coetser SE, Cloete TE. Biofouling and biocorrosion in industrial water systems. Crit Rev Microbiol 2005;31:213-232.
-
4Videla HA, Herrera LK. Microbiologically influenced corrosion: looking to the future. Int Microbiol 2005;8:169-180.
-
5Rajasekar A, Anandkumar B, Maruthamuthu S, Ting Y-P, Rahman PKSM. Characterization of corrosive bacterial consortia isolated from petroleum-product-transporting pipelines. Appl Microbiol Biotechnol 2010;85:1175-1188.
-
6Benka-Coker MO, Metseagharun W, Ekundayo JA. Abundance of sulphate-reducing bacteria in Niger Delta oilfield waters. Bioresour Technol 1995;54:151-154.
-
7Costerton JW, Boivin J. Biofilms and biocorrosion. In: Flemming H-C, Geesey GG, eds. Biofouling and Biocorrosion in Industrial Water Systems 1991:195–204. Heidelberg, Berlin.
-
8Beech IB. Corrosion of technical materials in the presence of biofilms - current understanding and state-of-the-art methods of study. Int Biodeter Biodegrad 2004;53:177-183.
-
9Voordouw G. Production-related petroleum microbiology: progress and prospects. Curr Opin Biotechnol 2011;22:401-405.
-
10Graves JW, Sullivan EH. International corrosion in gas gathering systems and transmission lines. Mater Prot 1966;5:33-37.
-
11Postgate JR. The Sulphate-reducing Bacteria 2nd ed. Cambridge, England: Cambridge University Press; 1984.
-
12Korenblum E, Goulart FRV, Rodrigues IA, et al. Antimicrobial action and anti-corrosion effect against sulfate reducing bacteria by lemongrass (Cymbopogon citratus) essential oil and its major component, the citral. AMB Express 2013;3:44.
-
13Zuo R. Biofilms: strategies for metal corrosion inhibition employing microorganisms. Appl Microbiol Biotechnol 2007;76:1245-1253.
-
14Videla HA. Prevention and control of biocorrosion. Int Biodeter Biodegrad 2002;49:259-270.
-
15Nemati T, Jenneman GE, Voordouw G. Impact of nitrate-mediated microbial control of souring in oil reservoirs on the extend of corrosion. Biotechnol Prog 2001;17:852-859.
-
16Stewart P. Mechanisms of antibiotics resistance in bacterial biofilms. Int J Med Microbiol 2002;292:107-113.
-
17Balagurunathan R, Radhakrishnan M. Biotechnological, genetic engineering and nanotechnological potential of actinomycetes. In: Maheshwari DK, Dubey RC, Saravanamurthu R, eds. Industrial Exploitation of Microorganisms 1st ed. 2010:302–436. New Delhi, India.
-
18Satheeja SV, Jebakumar SRD. Phylogenetic analysis and antimicrobial activities of Streptomyces isolates from mangrove sediment. J Basic Microbiol 2011;51:71-79.
-
19Kekuda TRP, Shobha KS, Onkarappa R. Studies on antioxidant and antihelmintic activity of two Streptomyces species isolated from Western Ghat soils of Agumbe, Karnataka. J Pharm Res 2010;3:26-29.
-
20Ravikumar S, Inbaneson SJ, Uthiraselvam M, Priya SR, Ramu A, Banerjee MB. Diversity of endophytic actinomycetes from Karangkadu mangrove ecosystem and its antibacterial potential against bacterial pathogens. J Pharm Res 2011;4:294-296.
-
21Berdy J. Bioactive microbial metabolites. J Antibiot (Tokyo) 2005;58:1-26.
-
22Rosa JP, Korenblum E, Franco-Cirigliano MN, et al. Streptomyces lunalinharesii strain 235 shows the potential to inhibit bacteria involved in biocorrosion process. BioMed Res Int 2013;2013:1-10.
-
23Coelho RRR, Lopes A, Semêdo LTAS, Cruz FS. Culture filtrates of actinomycetes isolated from tropical soils inhibit Trypanosoma cruzi replication in vitro. Rev Microbiol 1995;26:307-313.
-
24Semêdo LTAS, Linhares AA, Gomes RC, et al. Isolation and characterization of actinomycetes from Brazilian tropical soils. Microbiol Res 2001;155:291-299.
-
25Reis SA, Costa LV, Cavalcanti EDC, et al. Protein synthesis inhibitory activity in culture filtrates from new strains of Streptomyces isolated from Brazilian tropical soils. Lett Appl Microbiol 2003;37:138-143.
-
26Shirling EB, Gottlieb D. Methods for characterization of Streptomyces species. Int J Syst Bacteriol 1966;16:313-340.
-
27Feio MJ, Zinkevich V, Beech IB, et al. Desulfovibrio alaskensis sp. nov., a sulfate-reducing bacterium from a soured oil reservoir. Int J Syst Evol Microbiol 2004;54:1747-1752.
-
28von der Weid I, Alviano DS, Santos ALS, Soares RM, Alviano CS, Seldin L. Antimicrobial activity of Paenibacillus peoriae strain NRRL BD-62 against a broad spectrum of phytopathogenic bacteria and fungi. J Appl Microbiol 2003;95:1143-1151.
-
29Clark ME, Edelmann RE, Duley ML, Wall JD, Fields MW. Biofilm formation in Desulfovibrio vulgaris Hildenborough is dependent upon proteins filaments. Environ Microbiol 2007;9:2844-2854.
-
30Araujo JC, Téran FC, Oliveira RA, et al. Comparison of hexamethyldisilazane and critical point drying treatments for SEM analysis of anaerobic biofilms and granular sludge. J Electron Microsc (Tokyo) 2003;52:429-433.
-
31ASTM G-4/95. Standard guide for conducting corrosion coupon tests in field applications. In: Annual Book of American Society for Testing and Materials Standards 3.02; 2001:49–57. Philadelphia, PA, USA.
-
32Motta VT, Wagner MB. Bioestatística Caxias do Sul, RS/São Paulo, SP: Educs/Robe Editorial; 2003.
-
33Coutinho CMLM, Magalhães FC, Araújo Jorge TC. Scanning electron microscope study of biofilm formation on different flow rates over metal surfaces using sulfate-reducing bacteria. Biofouling 1993;7:19-27.
-
34Taylor J. The estimation of members of bacteria by tenfold dilution serial. J Appl Microbiol 1962;25:54-61.
-
35Odom JM, Peck HD. Hydrogenase, electron transfer proteins, and energy coupling in the sulfate-reducing bacteria Desulfovibrio. Annu Rev Microbiol 1984;38:551-592.
-
36Wagner M, Roger AJ, Flax JL, Brusseau GA, Stahl DA. Phylogeny of dissimilatory sulfite reductases supports an early origin of sulfate respiration. J Bacteriol 1998;180:2975-2982.
-
37Kondo J, Nedwell DB, Purdy KJ, Silva SQ. Detection and enumeration of sulphate-reducing bacteria in estuarine sediments by competitive PCR. Geomicrobiol J 2004;21:145-157.
-
38Medema MH, Breitling R, Takano E. Synthetic biology in Streptomyces bacteria. Methods Enzymol 2011;497:485-502.
-
39Christensen GD, Simpson WA, Younger JJ, et al. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol 1985;22:996-1006.
-
40Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic-Vlahovic M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods 2000;40:175-179.
-
41Li X, Yan Z, Xu J. Quantitative variation of biofilms among strains in natural populations of Candida albicans Microbiology 2003;149:353-362.
Publication Dates
-
Publication in this collection
Jul-Sep 2016
History
-
Received
25 June 2015 -
Accepted
08 Dec 2015